Abstract
A method for grafting ethylenediamine to a magnetic graphene oxide composite (EDA-GO@Fe3O4) was developed for Cr(VI) decontamination. The physicochemical properties of EDA-GO@Fe3O4 were characterized using HRTEM, EDS, FT-IR, TG-DSC, and XPS. The effects of pH, sorbent dose, foreign anions, time, Cr(VI) concentration, and temperature on decontamination process were studied. The solution pH can largely affect the decontamination process. The pseudo-second-order model is suitable for being applied to fit the adsorption processes of Cr(VI) with GO@Fe3O4 and EDA-GO@Fe3O4. The intra-particle diffusion is not the rate-controlling step. Isotherm experimental data can be described using the Freundlich model. The effects of multiple factors on the Cr(VI) decontamination was investigated by a 25−1 fractional factorial design (FFD). The adsorption process can significantly be affected by the main effects of A (pH), B (Cr(VI) concentration), and E (Adsorbent dose). The combined factors of AB (pH × Cr(VI) concentration), AE (pH × Adsorbent dose), and BC (Cr(VI) concentration × Temperature) had larger effects than other factors on Cr(VI) removal. These results indicated that EDA-GO@Fe3O4 is a potential and suitable candidate for treatment of heavy metal wastewater.
Introduction
Heavy metal pollution is a current worldwide environmental concern because it can harm ecosystems and endanger human health. Since the industrial revolution, chromium has been widely used in electroplating, tanning, dying, smelting, and corrosion protection [1–3]. Cr(VI), one form of chromium, is very harmful to most organisms due to its mammalian toxicity and carcinogenicity [4]. Therefore, it is necessary and important to separate Cr(VI) ions from aqueous solution before they are discharged into aquatic systems.
Compared with traditional chemical precipitation and ion exchange methods, adsorption is a simpler, faster, and more economically viable method for removing heavy metals from various wastewaters [5–7]. The nature of an adsorbent is critical to the adsorption process, and the efficiency and cost of which are determined by the efficiency of the adsorbent regarding contaminant removal and its solid-liquid separation ability [1]. Therefore, it is desirable to find adsorbents that possess both high adsorption ability and straightforward solid-liquid separation.
In recent years, graphene oxide (GO) has been used as an excellent adsorbent material due to its unique properties [8, 9]. GO has very high surface area and a large number of carboxyl, hydroxyl, carbonyl, and epoxy groups [8, 10], which can be used as anchoring sites for metal ions. GO and GO-based materials have been used as adsorbents for binding metals such as chromium [11], cadmium [12], lead [13], zinc [14], platinum [15], and copper [16]. However, due to its nanoscale and hydrophilic nature, GO is difficult to separate from aqueous solution following the adsorption process. The dispersion of magnetic nanomaterials on GO sheets is a topic of current research because it combines the advantages of high adsorption rate and easy phase separation [17, 18]. Therefore, it is important to integrate graphene oxide with magnetic nanomaterials for improving the solid-liquid separation capacity of the composite[19]. The adsorption capacity of an adsorbent for contaminants is partly determined by the number of functional groups [20]. Ethylenediamine is low-toxicity and low-cost, and contains two amino groups that can form stable chelates with metal ions. Therefore, grafting ethylenediamine to GO and GO-based materials may increase their adsorption ability. However, the adsorption behaviors of ethylenediamine modified magnetic graphene oxide composite (EDA-GO@Fe3O4) for Cr(VI) ions have not been fully investigated.
It is well known that environmental factors including pH, contact time, temperature, initial metal concentration, and background electrolyte species may affect the efficiency of an adsorbent for metal ions, and that this could be increased by optimizing these factors [21]. Traditional one-factor experimental design just study one factor at a time, which cannot investigate the interaction of factors [22]. Full factorial experimental design can give information about the interaction of factors, but it is only suitable for experiments with a small number of factors [21]. Fractional factorial design (FFD) can identify significant factors and assess interaction of factors only with a smaller number of experiments [21, 23]. Besides, the produced results can be easily analyzed without any complicated calculations. Therefore, it is significant to identify the key factors that have large effects on the Cr(VI) decontamination by EDA-GO@Fe3O4 using FFD.
In this study, a novel type of GO based composite named EDA-GO@Fe3O4 was developed for effective Cr(VI) decontamination. To the authors’ knowledge, few studies attempted to graft ethylenediamine to magnetic graphene oxide for improving the Cr(VI) removal efficiency. Moreover, there has not been any studies on the use of FFD to identify the main factors influencing adsorption efficiency of EDA-GO@Fe3O4 for Cr(VI) ions. The aims of this research are to: (1) synthesize and characterize magnetic hybrid adsorbent (EDA-GO@Fe3O4) and apply it for removing Cr(VI) ions from wastewater; (2) evaluate the effects of pH, sorbent dose, foreign anions, time, Cr(VI) concentration, and temperature on removal process; (3) investigate the reusability of EDA-GO@Fe3O4 composite; (4) apply kinetics and isotherm models for modelling the adsorption experiments; and (5) use FFD to identify significant factors and interactions for removing Cr(VI) ions with EDA-GO@Fe3O4.
Materials and methods
Materials
The chemicals such as H2SO4, P2O5, NaNO3, FeCl2·4H2O, FeCl3·6H2O, NH3·H2O, H2O2 were supplied by Guangzhou Chemical Reagent Factory. K2S2O8 was purchased from Tianjin Damao Chemical Reagent Factory. Graphite powder, ethylenediamine, K2Cr2O7, and KMnO4 were obtained from Tianjin Fuchen Chemical Reagent Factory. All reagents above were of analytical grade.
Synthesis of EDA-GO@Fe3O4
The GO was prepared using the modified Hummers procedure reported previously [17]. Natural graphite was first preoxidized with K2S2O8, P2O5, and H2SO4, then further oxidized with H2SO4, NaNO3, and KMnO4. Lastly, the graphite oxide layers were separated by ultrasonication to obtain a GO suspension.
