Abstract
The molecular mechanisms of chronic pain are poorly understood and effective mechanism-based treatments are lacking. Here we report that mice lacking adenosine deaminase (ADA), an enzyme necessary for the breakdown of adenosine, displayed unexpected chronic mechanical and thermal hypersensitivity due to sustained elevated circulating adenosine. Extending from Ada−/− mice, we further discovered that prolonged elevated adenosine contributed to chronic pain behaviors in two additional independent animal models:1) sickle cell disease mice, a model of severe pain with limited treatment, and 2) complete Freund’s adjuvant paw-injected mice, a well-accepted inflammatory model of chronic pain. Mechanistically, we revealed that activation of adenosine A2B receptors on myeloid cells caused nociceptor hyperexcitability and promoted chronic pain via soluble IL-6 receptor trans-signaling, our findings determined that prolonged accumulated circulating adenosine contributes to chronic pain by promoting immune-neuronal interaction and revealed multiple therapeutic targets.
ETOC BLURB

Hu et.al show that prolonged increase in plasma adenosine activates ADORA2B on myeloid cells leading to an increase in circulating IL-6 and sIL-6R. The IL-6/sIL-6R complex trans-activates gp130 on DRG cells, leading to STAT3 phosphorylation and induction of neuronal TRPV1 expression that results in increased sensitivity and chronic pain.
Introduction
Acute pain is beneficial for the organism under dangerous conditions and is relatively well controlled by available analgesics. In contrast, chronic pain is often debilitating and treated inadequately. As the most common chronic medical disorder and one commonly treated with highly addictive drugs, chronic pain incurs enormous clinical, economic, and social costs (Gereau et al., 2014; Goldberg and McGee, 2011). There is an urgent need to define critical molecular mechanisms that may disclose therapeutic targets for better treatment of chronic pain.
Chronic pain is associated with numerous clinical conditions, often having an inflammatory component (Chiu et al., 2012). Diverse cell types in the peripheral and central nervous systems, immune system, and other tissues have been implicated in the induction and maintenance of chronic pain. Particular attention has been paid to primary sensory neurons that have their cell bodies in dorsal root ganglia (DRG). These neurons detect mechanical, thermal, and chemical stimuli in most tissues, including skin, muscle, joints, and viscera, and they convey this information from the periphery to the spinal cord and brain. Sensory neurons detection of injury has been linked to many chronic pain conditions involving inflammation (Gold and Gebhart, 2010). While several pathways signaling to nociceptors have been implicated in inflammatory models (Ji et al., 2014; McMahon et al., 2015), the signaling pathways critical for inducing chronic inflammatory pain are not yet fully defined.
Extracellular adenosine is a purinergic ligand, induced by hypoxia and tissue injury, that signals via four G protein coupled receptors recognized as (ADORA1, ADORA2A, ADORA2B and ADORA3) to regulate multiple cellular and systemic functions under physiological and pathological conditions (Fredholm, 2007; Fredholm et al., 2001). Acutely elevated adenosine signaling via specific receptors is considered beneficial, producing vasodilation, reducing inflammation and vascular leakage (Eltzschig et al., 2006; Eltzschig et al., 2003; Hershfield, 2005). In contrast, persistently elevated adenosine has detrimental effects, promoting chronic inflammation, tissue fibrosis and multiple organ damage(Dai et al., 2011; Figler et al., 2011; Karmouty-Quintana et al., 2012; Zhang et al., 2011; Zhang and Xia, 2012). Supporting the beneficial role of acutely increased adenosine, early studies showed that acutely elevated adenosine signaling via peripheral and spinal ADORA1 inhibits pain-related behavior (Aley et al., 1995; Goldman et al., 2010; Wu et al., 2005). Additional studies showed that acute actions of adenosine via peripheral ADORA2A can sensitize pain-related behavior (Li et al., 2010; Taiwo and Levine, 1990). The role of sustained elevated adenosine in chronic pain remained unclear and cannot be fully understood using animal models with brief and transient elevation of adenosine. As a result, the underlying mechanism through which adenosine signaling contributes to chronic pain may be more complex than once thought and remains poorly understood. We demonstrated that sustained elevated blood adenosine plays an unexpectedly major role in the development of pain related behavioral responses in three distinct chronic pain models. Mechanistically, we revealed that prolonged elevated extracellular adenosine specifically activates ADORA2B on myeloid cells, subsequently transactivates nociceptors of sensory neurons and eventually causes hypersensitive neurons and chronic pain in an IL-6 and sIL-6R trans-signaling-dependent manner. Our findings reveal that sustained elevated circulating adenosine coupled with ADORA2B plays a major role in chronic pain by promoting immune-neuronal interaction and suggests therapeutic targets to counteract chronic pain.
