Abstract
Beyer, KS, Stout, JR, Fukuda, DH, Jajtner, AR, Townsend, JR, Church, DD, Wang, R, Riffe, JJ, Muddle, TWD, Herrlinger, KA, and Hoffman, JR. Impact of polyphenol supplementation on acute and chronic response to resistance training. J Strength Cond Res 31(11): 2945–2954, 2017—This study investigated the effect of a proprietary polyphenol blend (PPB) on acute and chronic adaptations to resistance exercise. Forty untrained men were assigned to control, PPB, or placebo. Participants in PPB or placebo groups completed a 4-week supplementation period (phase I), an acute high-volume exercise bout (phase II), and a 6-week resistance training program (phase III); whereas control completed only testing during phase II. Blood draws were completed during phases I and II. Maximal strength in squat, leg press, and leg extension were assessed before and after phase III. The exercise protocol during phase II consisted of squat, leg press, and leg extension exercises using 70% of the participant's strength. The resistance training program consisted of full-body exercises performed 3 d·wk−1. After phase I, PPB (1.56 ± 0.48 mM) had greater total antioxidant capacity than placebo (1.00 ± 0.90 mM). Changes in strength from phase III were similar between PPB and placebo. Polyphenol blend supplementation may be an effective strategy to increase antioxidant capacity without limiting strength gains from training.
Key Words: antioxidant supplementation, muscle damage, tea
Introduction
Reactive oxygen species are produced in the body after high-intensity bouts of exercise, which serve as messengers to regulate adaptation to exercise (4,23,28–30,33,36). The production of reactive oxygen species has been suggested to be a part of the cascade of events leading to muscle adaptation during exercise training (23,28). However, Reid and Durham (31,32) reported chronic elevations in reactive oxygen species may lead to muscle dysfunction and impaired force production, thus prolonging recovery from muscle damaging exercise (31,32). Consequently, several studies have examined a variety of supplementation strategies as a means to attenuate oxidative stress and muscle damage after exercise (4,23,33). Whether supplementation with polyphenols can attenuate or exacerbate recovery from exercise or adaptation from training remains ambiguous.
Previous research has shown that the acute consumption of polyphenol-containing supplements (1,5,8,9,12,14,16,17,19,22,24,35) will reduce oxidative stress and markers of muscle damage after high-intensity bouts of endurance and resistance exercise. Several studies have reported polyphenol supplementation to result in an attenuation in strength loss, muscle damage, and oxidative stress (1,3,8,14,16,17,22,35), whereas others have reported no differences between polyphenol-supplemented individuals and placebo (PL) (5,9,12,19,24,34). The discrepancy in the literature is most likely due to differences in the type of supplement being investigated, the supplementation protocol, and the specific exercise intervention. The studies showing a positive effect on muscle force recovery after resistance exercise have used tart cherry juice (3,8) or pomegranate juice (22,35). Conversely, the studies showing no effect of supplementation on strength recovery from resistance exercise have generally examined vitamins C and E (27,34), N-acetylcysteine and epigallocatechin gallate (19), quercetin (24), or a fruit-vegetable-berry juice blend (12). Two studies have examined the effect of green tea extracts after acute resistance exercise in trained and untrained men, but neither examined the recovery of muscular force (18,25). Panza et al. (25) observed that 7 days of green tea supplementation increased total polyphenols and total antioxidant capacity (TAC) while reducing circulating indicators of systemic oxidative stress after 4 sets of the bench press exercise in weight-trained men. The other study used a 4-week supplementation protocol with a green tea extract and observed an increase in total antioxidant status and total polyphenols at rest and after a muscular endurance test in untrained men (18). A study by Herrlinger et al. (14) used a proprietary polyphenol blend (tea extracts) after downhill running and reported enhanced recovery of force in active but not trained men; however, no study has examined the effect of this polyphenol blend after an acute high-volume lower-body resistance exercise session.
Numerous studies have examined the effect of chronic polyphenol and antioxidant supplementation on the adaptations to endurance (5,6,13,20,26) and resistance training (18,27,34). Previous studies have shown vitamin C (13,26) and green tea extract (20) to have no effect on maximal oxygen consumption after endurance training in trained or untrained men; however, there is evidence that quercetin or vitamin C supplementation will attenuate the increase in mitochondrial biogenesis after endurance training (6,26). In response to chronic resistance training, strength improvements were not hindered in untrained men by polyphenol supplementation after 4 weeks of training focused on muscular endurance (18) or vitamin C and E supplementation after 4 weeks of eccentric-only training (34). Conversely, vitamin C and E supplementation has been shown to significantly blunt strength gains after 10 weeks of resistance training in recreationally active men (27) and muscle hypertrophy after 12 weeks of resistance training in elderly men (2). However, no studies have examined the effects of a polyphenol blend on the adaptations from a progressive resistance training program.
The conflicting findings of previous research have brought into question the usefulness of antioxidant supplementation during resistance training. As polyphenolic antioxidants have shown promise as recovery strategies from fatiguing and damaging bouts of exercise, supplementation with polyphenols may be an appealing option to recover from an intense resistance exercise bout. However, it is important to determine whether polyphenol supplementation during a resistance training program will augment or diminish adaptations in muscular strength. Thus, the purpose of this study was to investigate the effects of a 4-week polyphenol supplementation period, using a proprietary polyphenol blend (containing green and black tea extracts), on resting hormonal concentrations and circulating indicators of systemic oxidative stress. Furthermore, this study aims to examine the hormonal and oxidative stress response after an acute high-volume lower-body resistance exercise session conducted at the end of the 4-week supplementation period. An additional aim of the study was to examine the ergogenic effect of the polyphenol-blend supplement during a 6-week progressive resistance training program.