Coprecipitation method was used to synthesize the magnetic graphene oxide (GO@Fe3O4). Fe2+and Fe3+ were added to the GO suspension and stirred vigorously for 2 min. Next, concentrated NaOH solution (100 g/L) was added into the mixture until the solution pH was 10, then the mixture was stirred constantly for 45 min at 85°C. The product was rinsed with Milli-Q water to obtain a black-colored GO@Fe3O4 suspension.
Grafting ethylenediamine to the magnetic graphene oxide composite (EDA-GO@Fe3O4) was achieved by modifying GO@Fe3O4 with ethylenediamine [24]. First, 9.0 mL ammonia solution was added to the GO@Fe3O4 suspension and stirred for 5 min. Then 36 mL ethylenediamine was added into the suspension and stirred for 10 min. Next, the suspension was stirred at 95°C for 6 h. Finally, ethanol and Milli-Q water were used to wash the product to neutral pH.
Characteristics of EDA-GO@Fe3O4
High-resolution transmission electron microscopy (HRTEM) of the EDA-GO@Fe3O4 was collected with a Tecnai G2-F20 (FEI, USA). The EDS spectrum was collected with an energy-dispersive X-ray spectrometer (FEI, USA). The FT-IR spectrum of EDA-GO@Fe3O4 was collected using a Magna-IR 170 spectrometer with KBr pellets at room temperature (Nicolet, USA). TG and DSC curves were recorded using a Q600 thermoanalyzer (TA, USA). The surface elemental composition was analyzed using an ESCALAB 250Xi X-ray photoelectron spectroscope with a resolution of 0.5 eV (Thermo, USA).
Adsorption experiments
Batch adsorption experiments
Adsorption experiments were study in a water bath shaker. The EDA-GO@Fe3O4 or GO@Fe3O4 and the Cr(VI) solution were added to 100 mL Erlenmeyer flasks. 0.01 or 0.1 M NaOH and HCl solution was used to adjust the pH values of the suspensions. Then, the Erlenmeyer flasks were shaken for 8 h at the desired temperature. After the adsorption process, a permanent magnet was used to separate the suspension. The concentration of Cr(VI) ions was determined by a UV spectrophotometer at 540 nm [20]. The adsorption capacities (qe, mg/g) and adsorption percentages (Ee, %) of EDA-GO@Fe3O4 or GO@Fe3O4 were calculated by the following equations:
| (1) |
| (2) |
where C0 (mg/L) is the initial Cr(VI) concentration; Ce (mg/L) is the equilibrium concentration of Cr(VI); V (L) is the volume of the Cr(VI) solution; and W (g) is the dosage of EDA-GO@Fe3O4 or GO@Fe3O4.
Two-level fractional factorial design
Five factors (A: pH, B: Cr(VI) concentration, C: temperature, D: time, E: adsorbent dose) were screened for their effects on qe (response) by a 25−1 FFD with resolution V. The experimental design matrix and corresponding values of each factor are shown in S1 Table. Design Expert 8.0.6 (Stat-Ease Inc., USA) and Minitab Release 16 (Minitab Inc., USA) were used for the FFD of the experiments and regression analysis of the experimental data obtained.
Modeling of adsorption kinetics and isotherm
Adsorption kinetics
The pseudo-first-order, pseudo-second-order, and intra-particle diffusion models can be expressed with Eqs 3, 4 and 5, respectively [25, 26].
| (3) |
| (4) |
| (5) |
where qt (mg/g) is the adsorption capacity of EDA-GO@Fe3O4 or GO@Fe3O4 at time t (h); k1 (1/min), k2 (g/mg min), and kp (mg/g min0.5) are the adsorption rate constants for the three kinetic models, respectively; qe (mg/g) is the adsorption capacities calculated by the kinetics models; C of adsorption constant is the intercept.
Adsorption isotherm
The nonlinear form of Langmuir, Freundlich, Temkin isotherm models are given by the Eqs 6, 7 and 8, respectively [27–29].
| (6) |
| (7) |
| (8) |
where qe (mg/g) is the adsorption amount of Cr(VI) ions; qmax (mg/g) is the maximum adsorption capacities of the adsorbent; KL (L/mg), KF and n, aT (L/g) and bT (kJ/mol) are the constants for the Langmuir, Freundlich, and Temkin isotherm models, respectively; Ce (mg/L) is the equilibrium concentration after the adsorption process; T (K) is the temperature; and R (8.314 × 10−3 kJ/mol K) is the gas constant.
Results and discussion
Characterization
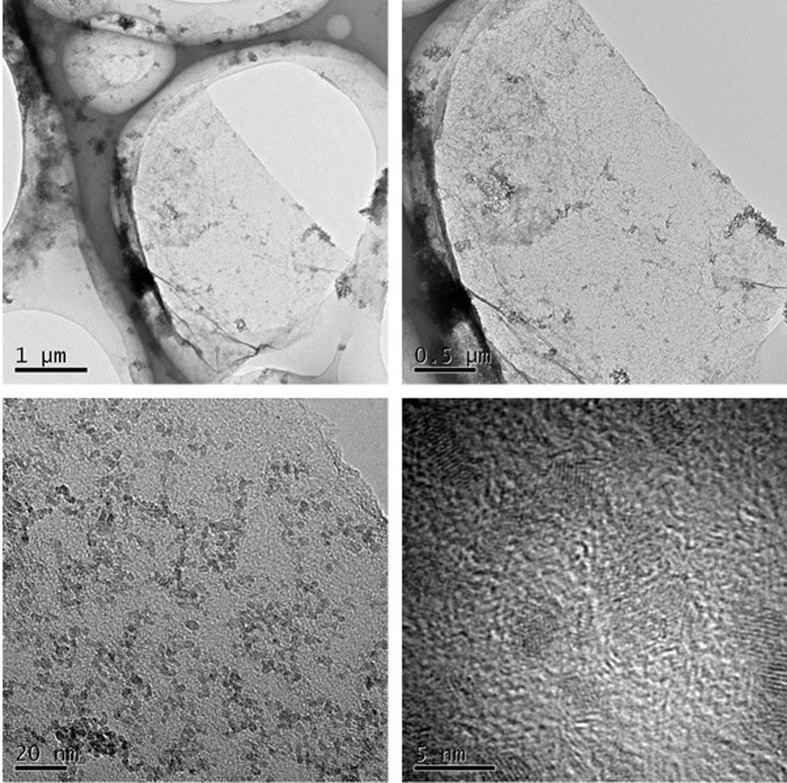
The morphology and microstructure of EDA-GO@Fe3O4 were characterized by HRTEM, and images at different magnifications are shown in Fig 1. EDA-GO@Fe3O4 revealed a typical fabric-like shape with a two-dimensional nanosheet structure (Fig 1A and 1B). From Fig 1, several small black spots (Fe3O4 nanoparticles) are dispersed on the GO nanosheets.
