Chronic granulomatous disease (CGD) is a primary immunodeficiency characterized by the deficient generation of reactive oxygen metabolites by phagocytic cells. In about 70% of patients, the disease is X-linked and caused by mutations in CYBB (cytochrome B beta subunit or gp91phox; OMIM 300481).1 If a large deletion includes the adjacent XK gene (OMIM + 314850), the disorder is associated with a condition called McLeod phenotype, which is defined by the reduced expression of the Kell blood-group antigens on red blood cells (RBCs) and the absence of the XK protein.2 As RBCs of any healthy donor express the XK protein and the associated Kx antigen, transfusions in these patients will lead to sensitization to Kx and may induce severe hemolytic transfusion reactions on repeat transfusion.3,4 Transfusions hence require donations from donors with the McLeod phenotype, which, however, are extremely rare.5
We report on a patient with CGD and the McLeod phenotype, who underwent successful hematopoietic stem cell transplantation (HSCT) in spite of previous sensitization to Kx. At the age of 3 months, the boy (blood group: A2, Rh positive, K negative) had been transfused with RBCs (A1, Rh positive, K positive) because of unexplained anemia. CGD was diagnosed at the age of 3 years because of a history of recurrent pneumonia and generalized lymphadenopathy. At 9 years of age, lung surgery was required because of pulmonary aspergillosis. Granulocyte transfusions from two donors (A2, Rh positive, K negative; A1, Rh positive, K positive) were administered preoperatively. When transfused 2 weeks later with RBCs (A1, Rh positive, K negative), the patient experienced an acute hemolytic reaction with hemoglobinuria and a marked elevation of lactate dehydrogenase (LDH) levels (950 U/l; normal: <250U/l). Besides the detection of anti-A1 antibodies (titer 1:16), antibodies against the Kx antigen (titer 1:8) and against the K antigen (titer 1:16) were noted. Patient RBCs revealed an absence of expression of the Kx-antigen, leading to the diagnosis of the McLeod phenotype. In spite of continued antifungal prophylaxis, the patient developed chronic pulmonary disease, and at 13 years of age, he was referred for treatment by allogeneic HSCT.
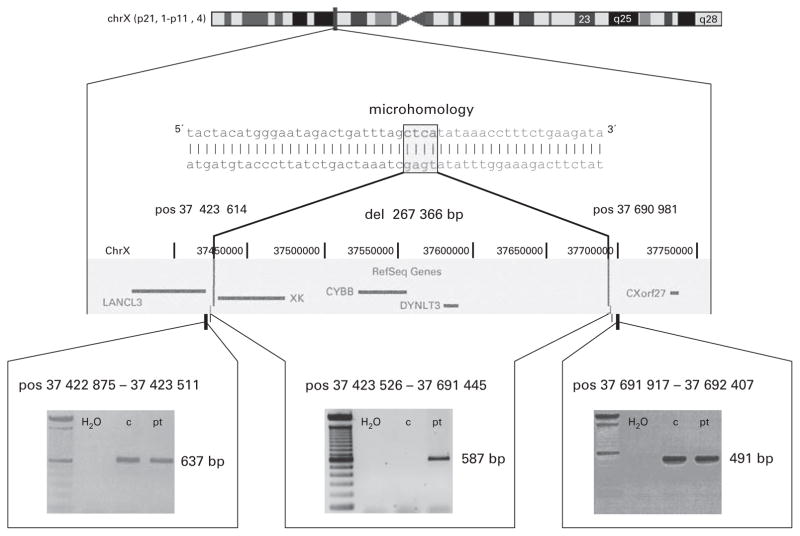
In addition to the initial phenotypic diagnosis of X-linked CGD, a sequential PCR strategy was used to localize the X-chromosomal deletion as the suspected basis for both diseases. This revealed a deletion of 267 366 bp, with complete absence of the genes XK, CYBB and DYNLT3 (Dynein light chain; OMIM 300302) (Figure 1).
Figure 1.