Methods
Experimental Approach to the Problem
The current study used a randomized, double-blind, PL-controlled study design. The study timeline is presented in Figure 1. The study consisted of 3 phases. Phase I included the proprietary polyphenol-blend (PPB) supplement and PL groups and consisted of a baseline blood draw (T0), a 4-week daily supplementation period, and a follow-up blood draw (T1). Phase II included control (CON), PPB, and PL groups and consisted of maximal strength testing and a resting blood draw before exercise (T1/Pre), an acute high-volume lower-body resistance exercise session, and follow-up blood draws up to 4 days after exercise. The CON group was included during phase II to determine when participants in PPB and PL returned to a nonexercising level, as the CON group did not perform the high-volume exercise session. Phase III included PPB and PL groups only and consisted of 6 weeks of resistance training and daily supplementation with maximal strength testing at T2 which was compared with the maximal strength values from T1. Throughout the duration of the study, dietary recalls and supplementation compliance were recorded. Supplement compliance had to be at least 80% to be included in the analysis.
Figure 1.
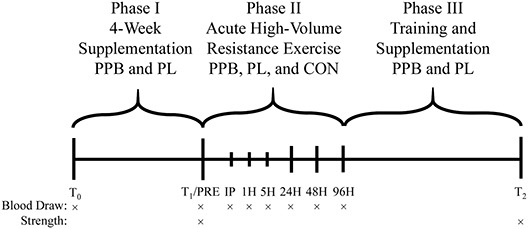
Study timeline. T0 = baseline; Pre/T1 = pre-exercise; IP = immediately after exercise; 1H = 1 hour after exercise; 5H = 5 hours after exercise; 24H = 24 hours after exercise; 48H = 48 hours after exercise; 96H = 96 hours after exercise; T2 = After 6 weeks of resistance training. CON = Control; PPB = proprietary polyphenol blend; PL = placebo.
Subjects
Fifty-eight previously untrained (no structured resistance training within the last year) men were enrolled in this study. Before enrolling in the study, all participants completed a Confidential Medical and Activity Questionnaire, as well as a Physical Activity Readiness Questionnaire, to determine whether they had any physical limitations that would keep them from performing the testing and/or training procedures. Eighteen participants who were enrolled were not included in the analysis. Of those 18 participants who were not included, 9 did not complete any testing, and another 9 did not meet supplement compliance or missed testing sessions. The participants included (18–31 years of age) in the analysis were randomly assigned (according to participant number) into either the CON (mean ± SD; n = 11, 23.3 ± 4.1 years, 1.74 ± 0.12 m, 78.3 ± 14.8 kg), the PPB (n = 14, 21.9 ± 2.5 years, 1.71 ± 0.05 m, 69.3 ± 7.5 kg), or PL (n = 15, 21.5 ± 2.3 years, 1.76 ± 0.05 m, 83.8 ± 15.8 kg). Participants and research staff remained blinded throughout the study and data analysis. Sample size was calculated using G*Power to achieve a power ≥0.80 based on data from previous research using the same supplement (14). Throughout the study, participants were not allowed to use any ergogenic nutritional supplements or engage in any outside structured training program. Written informed consent was obtained from all individual participants included in the study. This study was approved by the New England Institutional Review Board for the protection of human participants. The use of an external institutional review board was approved by the University of Central Florida. A CONSORT schematic outlining the overall study sample is presented in Figure 2.
Figure 2.

CONSORT diagram. Participant screening through study completion is shown for all study participants. Supplementation compliance was prospectively set at >80% throughout the duration of the study.
Supplementation Procedures
All participants in PPB and PL completed daily supplementation with PPB or PL (Kemin Foods, L.C., Des Moines, IA, USA), respectively. The PPB group consumed a proprietary blend of aqueous tea extracts (Camellia sinensis) containing a minimum of 40% total polyphenols, 1.3% theaflavins, 5–8% epigallocatechin-3-gallate (EGCG), 7–13% caffeine, 600 ppm manganese, and formulated under good manufacturing practices. The PL group consumed capsules of similar shape and size to PPB, but contained microcrystalline cellulose instead of the active ingredients. The study product was encapsulated in gelatin capsules and packaged in light-resistant plastic bottles (Five Star Compounding Pharmacy, Des Moines, IA, USA). The product lots were tested for toxins including heavy metals, pesticides, and excipients. Stability of the capsules was confirmed throughout the study period by measurement of the active components. After T0, participants were randomly assigned to PPB, 2,000 mg·d−1 of active supplement, or PL, 2,000 mg·d−1 of microcrystalline cellulose. Participants consumed the 2,000 mg·d−1 in 2 divided doses of 1,000 mg each.