Fig 1. HRTEM images of EDA-GO@Fe3O4 at different magnification.
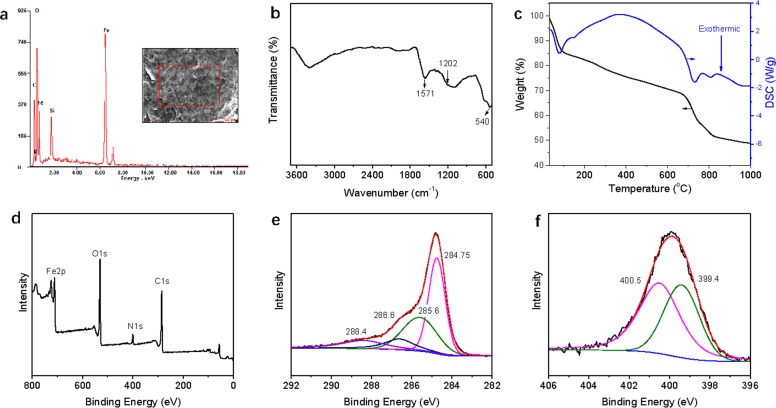
The EDS spectrum (Fig 2A) indicated that Fe, C, O, and N were present. Carbon came mainly from the GO nanosheets and the oxygen from the oxygen-containing functional groups in GO and Fe3O4 nanoparticles. N arose mainly from the amino groups in the grafted ethylenediamine, which indicated that ethylenediamine had been successfully introduced into the GO@Fe3O4.
Fig 2.
(a) EDS, (b) FT-IR, (c) TG-DSC, (d) XPS survey scan spectrum, (e) C1s and (f) N1s XPS spectra of EDA-GO@Fe3O4.
Fig 2B shows the FT-IR spectrum of EDA-GO@Fe3O4 composite. The peak at 540 cm-1 is attributed to Fe–O in Fe3O4, indicating successful connections between the Fe3O4 nanoparticles and the GO nanosheets. The peaks at 1571 cm-1 and 1202 cm-1 are attributable to the N–H stretching vibration (in the C–NH group) and C–N (in the–C–NH–C–and–NHCO–groups), respectively [24].
The TG-DSC curves of EDA-GO@Fe3O4 are shown in Fig 2C. The 15% loss of mass between 30°C and 106°C was attributed to the evaporation of water. The 51% loss of mass observed when the temperature ranged from 106°C to 1000°C was ascribed to pyrolysis of the grafted ethylenediamine and the oxygen-containing functional groups on the surfaces of the EDA-GO@Fe3O4 composite.
In order to gain further information on the chemical composition of EDA-GO@Fe3O4, XPS analysis was performed; the results are shown in Fig 2D–2F. From the XPS survey scan spectrum of EDA-GO@Fe3O4 (Fig 2D), four distinct peaks corresponding to C1s, O1s, Fe2p, and N1s were obtained. The N1s originated largely from the grafted ethylenediamine. The C1s spectrum of EDA-GO@Fe3O4 (Fig 2E) was separated into four different peaks at binding energies of 284.75, 285.6, 286.6, and 288.4 eV, attributing to C–C, C–N, C–O and C = O groups, respectively [24]. Fig 2F shows that the N1s spectrum of EDA-GO@Fe3O4 separated into two peaks centered at 399.4 eV and 400.5 eV, corresponding to the N in the amine and amide, respectively [30]. These results indicated that the surfaces of GO@Fe3O4 were successfully modified by ethylenediamine.
Effect of pH
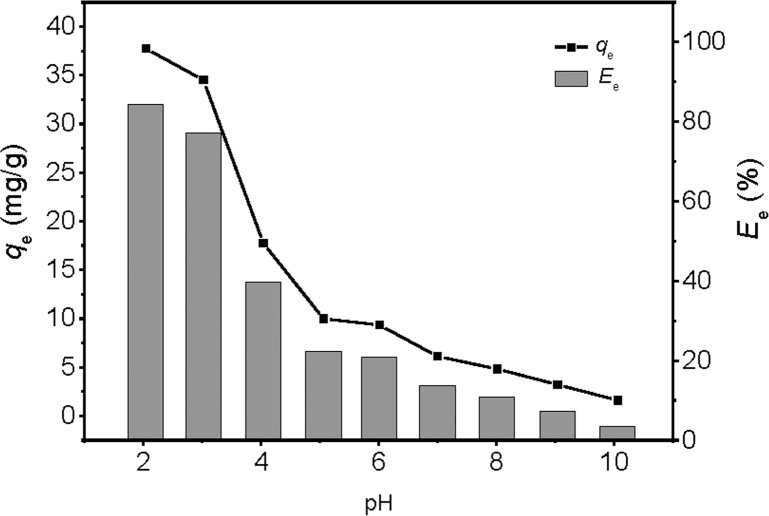
Solution pH is a very important factor for affecting sorption efficiency. In Fig 3, the sorption capacities and adsorption percentages of EDA-GO@Fe3O4 decreased significantly when the solution pH value increased from 2 to 10. For instance, the adsorption capacity and adsorption percentages were 37.73 mg/g and 84% at pH = 2, but only 1.60 mg/g and 4% at pH = 10, respectively. This result indicated clearly that the adsorption process was pH dependent, which may be due to that pH can affect the surface binding sites of EDA-GO@Fe3O4 and the aqueous chemistry. HCrO4−, KCrO4−, H2CrO4(aq), CrO42−, and Cr2O7− are the main Cr(VI) species at low pH levels, and the HCrO4− is the predominant form [1, 31]. The zeta potentials of EDA-GO@Fe3O4 were determined at different pH (ZEN3690, Malvern, UK), and the results is shown in S1 Fig. The pHpzc value was 4.73. At pH < 4.73, EDA-GO@Fe3O4 surfaces were positively charged due to protonation reactions on the functional groups of the composite. Therefore, the positively charged EDA-GO@Fe3O4 was more likely to attract negatively charged Cr(VI) ions (HCrO4−) electrostatically. However, At pH > 4.73, the negative charge on the EDA-GO@Fe3O4 surface increased because of the deprotonation of the groups at high OH− concentrations [32]. Therefore, the electrostatic repulsion between EDA-GO@Fe3O4 and Cr(VI) ions (CrO42−) was very strong, reducing the Cr(VI) adsorption capacity. In addition, at higher pH levels, as OH− concentrations increased the OH− could compete with CrO42− ions for adsorption sites, decreasing the Cr (VI) adsorption capacity [33].