Schematic view of the deletion. The deletion (del) on the X chromosome (chr) includes the genes XK, CYBB and DYNLT3. Breakpoint positions (pos) are given according to UCSC Human Genome Browser database (http://genome.ucsc.edu/).11 3′ and 5′ ends of the breakpoints are characterized by a microhomology region of four nucleotides. Gels indicate the presence of PCR products in patient (pt) and control (c) in genomic DNA flanking the deletion (lower left and right panels) and the detection of a patient-specific PCR product in a PCR spanning the deletion (lower panel, center).
Before conditioning, which was done 4 years after the hemolytic transfusion reaction, anti-K-antibodies were still detectable (titer: 1:16). In addition, the patient’s serum revealed a weak reaction against all erythrocytes tested. The concentration of this antibody was too low to allow further characterization, but considering the patient’s transfusion history, the reaction was most probably caused by Kx-antibodies persisting at low concentrations. A further reduction of this low antibody titer by plasmapheresis before HSCT was not considered helpful.
To prevent or to control potential complications arising from the inevitable re-exposure to Kx-antigen-expressing cells during transplantation, several precautions were taken. Four units of patient RBCs were cryopreserved before conditioning, as a readily available source for Kx-negative RBCs in case of an alloreaction against this antigen. As Kx has been shown to be expressed also by hematopoietic precursor cells, graft failure due to antibody-mediated rejection was also considered as a potential risk.6,7 Thus, granulocyte-colony stimulating factor (GCSF)-mobilized autologous peripheral blood stem cells (PBSCs) were harvested and cryopreserved as back-up in case of graft failure. To limit the boosting of an antibody response against Kx on exposure to Kx-positive blood cells, the patient’s B cells were depleted in vivo by treatment with an anti-CD20 antibody (Rituximab, 2 × 375 mg/m2) 2 weeks before HSCT. The conditioning regimen consisted of total body irradiation (TBI; 6 × 2 Gy), Fludarabine (160 mg/m2) and anti-thymocyte-globulin (ATG; rabbit 10 mg/kg, Sangstat), because of its expected effective myeloablative and immunosuppressive activity. Reduced-intensity conditioning has been explored in two cases with CGD/McLeod, who were not sensitized. In one patient, this led to complete donor chimerism;8 in the other, however, mixed chimerism resulted, and the transplant course was complicated by severe prolonged hemolytic anemia.9 Thus, myeloablation in our patient was considered essential to avoid the ongoing coexistence of the sensitized immune system of the recipient and donor blood cells expressing the target antigen after HSCT. The graft (peripheral-blood stem cells) was obtained from a K-antigen-negative, HLA-identical unrelated donor (10/10 match; blood group: A, Rh positive, K negative). The transplant was depleted of RBCs by a positive selection of CD34 + cells, and the patient received a total of 1 × 107 CD34 + cells/kg. The graft was supplemented with donor T cells at a concentration of 1 × 107/kg. For graft-versus-host disease (GvHD) prophylaxis, CsA was used starting on day −3, in combination with methotrexate (MTX) (10 mg/m2) on days + 3, + 6, + 9 and + 11.
The first transfusion of RBCs from a K-negative donor was required on day + 7 and was tolerated without complications, as were subsequent transfusions (4 × RBCs, 10 × platelets). Hematological recovery was prompt (neutrophils >500/μl on day + 14, platelets >20 000/μl on day + 22, reticulocytes >20‰ on day + 24) and was followed by complete hematological and immunological reconstitution. Antibodies with specificity to the K antigen remained detectable at decreasing concentrations until 20 months after transplantation. Anti-Kx antibodies were never detected after transplantation. Repeat chimerism analysis revealed the complete donor origin of peripheral blood cells (STR analysis). The blood group completely changed to donor phenotype.
Hemolytic anemia after HSCT is frequently caused by incompatibilities in the ABO- or the Rh-blood-group systems, with the persistence of antibody-producing cells of host origin directed against donor red cells.10 In contrast to this constellation, the supply of compatible RBC products in our patient would be extremely difficult or impossible because of the shortage of compatible Kx-negative blood products.3,4 To our knowledge, HSCT in a patient with CGD and the McLeod phenotype sensitized against the Kx antigen has never been reported.