During all phases, participants reported to the Human Performance Lab 5 days per week for their supplement. Supplements that were to be taken on the other 2 days of the week were given in individual containers for later consumption. During phase I, participants took one dose of the supplement in the lab, and the second dose was given to the participant in individual containers for consumption later in the day with a meal at least 6 hours later. During phase II, participants took one dose before completing the acute high-volume lower-body resistance exercise session and the second dose after the 5H testing (which was approximately 6 hours between doses). Throughout the recovery days of phase II, participants took one dose immediately after testing, and the second dose was given to the participant in individual containers for consumption later in the day with a meal at least 6 hours later. During the 6-week resistance training program (phase III), participants consumed one dose of their respective supplement 1 hour before each training session, and the second dose was given to the participant in individual containers for consumption later in the day with a meal at least 6 hours later. For the days they were unable report to the laboratory, participants were given their specified supplement in individual containers to be consumed in the morning with a meal and then 6 hours later with a meal. Participants were asked to return all empty containers upon their next visit to the laboratory. The returned containers were used to determine supplement compliance. No participants withdrew because of or reported serious long-term adverse events related to either treatment.
Maximal Strength Testing Procedures
To assess lower-body strength, 1 repetition maximum (1RM) tests were completed on the squat, leg press, and leg extension exercises at T1 (phase II) and T2 (phase III). The 1RM tests were performed using methods previously described (15). Before beginning strength testing, each participant completed a general and specific warm-up. The general warm-up consisted of riding a cycle ergometer for 5 minutes at the participant's preferred resistance. The specific warm-up consisted of 10 body weight squats, 10 alternating lunges, 10 walking knee hugs, and 10 walking butt kicks. Each participant performed 2 warm-up sets using a resistance level that was approximately 40–60% and 60–80% of his perceived maximum. The third set was the first attempt at the participant's 1RM. If the set was successfully completed, then weight was added and another set was attempted. If the set was not successfully completed, then the weight was reduced and another set was attempted. A 3–5 minutes rest period was provided between each set. This process of adding and removing weight continued until a 1RM was reached. Attempts not meeting the range of motion criterion for each exercise, as determined by the trainer, were discarded. All 1RM tests were completed under the supervision of a Certified Strength and Conditioning Specialist (CSCS).
Acute High-Volume Lower-Body Resistance Exercise Procedures
During phase II, blood samples and visual analog scales were obtained from each participant at T1/Pre, immediately after (IP), 1 hour after (1H), 5 hours after (5H), 24 hours after (24H), 48 hours after (48H), and 96 hours after (96H) an acute high-volume lower-body resistance exercise session. Participants were instructed to abstain from exercise and alcohol consumption for 72 hours before T1/Pre, and food/caffeine intake for 12 hours before T1/Pre. In addition, participants were asked to ensure at least 8 hours of sleep the night before T1/Pre. After the T1/Pre, a blood sample was obtained, and participants were provided a standardized low protein, low carbohydrate breakfast bar (Atkins Nutritionals, Inc., Denver, CO, USA: 7 g protein, 3 g carbohydrate, and 3 g fat) and 1 dose of their respective study supplement. Immediately after consumption, participants completed the same general and specific warm-up as previously described in strength testing. The acute high-volume lower-body resistance exercise session included the 6 sets of squat and 4 sets of leg press and leg extension exercises, in that order. The load for each exercise was 70% of each participant's previously determined 1RM. Each set required participants to complete 10 repetitions. When participants were unable to complete all repetitions within a set by themselves, the CSCS assisted the participant with force repetitions until 10 total repetitions were completed. The rest interval between each set and each exercise was 90 seconds.
Six-Week Resistance Training Procedures
The week after completing phase II, PPB and PL groups reported to the Strength and Conditioning Lab to complete the 6 weeks of resistance training (phase III). Training took place 3 days per week, with at least 1 day of rest in between exercise sessions. If a participant missed an exercise session, a make-up session was scheduled on the weekend with laboratory staff to ensure that 17 total sessions were completed during the 6-week period while still maintaining appropriate rest periods between sessions. One hour before each session, participants reported to the Human Performance Lab for consumption of 1 dose of supplement. Participants completed the same general and specific warm-up as previously described in strength testing. The training was a full-body progressive resistance training protocol focusing on all major muscle groups of the body. The exercises prescribed for the training sessions were squat, leg press, leg extension, hamstrings curl, seated calf raise, step ups, bench press, shoulder press, low row, and lat pulldown. The assigned load for each core exercise was 70% of the previously determined 1RM. For the remaining assistance exercises, trainers adjusted the load to achieve an 8–10RM (approximately 70% of their maximal strength per exercise). Each training session was monitored by a CSCS. All repetitions and loads were charted in a log book. The CSCS adjusted the training load for next training session based on the participant's performance. On successful completion of all required repetitions per exercise (i.e., 3 sets of 10), the training load was increased, by 5–10 lbs (2.3–4.5 kg) for upper-body exercises and by 10–20 lbs (4.5–9.0 kg) for lower-body exercises.
Blood Draw Procedures
Blood samples were obtained at T0 (phase I) from a forearm vein using a 20-gauge disposable needle equipped with a Vacutainer tube holder (Becton Dickinson, Broken Bow, NE, USA). During phase II, blood samples were drawn from a participant's forearm vein using a Teflon cannula (Becton Dickinson, Broken Bow, NE, USA) at T1/Pre, IP, 1H, 5H, 24H, 48H, and 96H exercise. Participants were instructed not to eat or drink (except water) within 10 hours before each blood draw. Blood samples were drawn into untreated (for serum collection), as well as ethylenediaminetetraacetic acid- and heparin-treated (for plasma collection) Vacutainer tubes. Untreated tubes were allowed to clot for 30 minutes before centrifugation, whereas treated tubes were centrifuged immediately for 15 minutes at 1,500g at 4°C. The resulting serum and plasma samples were aliquoted and stored at −80°C until analysis.