Fig 3. Effect of solution pH on Cr(VI) adsorption onto the EDA-GO@Fe3O4: (initial Cr(VI) concentration = 10 mg/L; sorbent dose = 2 mL; temperature = 25°C; time = 8 h).
Effect of adsorbent dose on adsorption performance
S2 Fig illustrates the influence of adsorbent dose on the adsorption process of the EDA-GO@Fe3O4 for Cr(VI) ions. Adsorption capacity decreased as the EDA-GO@Fe3O4 dose increased from 1 mL to 8 mL. This may have been due to the fact that the higher EDA-GO@Fe3O4 dose provided more adsorption sites for Cr(VI) ions, but the amount of Cr(VI) ions in the system was constant, therefore the Cr(VI) ions were not sufficient for all adsorption sites, resulting in the decrease of adsorption capacity. Increasing EDA-GO@Fe3O4 dosage might also result in the aggregation of adsorbent particles, thereby decreasing the total surface area of the EDA-GO@Fe3O4 composite and increasing the diffusion path length of the Cr(VI) ions [34].
Effect of foreign anions
S3 Fig shows the effect of 0.01 M Cl−, NO3−, ClO4−, and SO42− on Cr(VI) adsorption by EDA-GO@Fe3O4 at pH 2. We can see that these foreign anions have different effects on the Cr(VI) removal. The adsorption capacity of EDA-GO@Fe3O4 for Cr(VI) ions is the highest in the system with addition of ClO4−, which may be due to that the ClO4− ions might not interact with the adsorption sites on the EDA-GO@Fe3O4 surfaces. The Cr(VI) adsorption capacity of EDA-GO@Fe3O4 in the present of 0.01 M NO3− was lower than those in the systems with Cl− and SO42−, which may be mainly ascribed to the NO3− competing with the HCrO4− ions for the adsorption sites [31]. Besides, the NO3− might decrease the zeta potentials of the EDA-GO@Fe3O4, which reduced the electrostatic attraction forces between the negative HCrO4− and the positively charged surfaces of EDA-GO@Fe3O4, thereby decreasing the Cr(VI) removal[35, 36].
Desorption and regeneration analysis
In order to determine the reusability of EDA-GO@Fe3O4, the adsorption-desorption cycles were conducted for four times, and the results are illustrated in S4 Fig. After adsorption experiments, the EDA-GO@Fe3O4 was regenerated by 0.1 M NaOH, and then rinsed with Milli-Q water. From S4 Fig, the adsorption capacity of EDA-GO@Fe3O4 for Cr(VI) decreased slightly from 39.67 mg/g to 34.83 mg/g after four cycling runs, which indicated that EDA-GO@Fe3O4 shows good stability in the adsorption process.
Adsorption kinetics
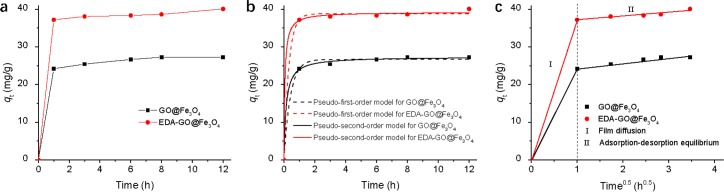
Fig 4 demonstrates the adsorption kinetics of Cr(VI) ions by GO@Fe3O4 and EDA-GO@Fe3O4. Fig 4A shows that adsorption equilibrium could be reached at 1 h for GO@Fe3O4 and EDA-GO@Fe3O4. The adsorption of Cr(VI) ions did not increase significantly after 1 h, which might have been due to the complete occupation of available adsorption sites on the surfaces of GO@Fe3O4 and EDA-GO@Fe3O4 [37]. Fig 4A also shows that the adsorption capacities of EDA-GO@Fe3O4 for Cr(VI) ions were higher than those of GO@Fe3O4, indicating that the ethylenediamine grafted on the GO@Fe3O4 surface enhances the adsorption capacity of EDA-GO@Fe3O4.
Fig 4.
(a) Time profiles of Cr(VI) adsorption with GO@Fe3O4 and EDA-GO@Fe3O4; Kinetics of Cr(VI) adsorption by fitting (b) pseudo-first-order and pseudo-second-order models, and (c) intraparticle diffusion model, respectively (initial Cr(VI) concentration = 10 mg/L; sorbent dose = 2 mL; temperature = 25°C; pH = 2).
The adsorption experimental data was interpreted by the kinetics models of pseudo-first-order, pseudo-second-order, and intra-particle diffusion (Fig 4B and 4C). The parameters of pseudo-first-order and pseudo-second-order adsorption kinetics are summarized in Table 1. In both Fig 4B and Table 1, the correlation coefficient (R2) values of the GO@Fe3O4 and EDA-GO@Fe3O4 for the pseudo-second-order model were higher than those for the pseudo-first-order model. In addition, the calculated qe values for the pseudo-second-order model were very close to the experimental data. These results indicate that the pseudo-second-order model is more suitable to describe the adsorption experimental data, indicating that chemical adsorption reaction is the dominant rate-limiting step for both adsorption processes.