The transplantation course was entirely uneventful, with prompt hematological reconstitution and development of complete and stable donor-cell chimerism. Although it remains a matter of debate as to what degree the precautions taken to prevent transfusion reactions after transplantation—in particular the administration of anti-CD20 antibody or the intensive TBI-containing conditioning to achieve complete donor chimerism–contributed to the observed uneventful transplantation course, this case report demonstrates that patients with CGD and the McLeod phenotype sensitized to the Kx antigen can nevertheless undergo curative treatment by HSCT. It is, however, important to emphasize that the situation described here can be avoided by systematic screening for the expression of Kx and Kell antigens of all patients with X-linked CGD.
References
- 1.Roos D, Kuijpers T, Curnutte J. Chronic granulomatous disease. In: Ochs H, Smith E, Puck J, editors. Primary Immunodeficiency Diseases. 2. Oxford University Press; Oxford, New York: 2007. pp. 525–549. [Google Scholar]
- 2.Lee S, Russo D, Redman CM. The Kell blood group system: Kell and XK membrane proteins. Semin Hematol. 2000;37:113–121. doi: 10.1016/s0037-1963(00)90036-2. [DOI] [PubMed] [Google Scholar]
- 3.Brzica SM, Jr, Pineda AA, Taswell HF, Rhodes KH. Chronic granulomatous disease and the McLeod phenotype. Successful treatment of infection with granulocyte transfusions resulting in subsequent hemolytic transfusion reaction. Mayo Clin Proc. 1977;52:153–156. [PubMed] [Google Scholar]
- 4.van der Hart M, Szaloky A, van Loghem JJ. A ‘new’ antibody associated with the Kell blood group system. Vox Sang. 1968;15:456–458. doi: 10.1111/j.1423-0410.1968.tb04090.x. [DOI] [PubMed] [Google Scholar]
- 5.Bansal I, Jeon HR, Hui SR, Calhoun BW, Manning DW, Kelly TJ, et al. Transfusion support for a patient with McLeod phenotype without chronic granulomatous disease and with antibodies to Kx and Km. Vox Sang. 2008;94:216–220. doi: 10.1111/j.1423-0410.2007.01021.x. [DOI] [PubMed] [Google Scholar]
- 6.Pu JJ, Redman CM, Visser JW, Lee S. Onset of expression of the components of the Kell blood group complex. Transfusion. 2005;45:969–974. doi: 10.1111/j.1537-2995.2005.04289.x. [DOI] [PubMed] [Google Scholar]
- 7.Wagner T, Berer A, Lanzer G, Geissler K. Kell is not restricted to the erythropoietic lineage but is also expressed on myeloid progenitor cells. Br J Haematol. 2000;110:409–411. doi: 10.1046/j.1365-2141.2000.02195.x. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki N, Hatakeyama N, Yamamoto M, Mizue N, Kuroiwa Y, Yoda M, et al. Treatment of McLeod phenotype chronic granulomatous disease with reduced-intensity conditioning and unrelated-donor umbilical cord blood transplantation. Int J Hematol. 2007;85:70–72. doi: 10.1532/IJH9706129. [DOI] [PubMed] [Google Scholar]
- 9.Kordes U, Binder TMC, Eiermann TH, Hassenpflug-Diederich B, Hassan MA, Beutel K, et al. Successful donor-lymphocyte infusion for extreme immune-hemolysis following unrelated BMT in a patient with X-linked chronic granulomatous disease and McLeod phenotype. Bone Marrow Transplant. 2008;42:219–220. doi: 10.1038/bmt.2008.159. [DOI] [PubMed] [Google Scholar]
- 10.Franchini M, Gandini G, Aprili G. Non-ABO red blood cell alloantibodies following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33:1169. doi: 10.1038/sj.bmt.1704524. [DOI] [PubMed] [Google Scholar]
- 11.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]