Blood Analysis Procedures
Blood lactate concentrations (mM) were analyzed in real-time from plasma using an automated analyzer (Analox GM7 enzymatic metabolite analyzer; Analox Instruments USA, Lunenburg, MA, USA). Plasma concentrations of TAC, total testosterone, ferric-reducing ability of plasma (FRAP), and thiobarbituric reactive substances (TBARS), as well as serum concentrations of creatine kinase (CK), cortisol and myoglobin, were assayed using commercially available enzyme-linked immunosorbent assay kits. The TAC was assessed as the combined antioxidant ability of the sample to prevent the oxidation of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) and is expressed in Trolox equivalents. Assay absorbance was read according to manufacturer specifications on a BioTek Eon Microplate Spectrophotometer (BioTek Instruments, Inc., Winooska, VT, USA). To eliminate interassay variance, all samples for a particular assay remained frozen until analysis, were thawed only once, and were measured in duplicate by a single technician. The coefficient of variation for each assay was 4.7% for TAC, 1.5% for lactate, 7.6% for myoglobin, 3.7% for CK, 4.3% for testosterone, 3.4% for cortisol, 14.4% for FRAP, and 6.5% for TBARS. All assay procedures followed the manufacturers' guidelines.
Visual Analog Scale Procedures
During phase II, participants in PPB and PL were asked to rate their perceived levels of soreness and fatigue from T1/Pre-96H. The scale was a 10-cm line anchored by the words “Lowest” and “Highest.” Participants made a mark on the line to indicate their feeling for each question. Questions were structured as “My level of leg soreness is:” and “My level of fatigue is:”. The validity and reliability of this scale has been previously reported (21).
Dietary Recall Procedures
Participants were instructed to remember as accurately as possible everything they consumed for the 2 days preceding T0, T1, and T2. Participants were asked to mimic their food intake before T0 for both T1 and T2. During phase III, participants were asked to complete a 3-day food log (2 weekdays, 1 weekend day) for each of the 6 weeks of training. FoodWorks Dietary Analysis software (The Nutrition Company, Long Valley, NJ) was used to analyze the dietary recalls for total kilocalorie intake (kcal) and macronutrient distributions (carbohydrate, protein, and fat).
Statistical Analyses
All data were analyzed with separate group × time mixed factorial analysis of variance (ANOVA). For evaluation of effects of supplementation in phase I, resting hormonal concentrations and indicators of systemic oxidative stress at T0 and T1 were assessed with separate 2 × 2 mixed factorial ANOVAs. Phase III evaluation of squat, leg press, and leg extension strength at T1 and T2 were assessed with separate 2 × 2 mixed factorial ANOVAs. In phase II, circulating lactate and myoglobin from Pre-5H were assessed with separate 3 × 4 mixed factorial ANOVAs. Creatine kinase activity at Pre, 24H, 48H, and 96H were assessed with 3 × 4 mixed factorial ANOVAs and circulating testosterone, cortisol, FRAP, TBARS, and TAC from Pre-96H were assessed with separate 3 × 7 mixed factorial ANOVAs. If a significant group × time interaction was observed, relevant post hoc procedures (1-way ANOVA, t-test) were conducted. If significant main effects of time were observed, post hoc least significant differences pairwise comparisons were conducted. Furthermore, Cohen's d was calculated for between-group differences when comparing PPB and PL, and effect sizes were interpreted as small (0.2), medium (0.5), and large (0.8) (7). Statistical software (SPSS; V. 20.0; SPSS, Inc., Chicago, IL, USA) was used for all analyses. Results were considered significant at an alpha level of p ≤ 0.05. Also, results were considered a trend at a p value ≤0.10. All data are reported as mean ± SD.
Results
At baseline, no differences were observed between CON, PPB, and PL in age (p = 0.303) or height (p = 0.230). There was a significant group difference in body mass at T0 (p = 0.019) with PPB (69.3 ± 7.5 kg) being significantly less than PL (83.8 ± 15.8 kg). As body mass was different between PPB and PL, all 1RM values were calculated relative to body mass. Relative squat (p = 0.028) and leg extension (p = 0.007) 1RM were significantly greater in PPB when compared with PL at T1. Furthermore, there was a trend (p = 0.091) toward PPB being greater than PL for relative leg press at T1. In terms of diet, no differences were noted for daily average calories (p = 0.854), protein (p = 0.797), carbohydrates (p = 0.634), or fat (p = 0.986) consumed during the acute high-volume lower-body resistance exercise session between CON, PPB, and PL. Furthermore, no differences were observed in daily average caloric (p = 0.712), protein (p = 0.885), carbohydrate (p = 0.784), and fat (p = 0.827) intake throughout the training period between PPB and PL.
Phase I: 4-Week Supplementation Period
The circulating hormonal concentrations and indicators of systemic oxidative stress before and after the 4-week supplementation period in phase I are presented in Table 1. There was a significant group × time interaction (p = 0.013) for TAC, with differences identified at T1 (p = 0.025). The between-group differences at T1 were large (d = 0.89) in favor of PPB. Furthermore, PPB had a significant increase (p = 0.010) from T0 to T1, whereas PL did not significantly change (p = 0.297). Changes in TAC from presupplementation to postsupplementation in PPB and PL can be seen in Figure 3. No significant group × time interactions (p = 0.302 and p = 0.773) or main effects for time (p = 0.850 and p = 0.818) were noted after 4 weeks of supplementation period in FRAP and TBARS, respectively, with small effects between PPB and PL.