Table 1. Adsorption kinetics parameters for Cr(VI) adsorption onto GO@Fe3O4 and EDA-GO@Fe3O4.
| Adsorbents | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|
| qe,1 | k1 | R2 | qe,2 | k2 | R2 | |
| GO@Fe3O4 | 26.675 | 2.367 | 0.995 | 27.382 | 0.258 | 0.998 |
| EDA-GO@Fe3O4 | 38.791 | 3.198 | 0.997 | 39.247 | 0.424 | 0.998 |
Fig 4C illustrates the qt vs. t0.5 plot. The multi-linear plot can be separated into two largely linear regions, indicating that intra-particle diffusion of the Cr(VI) ions was not the rate-controlling step for the overall adsorption process. The first region in Fig 4C (labeled “I”) might be assigned to film diffusion corresponding to transportation of Cr(VI) ions from the aqueous solution to the external surfaces of GO@Fe3O4 and EDA-GO@Fe3O4. The second region (labeled “II”) includes the gradual sorption and equilibrium stages [38].
Adsorption isotherm
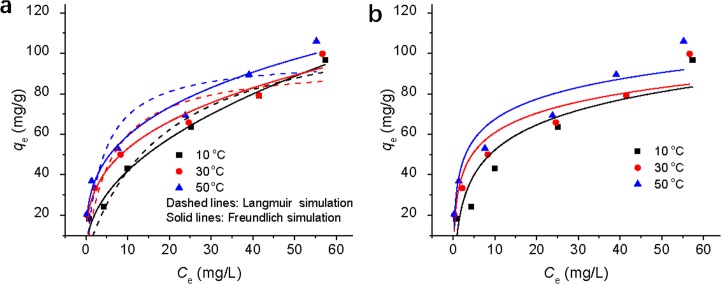
The decontamination of Cr(VI) ions by EDA-GO@Fe3O4 was studied at 15°C, 30°C, and 50°C to determine the relative parameters of the adsorption isotherms, and the results are demonstrated in Fig 5. The adsorption capacities of EDA-GO@Fe3O4 for Cr(VI) ions increased when the temperature increased from 10 to 50°C, implying an endothermic process. The nonlinear form of Langmuir, Freundlich, and Temkin adsorption isotherms at different temperatures are also illustrated in Fig 5. The parameters for the three isotherm models are demonstrated in Table 2. The Freundlich model clearly describes the isotherm adsorption data better than the Langmuir and Temkin models within the studied temperature range. The Freundlich constants of n (2.150 for 10°C, 3.086 for 30°C, and 3.259 for 50°C) are within the beneficial adsorption range (1–10) [39], indicating that EDA-GO@Fe3O4 can be applied as an effective adsorbent.
Fig 5.
(a) Langmuir and Freundlich and (b) Temkin plots for Cr(VI) ions onto EDA-GO@Fe3O4 at 10, 30, and 50°C, respectively (time = 8 h; sorbent dose = 223 mg/L; pH = 2).
Table 2. Isotherm parameters for Cr(VI) ions adsorption onto EDA-GO@Fe3O4.
| Models | Parameters | Temperature (°C) | ||
|---|---|---|---|---|
| 10 | 30 | 50 | ||
| Langmuir | qmax | 120.327 | 95.222 | 97.808 |
| KL | 0.054 | 0.167 | 0.241 | |
| R2 | 0.931 | 0.813 | 0.781 | |
| Freundlich | n | 2.150 | 3.086 | 3.259 |
| KF | 14.363 | 25.085 | 29.222 | |
| R2 | 0.984 | 0.970 | 0.970 | |
| Temkin | aT | 1.848 | 8.083 | 10.951 |
| bT | 0.132 | 0.182 | 0.187 | |
| R2 | 0.844 | 0.869 | 0.885 | |
FFD for assessing the effects of multiple factors on adsorption
The profiles of Cr(VI) removal by EDA-GO@Fe3O4 under varying levels of multiple experimental factors are illustrated in S5 Fig. Higher Cr(VI) removal was clearly found in Runs 3 and 11. Both of these experiments had low pH values (2) and adsorbent dose (1 mL) and high initial concentration of Cr(VI) ions (80 mg/L). In contrast, runs 1, 7, 15, and 16, which involved high pH values (10) and low Cr(VI) concentration (20 mg/L), showed lower Cr(VI) removal efficiency.
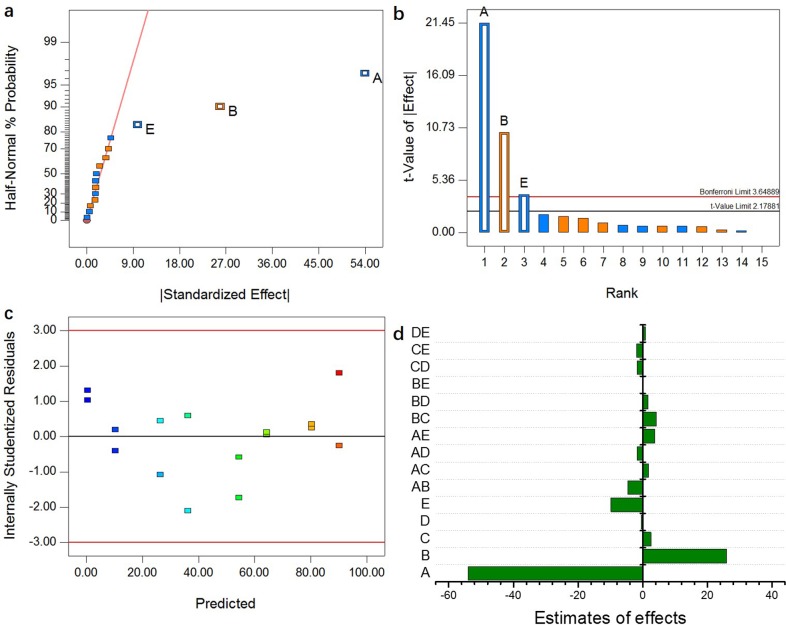
The effects of experimental factor variation and factor interactions on Cr(VI) decontamination were evaluated by significance testing of the FFD model [40]. The half-normal probability plot is shown in Fig 6A. Significant effects were observed in connection with variations of factors A, B, and E, while factor interactions have nonsignificant effects on the adsorption process. The Pareto chart (Fig 6B) was used to verify the results. The effect terms of A, B, and E are above the Bonferroni limit, indicating that these factorial effects are important factors in the removal process. Based on these findings, and the calculated coefficients, the model for predicting Cr(VI) removal can be represented by the following equation in terms of the factors:
| (9) |
Fig 6.