Table 1.
Indicators of systemic oxidative stress and hormonal biomarkers (mean ± SD) during phase I.*
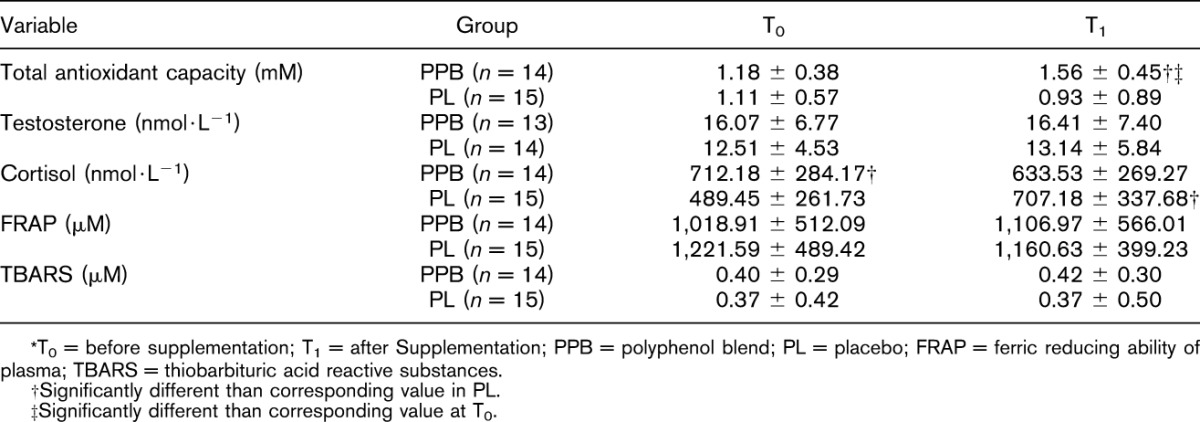
Figure 3.

Change in total antioxidant capacity after 28 days of supplement from baseline to pretraining. T0 = baseline; T1 = pretraining; *significant difference between PPB and PL at T1. †Significant increase from T0 to T1 for PPB group. PPB = proprietary polyphenol blend; PL = placebo.
Comparison of resting cortisol concentrations after the 4-week of supplementation period in phase I revealed a significant group × time interaction (p = 0.010). At T0, PPB was significantly (p = 0.037) greater than PL. Furthermore, evaluation of within-group differences showed that PPB did not significantly change (p = 0.220) from T0 to T1, whereas PL did experience a significant increase (p = 0.023) in resting cortisol concentrations from T0 to T1. No significant interaction (p = 0.770) or main effect of time (p = 0.335) was observed in resting testosterone concentrations after the 4-week supplementation period. At T1, there were only small effects between PPB and PL for cortisol and testosterone concentrations.
Phase II: Acute High-Volume Lower-Body Resistance Exercise Session
Total training volume completed during the acute high-volume lower-body resistance exercise session relative to body mass was not significantly different (p = 0.130) between PPB (312 ± 92 kg per kg body mass) and PL (264 ± 73 kg per kg body mass). Changes in circulating biomarker concentrations during phase II are presented in Table 2. A significant group × time interaction (p < 0.001) for changes in blood lactate was observed. Lactate concentrations during PPB and PL were significantly greater than CON at IP and 1H. Furthermore, there was a medium effect (d = 0.61) of PPB when compared with PL for lactate at 1H. A significant group × time interaction (p < 0.001) was also noted for changes in myoglobin concentrations between CON, PPB, and PL. Myoglobin concentrations were significantly elevated in PPB and PL at IP, 1H, and 5H compared with CON. A significant group × time interaction (p = 0.027) was noted in changes in CK activity between CON, PPB, and PL. Polyphenol blend had significantly greater CK activity when compared with CON at 24H, 48H, and 96H. At 96H, PPB had a medium effect (d = 0.62) when compared with PL for CK activity. No other group differences were noted, and all effect sizes between PPB and PL were less than 0.50.
Table 2.
Mean ± SD of circulating biomarkers during phase II.*
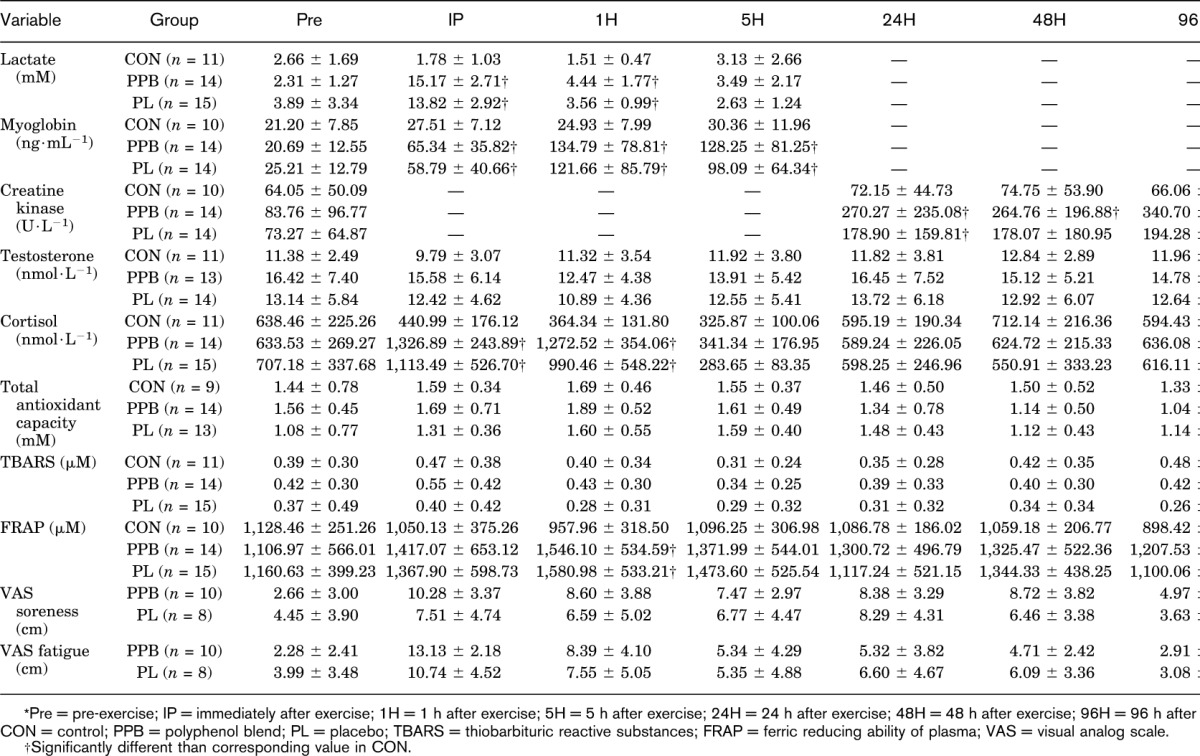
No significant group × time interaction (p = 0.067) was noted in changes in testosterone concentrations between CON, PPB, and PL. However, a significant main effect of time (p = 0.001) was observed. When averaged across groups, testosterone concentration was significantly reduced at IP (12.6 ± 5.3 nmol·L−1) and 1H (11.6 ± 4.1 nmol·L−1) when compared with PRE (13.8 ± 6.0 nmol·L−1). At IP, there was a medium effect (d = 0.58) of PPB when compared with PL for testosterone concentration. A significant group × time interaction (p < 0.001) was observed in the cortisol response between CON, PPB, and PL. Cortisol concentrations were significantly elevated in PPB and PL when compared with CON at IP and 1H. Furthermore, there were medium effects of PPB when compared with PL for cortisol concentrations at IP and 1H.
A significant group × time interaction (p = 0.041) was noted in FRAP. Ferric-reducing ability of plasma was significantly greater at 1H in PPB and PL compared with CON. In addition, no significant group × time interaction (p = 0.501) was noted for TBARS, but a significant main effect of time (p = 0.003) was observed. When averaged across groups, TBARS was significantly elevated at IP when compared with PRE. At 1H and 96H, there were medium effects of PPB when compared with PL for TBARS. Furthermore, there was a trend toward a significant group × time interaction (p = 0.087) and a significant main effect of time (p < 0.001) for TAC. When averaged across groups, TAC was significantly elevated at 1H and 5H when compared with PRE. Moreover, there were medium effects of PPB when compared with PL for TAC at IP and 1H.
No significant group × time interaction (p = 0.356) was observed for soreness, but a significant main effect of time (p < 0.001) was seen. When averaged across groups, soreness at all time points were greater than PRE. No significant group × time interaction (p = 0.384) was observed in fatigue, but a significant main effect of time (p < 0.001) was noted. When averaged across groups, fatigue at PRE was significantly less than IP, 1H, 24H, and 48H.
Phase III: 6-Week Resistance Training and Supplementation Protocol
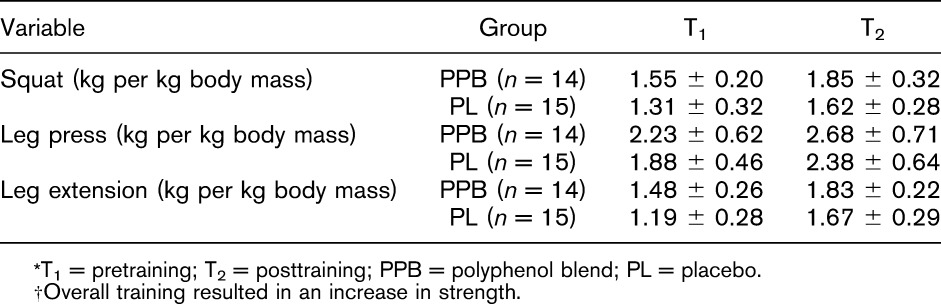
Changes in strength from pretraining (T1) to posttraining (T2) are presented in Table 3. There was no significant difference (p = 0.321) in average weekly training volume between PPB (10,678.98 ± 1,429.61 kg) and PL (11,419.23 ± 2,363.01 kg) during the 6-week resistance training protocol. A significant main effect of time (p < 0.001), but no significant interaction (p = 0.957) was noted when comparing relative squat strength between PPB and PL. When averaged across groups, there was a significant increase from T1 to T2. For leg press, there was a significant main effect of time (p < 0.001), but no significant interaction (p = 0.559) between PPB and PL from T1 to T2. When averaged across groups, a significant increase in leg press was observed from T1 to T2. For leg extension, a significant main effect of time (p < 0.001) was noted, but no significant interaction (p = 0.179) between PPB and PL from T1 to T2. When averaged across groups, a significant increase in leg extension was seen from T1 to T2.
Table 3.