(a) Half-normal probability plot; (b) Pareto chart; (c) Plots of internally standardized residuals with predicted values; (d) Identification of main effective factors and interaction factors on Cr(VI) adsorption by EDA-GO@Fe3O4: (A) pH; (B) Cr(VI) concentration; (C) Temperature; (D) Time; (E) Adsorbent dose.
The plot of normal probability of residuals for Cr(VI) decontamination is demonstrated in S6 Fig. All internally studentized residuals lie close to a straight line, indicating a normal pattern for the regression residuals [41]. S7 Fig shows predicted values versus actual values. The predicted values are very close to the experimental measurements, implying that the Cr(VI) adsorption process can be predicted by the FFD models obtained. Fig 6C shows that the internally studentized residuals were equally scattered between −3 and +3, which indicated that the obtained FFD model in this study was adequate [40].
The identification of important factors, and factor interaction on Cr(VI) adsorption by EDA-GO@Fe3O4, is illustrated in Fig 6D. The factors with negative effects were A (−54.00), D (−0.51), and E (−9.85), while positive estimates of the effects of B (25.89) and C (2.52) were observed. This indicates that factor A is very important in the removal process. The effects of the six selected factors on Cr(VI) adsorption by EDA-GO@Fe3O4 were found to lie in the order A > B > E > C > D.
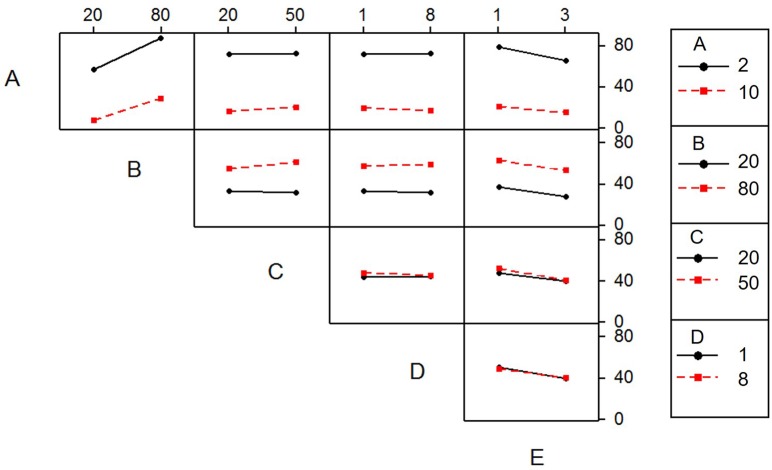
Fig 7 shows factor interaction effects for Cr(VI) decontamination. Lines in cells AB, AE, and BC were non-parallel, indicating that these factors could affect each other significantly [35]. In row A, Cr(VI) adsorption decreased slightly with the increase of pH values, indicating that factor A had a large impact on Cr(VI) decontamination. The two lines in row D coincided, suggesting that factor D had a slight or no impact on Cr(VI) removal.
Fig 7.
Interaction effects plot for Cr(VI) decontamination: (A) pH; (B) Cr(VI) concentration; (C) Temperature; (D) Time; (E) Adsorbent dose.
Conclusions
The HRTEM, EDS, FT-IR, TG-DSC, and XPS analyses indicate successful preparation of a novel ethylenediamine graft to a magnetic graphene oxide composite (EDA-GO@Fe3O4) using a simple chemical synthesis method. EDA-GO@Fe3O4 showed higher adsorption capacity for Cr(VI) ions than unmodified GO@Fe3O4, and the removal process was found to be affected by the operational parameters. The sorption capacities of EDA-GO@Fe3O4 decreased significantly with increased pH values due to the fact that pH affected both the aqueous chemistry and the surface binding-sites of EDA-GO@Fe3O4. The foreign anion of NO3− can compete with the HCrO4− ions for the adsorption sites. The EDA-GO@Fe3O4 has a good reusability. Adsorption equilibrium was reached within 1 h for both GO@Fe3O4 and EDA-GO@Fe3O4. The pseudo-second-order model is suitable for being used to describe the adsorption kinetics experimental data. The Freundlich model fits the adsorption isotherm data better than the Langmuir and Temkin models. Operational parameters A (pH), B (Cr(VI) concentration), and E (adsorbent dose) have significant effects on the removal process. The effects of the six selected factors on the decontamination process follow the order of A > B > E > C > D. The combined factors AB, AE, and BC have larger effects on Cr(VI) ions removal than other interactions. Consequently, the experimental results indicate that EDA-GO@Fe3O4 will have broad applications in cleaning up chromium pollution.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was financially supported by the National Natural Science Foundation of China (Grant No. 5160820 to Xinjiang Hu), the Project funded by China Postdoctoral Science Foundation (Grant No. 2017M610513 Xinjiang Hu), the Research Foundation of Education Department of Hunan Province, China (Grant No. 17K105 to Xinjiang Hu), the Natural Science Foundation of Guangdong Province (grant numbers 2016A030310246 and 2016A030310456 to Xinjiang Hu), the Science and Technology Planning Project of Hunan Province (grant numbers 2016TP2007 and 2016TP1014 to Yunlin Zhao), and the Scientific Research Staring Foundation for the attracted talent of Central South University of Forestry and Technology (grant number 2016YJ001 to Xinjiang Hu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hu X, Wang H, Liu Y. Statistical Analysis of Main and Interaction Effects on Cu(II) and Cr(VI) Decontamination by Nitrogen-Doped Magnetic Graphene Oxide. Sci Rep. 2016;6:34378 doi: 10.1038/srep34378 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y-g, Hu X-j, Wang H, Chen A-w, Liu S-m, Guo Y-m, et al. Photoreduction of Cr(VI) from acidic aqueous solution using TiO2-impregnated glutaraldehyde-crosslinked alginate beads and the effects of Fe(III) ions. Chem Eng J. 2013;226:131–8. doi: 10.1016/j.cej.2013.04.048 [Google Scholar]