Maximal strength relative to body mass during phase III (mean ± SD).*†
Discussion
The primary finding of this study was that a 4-week supplementation period with PPB resulted in an increase in TAC when compared with PL. Furthermore, an additional 6 weeks of supplementation in conjunction with progressive resistance training yielded similar strength gains between groups.
While the current study observed an increase in TAC in plasma after the 4-week supplementation of PPB, previous research on polyphenol supplementation has yielded equivocal results, most likely because of variable supplementation protocols. A study by Erba et al. (10) used 42 days of green tea extract (320 mg·d−1, 214 mg EGCG per day) and reported an increase in total antioxidant activity compared with baseline in untrained men. Another study by Jówko et al. (18) used untrained men and reported an increase from baseline in total antioxidant status after a 4-week supplementation protocol of green tea extract, defined as 640 mg polyphenols per day; however, this adaptation was not different than a PL group. Furthermore, Jówko et al. (17) observed an increase in TAC after a 4-week supplementation period with green tea extract supplementation (1,000 mg·d−1, 980 mg polyphenols per day, 548 mg EGCG per day) in trained sprinters when compared with a PL group. By contrast, a recent study by Kuo et al. (20) observed no change in antioxidant status from a 4-week green tea extract supplementation using a smaller dosage (250 mg·d−1, 120.5 mg EGCG per day) in untrained men. A study examining a supplement containing both vitamins C (1,000 mg·d−1) and E (400 IU·d−1) reported no effect on TAC after 4 weeks of daily supplementation when compared with PL (34). The change in TAC is most likely dependent on dosage and composition, as the current study used 2,000 mg·d−1 of a proprietary water-extracted polyphenol supplement containing tea components.
In the current study, the elevated TAC at the start of the exercise session and the medium effect of PPB when compared with PL for TAC at IP and 1H may have blunted the increase in reactive oxygen species in response to exercise. The acute high-volume lower-body resistance exercise session resulted in increased lactate, myoglobin, cortisol, and FRAP in PPB and PL when compared with CON, indicating that the exercise session did result in a stress response. Both PPB and PL had significant increases in myoglobin, lactate, cortisol, FRAP, TAC, and perceived levels of soreness and fatigue following an acute high-volume lower-body resistance exercise session; however, no significant differences in these measures were observed between PPB and PL during the recovery period. However, there were medium effects of PPB when compared with PL for TBARS and TAC during the recovery from the exercise session. While the PPB supplement used in this study did contain caffeine, an average of 1.9 ± 0.2 mg·kg−1 of body mass−1 per dose, previous research has shown that acute caffeine ingestion of 6 mg·kg−1 of body mass−1 did not enhance recovery after fatiguing contractions (11). Despite no significant differences between PPB and PL during the acute high-volume resistance exercise session, supplementation with PPB may have a role in augmenting the TBARS and TAC response after an acute high-volume lower-body resistance exercise session in previously untrained men.
In the current study, 6 weeks of progressive resistance training resulted in significant increases in squat, leg press, and leg extension strength for both PPB and PL treated groups, but no differences were noted between the groups. Similar to our findings, previous research has reported conflicting results regarding resistance training adaptations with other antioxidant supplements. Paulsen et al. (27) showed that vitamin C and E supplementation may attenuate the strength gains obtained from 10 weeks of resistance training. However, others have reported that 4 weeks of eccentric training with vitamin C and E supplementation did not affect gains in isometric strength (34). Furthermore, green tea extract supplementation did not alter the changes in 1RM after a 4-week muscular endurance resistance training program (18). The current study provides additional evidence that polyphenol-blend supplementation during a 6-week progressive resistance training program did not attenuate strength improvements. Future research should examine the effect of polyphenol supplementation over longer training durations. Also, the current study only assessed the adaptations to lower-body strength; future research should investigate strength and muscle size adaptations throughout the body. In addition, no blood draws were taken after completing the 6-week training program; therefore, future research is needed to determine any potential changes in markers of systemic oxidative stress or hormone concentrations at rest or in response to exercise after resistance training polyphenol supplementation.
Practical Applications
In conclusion, a 4-week daily supplementation period with PPB significantly increased resting TAC and did not hinder strength gains in untrained male participants after a 6-week resistance training program. Furthermore, supplementation with this PPB may have an impact on increasing that antioxidant response after an acute high-volume lower-body resistance exercise session. However, more research needs to be conducted on this PPB to fully elucidate its role during an acute exercise session. Polyphenol blend may be a useful supplement for individuals looking to increase antioxidant capacity without affecting strength gains from a resistance training program.
Acknowledgments
This study was funded by a grant from Kemin Foods, L.C. Kemin Foods L.C. is the manufacturer and distributor of the active supplement (PPB) which was tested in this study. One of the coauthors, K. A. Herrlinger, is affiliated with Kemin Foods, L.C. Kemin Foods, L.C. approved the study design and decision to publish, but had no role in the collection, analysis, or interpretation of the data.