- 3.Huang D-L, Zeng G-M, Feng C-L, Hu S, Jiang X-Y, Tang L, et al. Degradation of Lead-Contaminated Lignocellulosic Waste by Phanerochaete chrysosporium and the Reduction of Lead Toxicity. Environ Sci Technol. 2008;42(13):4946–51. doi: 10.1021/es800072c [DOI] [PubMed] [Google Scholar]
- 4.Bertoni FA, Bellu SE, Gonzalez JC, Sala LF. Reduction of hypervalent chromium in acidic media by alginic acid. Carbohydr Polym. 2014;114:1–11. doi: 10.1016/j.carbpol.2014.07.065 . [DOI] [PubMed] [Google Scholar]
- 5.Parida K, Mishra KG, Dash SK. Adsorption of Copper(II) on NH2-MCM-41 and Its Application for Epoxidation of Styrene. Ind Eng Chem Res. 2012;51(5):2235–46. doi: 10.1021/Ie200109h [Google Scholar]
- 6.Tyagi A, Tripathi KM, Singh N, Choudhary S, Gupta RK. Green synthesis of carbon quantum dots from lemon peel waste: applications in sensing and photocatalysis. RSC Advances. 2016;6(76):72423–32. doi: 10.1039/C6RA10488F [Google Scholar]
- 7.Tripathi KM, Tyagi A, Ashfaq M, Gupta RK. Temperature dependent, shape variant synthesis of photoluminescent and biocompatible carbon nanostructures from almond husk for applications in dye removal. RSC Advances. 2016;6(35):29545–53. doi: 10.1039/C5RA27432J [Google Scholar]
- 8.Zeng W, Liu Y-g, Hu X-j, Liu S-b, Zeng G-m, Zheng B-h, et al. Decontamination of methylene blue from aqueous solution by magnetic chitosan lignosulfonate grafted with graphene oxide: effects of environmental conditions and surfactant. RSC Advances. 2016;6(23):19298–307. doi: 10.1039/c5ra27657h [Google Scholar]
- 9.Tripathi KM, Singh A, Bhati A, Sarkar S, Sonkar SK. Sustainable Feasibility of the Environmental Pollutant Soot to Few-Layer Photoluminescent Graphene Nanosheets for Multifunctional Applications. ACS Sustainable Chemistry & Engineering. 2016;4(12):6399–408. doi: 10.1021/acssuschemeng.6b01045 [Google Scholar]
- 10.Stankovich S, Dikin DA, Dommett GH, Kohlhaas KM, Zimney EJ, Stach EA, et al. Graphene-based composite materials. Nature. 2006;442(7100):282–6. doi: 10.1038/nature04969 . [DOI] [PubMed] [Google Scholar]
- 11.Li L, Fan L, Sun M, Qiu H, Li X, Duan H, et al. Adsorbent for chromium removal based on graphene oxide functionalized with magnetic cyclodextrin-chitosan. Colloids Surf B Biointerfaces. 2013;107:76–83. doi: 10.1016/j.colsurfb.2013.01.074 . [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Chen B. Adsorption and coadsorption of organic pollutants and a heavy metal by graphene oxide and reduced graphene materials. Chem Eng J. 2015;281:379–88. doi: 10.1016/j.cej.2015.06.102 [Google Scholar]
- 13.Madadrang CJ, Kim HY, Gao GH, Wang N, Zhu J, Feng H, et al. Adsorption Behavior of EDTA-Graphene Oxide for Pb (II) Removal. Acs Appl Mater Interfaces. 2012;4(3):1186–93. doi: 10.1021/am201645g [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Yuan X, Wu Y, Huang H, Zeng G, Liu Y, et al. Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl Surf Sci. 2013;279:432–40. doi: 10.1016/j.apsusc.2013.04.133 [Google Scholar]
- 15.Liu L, Liu S, Zhang Q, Li C, Bao C, Liu X, et al. Adsorption of Au(III), Pd(II), and Pt(IV) from Aqueous Solution onto Graphene Oxide. Journal of Chemical & Engineering Data. 2013;58(2):209–16. doi: 10.1021/je300551c [Google Scholar]
- 16.Hu X-j, Liu Y-g, Wang H, Zeng G-m, Hu X, Guo Y-m, et al. Adsorption of copper by magnetic graphene oxide-supported β-cyclodextrin: Effects of pH, ionic strength, background electrolytes, and citric acid. Chemical Engineering Research and Design. 2015;93:675–83. doi: 10.1016/j.cherd.2014.06.002 [Google Scholar]
- 17.Hu X-j, Liu Y-g, Wang H, Chen A-w, Zeng G-m, Liu S-m, et al. Removal of Cu(II) ions from aqueous solution using sulfonated magnetic graphene oxide composite. Sep Purif Technol. 2013;108:189–95. doi: 10.1016/j.seppur.2013.02.011 [Google Scholar]
- 18.Zhang Y, Yan L, Xu W, Guo X, Cui L, Gao L, et al. Adsorption of Pb(II) and Hg(II) from aqueous solution using magnetic CoFe2O4-reduced graphene oxide. J Mol Liq. 2014;191:177–82. doi: 10.1016/j.molliq.2013.12.015 [Google Scholar]
- 19.Jiang L, Liu Y, Liu S, Hu X, Zeng G, Hu X, et al. Fabrication of β-cyclodextrin/poly (l-glutamic acid) supported magnetic graphene oxide and its adsorption behavior for 17β-estradiol. Chem Eng J. 2017;308:597–605. doi: 10.1016/j.cej.2016.09.067 [Google Scholar]
- 20.Hu X-j, Wang J-s, Liu Y-g, Li X, Zeng G-m, Bao Z-l, et al. Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: Isotherms, kinetics and thermodynamics. J Hazard Mater. 2011;185(1):306–14. doi: 10.1016/j.jhazmat.2010.09.034 [DOI] [PubMed] [Google Scholar]
- 21.Chang SH, Teng TT, Ismail N. Screening of factors influencing Cu(II) extraction by soybean oil-based organic solvents using fractional factorial design. J Environ Manage. 2011;92(10):2580–5. doi: 10.1016/j.jenvman.2011.05.025 . [DOI] [PubMed] [Google Scholar]
- 22.Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76(5):965–77. doi: 10.1016/j.talanta.2008.05.019 [DOI] [PubMed] [Google Scholar]
- 23.Myers RH, Montgomery DC, Anderson-Cook CM. Response Surface Methodology: Process and Product Optimization Using Designed Experiments. New Jersey: Wiley; 2008. [Google Scholar]
- 24.Ma HL, Zhang YW, Hu QH, Yan D, Yu ZZ, Zhai ML. Chemical reduction and removal of Cr(VI) from acidic aqueous solution by ethylenediamine-reduced graphene oxide. J Mater Chem. 2012;22(13):5914–6. doi: 10.1039/c2jm00145d [Google Scholar]
- 25.Chen Z, Ma W, Han M. Biosorption of nickel and copper onto treated alga (Undaria pinnatifida): Application of isotherm and kinetic models. J Hazard Mater. 2008;155(1–2):327–33. doi: 10.1016/j.jhazmat.2007.11.064 [DOI] [PubMed] [Google Scholar]
- 26.Ho Y-S, McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999;34(5):451–65. [Google Scholar]
- 27.Aksu Z. Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions onto Chlorella vulgaris. Process Biochem. 2002;38(1):89–99. [Google Scholar]
- 28.Kiran B, Kaushik A. Chromium binding capacity of Lyngbya putealis exopolysaccharides. Biochem Eng J. 2008;38(1):47–54. [Google Scholar]
- 29.Yang C-h. Statistical Mechanical Study on the Freundlich Isotherm Equation. J Colloid Interface Sci. 1998;208(2):379–87. http://dx.doi.org/10.1006/jcis.1998.5843. doi: 10.1006/jcis.1998.5843 [DOI] [PubMed] [Google Scholar]
- 30.Lawrie G, Keen I, Drew B, Chandler-Temple A, Rintoul L, Fredericks P, et al. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules. 2007;8(8):2533–41. doi: 10.1021/bm070014y . [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Liu Y-g, Zeng G-m, Hu X-j, Hu X, Li T-t, et al. Grafting of β-cyclodextrin to magnetic graphene oxide via ethylenediamine and application for Cr(VI) removal. Carbohydr Polym. 2014;113:166–73. doi: 10.1016/j.carbpol.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 32.Lei Y, Chen F, Luo Y, Zhang L. Three-dimensional magnetic graphene oxide foam/Fe3O4 nanocomposite as an efficient absorbent for Cr(VI) removal. J Mater Sci. 2014;49(12):4236–45. doi: 10.1007/s10853-014-8118-2 [Google Scholar]
- 33.Dinda D, Gupta A, Saha SK. Removal of toxic Cr(VI) by UV-active functionalized graphene oxide for water purification. Journal of Materials Chemistry A. 2013;1(37):11221–8. doi: 10.1039/C3TA12504A [Google Scholar]
- 34.Lu M, Liu Y-g, Hu X-j, Ben Y, Zeng X-x, Li T-t, et al. Competitive adsorption of Cu(II) and Pb(II) ions from aqueous solutions by Ca-alginate immobilized activated carbon and Saccharomyces cerevisiae Journal of Central South University. 2013;20:2478–88. [Google Scholar]
- 35.Hu X-j, Liu Y-g, Zeng G-m, Wang H, You S-h, Hu X, et al. Effects of inorganic electrolyte anions on enrichment of Cu(II) ions with aminated Fe3O4/graphene oxide: Cu(II) speciation prediction and surface charge measurement. Chemosphere. 2015;127:35–41. doi: 10.1016/j.chemosphere.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 36.Jiang L, Liu Y, Liu S, Zeng G, Hu X, Hu X, et al. Adsorption of Estrogen Contaminants by Graphene Nanomaterials under Natural Organic Matter Preloading: Comparison to Carbon Nanotube, Biochar, and Activated Carbon. Environ Sci Technol. 2017;51(11):6352–9. doi: 10.1021/acs.est.7b00073 . [DOI] [PubMed] [Google Scholar]
- 37.Nguyen TK, Beak MW, Huy BT, Lee YI. Adsorption and photodegradation kinetics of herbicide 2,4,5-trichlorophenoxyacetic acid with MgFeTi layered double hydroxides. Chemosphere. 2016;146:51–9. doi: 10.1016/j.chemosphere.2015.12.008 . [DOI] [PubMed] [Google Scholar]
- 38.Huang B, Liu Y, Li B, Zeng G, Hu X, Zheng B, et al. Synthesis of graphene oxide decorated with core@double-shell nanoparticles and application for Cr(VI) removal. RSC Advances. 2015;5(129):106339–49. doi: 10.1039/c5ra22862j [Google Scholar]
- 39.Wang XS, Huang J, Hu HQ, Wang J, Qin Y. Determination of kinetic and equilibrium parameters of the batch adsorption of Ni(II) from aqueous solutions by Na-mordenite. J Hazard Mater. 2007;142(1–2):468–76. doi: 10.1016/j.jhazmat.2006.08.047 [DOI] [PubMed] [Google Scholar]
- 40.Panic S, Rakic D, Guzsvany V, Kiss E, Boskovic G, Konya Z, et al. Optimization of thiamethoxam adsorption parameters using multi-walled carbon nanotubes by means of fractional factorial design. Chemosphere. 2015;141:87–93. doi: 10.1016/j.chemosphere.2015.06.042 . [DOI] [PubMed] [Google Scholar]
- 41.Liang J, Cheng L, Struckhoff JJ, Ravi N. Investigating triazine-based modification of hyaluronan using statistical designs. Carbohydr Polym. 2015;132:472–80. doi: 10.1016/j.carbpol.2015.06.067 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.