References
- 1.Arent SM, Senso M, Golem DL, McKeever KH. The effects of theaflavin-enriched black tea extract on muscle soreness, oxidative stress, inflammation, and endocrine responses to acute anaerobic interval training: A randomized, double-blind, crossover study. J Int Soc Sports Nutr 7: 11, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjørnsen T, Salvesen S, Berntsen S, Hetlelid KJ, Stea TH, Lohne-Seiler H, Rohde G, Haraldstad K, Raastad T, Køpp U, Haugeberg G. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand J Med Sci Sports 26: 755, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Bowtell JL, Sumners DP, Dyer A, Fox P, Mileva KN. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med Sci Sports Exerc 43: 1544–1551, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Braakhuis AJ, Hopkins WG. Impact of dietary antioxidants on sport performance: A review. Sports Med 45: 939–955, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Braakhuis AJ, Hopkins WG, Lowe TE. Effects of dietary antioxidants on training and performance in female runners. Eur J Sport Sci 14: 160–168, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Casuso RA, Martínez-López EJ, Nordsborg NB, Hita-Contreras F, Martínez-Romero R, Cañuelo A, Martínez-Amat A. Oral quercetin supplementation hampers skeletal muscle adaptations in response to exercise training. Scand J Med Sci Sports 24: 920–927, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Statistical Power Analysis for the Behavioral Sciences. (2nd ed.) Hillsdale, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 8.Connolly DAJ, McHugh MP, Padilla-Zakour OI. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br J Sports Med 40: 679–683, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eichenberger P, Colombani PC, Mettler S. Effects of 3-week consumption of green tea extracts on whole-body metabolism during cycling exercise in endurance-trained men. Int J Vitam Nutr Res 79: 24–33, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Erba D, Riso P, Bordoni A, Foti P, Biagi PL, Testolin G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J Nutr Biochem 16: 144–149, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Fimland MS, Helgerud J, Knutsen A, Ruth H, Leivseth G, Hoff J. No effect of prior caffeine ingestion on neuromuscular recovery after maximal fatiguing contractions. Eur J Appl Physiol 108: 123–130, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Goldfarb AH, Garten RS, Cho C, Chee PD, Chambers LA. Effects of a fruit/berry/vegetable supplement on muscle function and oxidative stress. Med Sci Sports Exerc 43: 501–508, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142–149, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Herrlinger KA, Chirouzes DM, Ceddia MA. Supplementation with a polyphenolic blend improves post-exercise strength recovery and muscle soreness. Food Nutr Res 59: 30034, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman J. Norms for Fitness, Performance, and Health. Champaign, IL: Human Kinetics, 2006. [Google Scholar]
- 16.Howatson G, McHugh MP, Hill JA, Brouner J, Jewell AP, Van Someren KA, Shave RE, Howatson SA. Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sci Sports 20: 843–852, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Jówko E, Dlugolęcka B, Makaruk B, Cieśliński I. The effect of green tea extract supplementation on exercise-induced oxidative stress parameters in male sprinters. Eur J Nutr 54: 783–791, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jówko E, Sacharuk J, Balasińska B, Ostaszewski P, Charmas M, Charmas R. Green tea extract supplementation gives protection against exercise-induced oxidative damage in healthy men. Nutr Res 31: 813–821, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Kerksick CM, Kreider RB, Willoughby DS. Intramuscular adaptations to eccentric exercise and antioxidant supplementation. Amino Acids 39: 219–232, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Kuo YC, Lin JC, Bernard JR, Liao YH. Green tea extract supplementation does not hamper endurance-training adaptation but improves antioxidant capacity in sedentary men. Appl Physiol Nutr Metab 40: 990–996, 2015. [DOI] [PubMed] [Google Scholar]
- 21.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res 36: 291–298, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Machin DR, Christmas KM, Chou TH, Hill SC, Van Pelt DW, Trombold JR, Coyle EF. Effects of differing dosages of pomegranate juice supplementation after eccentric exercise. Physiol J 2014: 271959, 2014. [Google Scholar]
- 23.Merry TL, Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol 594: 5135–5147, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Fallon KS, Kaushik D, Michniak-Kohn B, Patrick Dunne C, Zambraski EJ, Clarkson PM. Effects of quercetin supplementation on markers of muscle damage and inflammation after eccentric exercise. Int J Sport Nutr Exerc Metab 22: 430, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Panza VSP, Wazlawik E, Schütz GR, Comin L, Hecht KC, da Silva EL. Consumption of green tea favorably affects oxidative stress markers in weight-trained men. Nutrition 24: 433–442, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Paulsen G, Cumming KT, Holden G, Hallén J, Rønnestad BR, Sveen O, Skaug A, Paur I, Bastani NE, Østgaard HN, Buer C. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J Physiol 592: 1887–1901, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulsen G, Hamarsland H, Cumming KT, Johansen RE, Hulmi JJ, Børsheim E, Wiig H, Garthe I, Raastad T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J Physiol 592: 5391–5408, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol 95: 1–9, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic Biol Med 51: 942–950, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Reid MB. Invited review: Redox modulation of skeletal muscle contraction: What we know and what we don't. J Appl Physiol 90: 724–731, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Reid MB, Durham WJ. Generation of reactive oxygen and nitrogen species in contracting skeletal muscle. Ann N Y Acad Sci 959: 108–116, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Slattery K, Bentley D, Coutts AJ. The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: Implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Med 45: 453–471, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Theodorou AA, Nikolaidis MG, Paschalis V, Koutsias S, Panayiotou G, Fatouros IG, Koutedakis Y, Jamurtas AZ. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am J Clin Nutr 93: 1373–1383, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Trombold JR, Reinfeld AS, Casler JR, Coyle EF. The effect of pomegranate juice supplementation on strength and soreness after eccentric exercise. J Strength Cond Res 25: 1782–1788, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189: 41–54, 2003. [DOI] [PubMed] [Google Scholar]