Results
ADA-deficient mice display chronic pain behavior due to sustained elevation of circulating adenosine
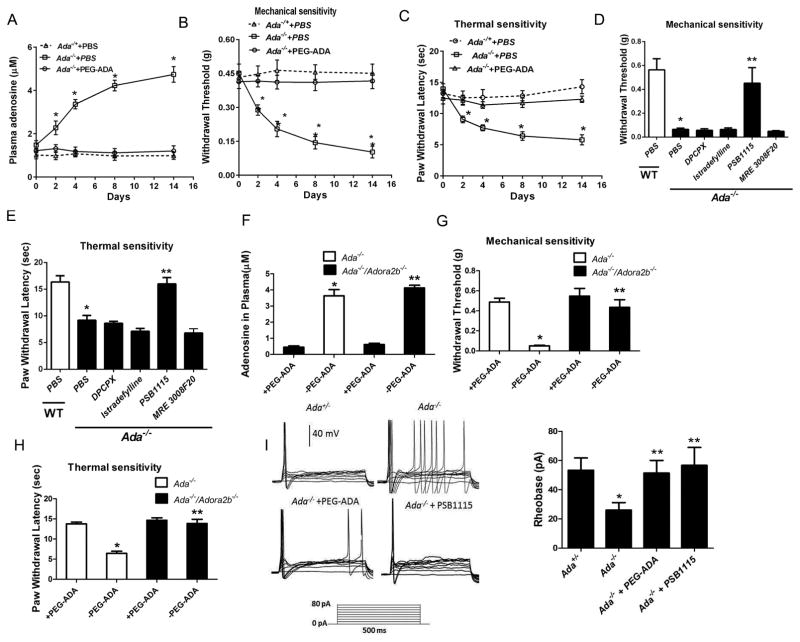
ADA deficiency in humans and mice is lethal due to severe purine metabolic disturbances. Fortunately, polyethylene glycol-ADA (PEG-ADA) enzyme therapy is used to treat ADA-deficient humans and mice to prevent mortality by lowering, but not eliminating the accumulation of adenosine (Blackburn et al., 2000; Hershfield, 1995). We routinely inject PEG-ADA weekly into Ada−/− mice from birth to prevent excessive elevation of adenosine to maintain them alive. Surprisingly, we observed that Ada−/− mice were unusually more agitated and aggressive compared to Ada−/+ littermates when we injected them with PEG-ADA, suggesting that these mice might experience chronic pain. As expected, without continuous PEG-ADA treatment and only with PBS, we found the plasma adenosine began to increase on day 2, continued to increase on day 4, peaked around day 8 and remained high at 2 weeks (Figure 1A). The rise in adenosine after stopping PEG-ADA treatment was accompanied with significantly increased sensitivity to both thermal and mechanical stimuli from day 2 (Figure 1B–C). An additional increase in response to both stimuli was observed on day 4–8, with a further increase on day 14 (Figure 1B–C). In contrast, to keep constant low adenosine within 2 week experimental period, we injected PEG-ADA every other days instead of weekly prior to our pain behavior measurement. Ada−/− mice receiving continuous PEG-ADA treatment maintained much lower levels of plasma adenosine and displayed no significant pain behavior (Figure 1A–C). Overall, our findings provide solid genetic evidence that prolonged elevation of circulating adenosine contributes to chronic sensory hypersensitivity in Ada−/− mice. More importantly, Ada−/− mice is a genetic tool to further dissect the molecular basis of chronic pain resulting from prolonged elevated adenosine.
Figure 1.
Ada −/− mice display reflex hypersensitivity and hyperexcitable sensory neurons after prolonged adenosine-ADORA2B signaling. (A) Plasma adenosine levels in the Ada+/− and Ada−/− mice treated with PBS or PEG-ADA every other day for 2 weeks (*P<0.01, n=6). (B–C)Paw withdrawal threshold to mechanical and thermal test stimuli were lower in Ada−/− compared to Ada−/+ mice (*P<0.01, n=10). PEG-ADA treatment prevented mechanical and thermal hypersensitivity in Ada−/− (*P<0.001, n=10). (D–E) Hypersensitivity were observed in Ada−/− compared to WT mice treated with PBS. However, hypersensitivity in Ada−/− mice were prevented by 2 week treatment with an ADORA2B antagonist, PSB1115, but not by other adenosine receptor antagonists (*P<0.001, n=10). (F) PEG-ADA treatment normalized plasma adenosine in Ada−/− and Ada−/−/Adora2b−/− mice (*P<0.05, **P<0.01, n=6). (G–H) Mechanical and thermal hypersensitivity was attenuated in Ada−/−/Adora2b−/− mice (*P<0.05 **P< 0.01, n=6). (I) Action potentials evoked by depolarizing test currents in DRG neurons isolated from Ada−/− mice previously treated in vivo with or without PEG-ADA or PSB1115. The threshold current (rheobase) was lower in DRG neurons from Ada−/− than Ada−/+ mice (*P<0.01, n=6). PEG-ADA or PSB1115 treatment in vivo attenuated sensory neuron hyperexcitability in Ada−/− mice (**P<0.01, n=5).
ADORA2B is essential for adenosine-induced chronic pain in Ada−/− mice
To determine which adenosine receptors are responsible for adenosine-induced pain behavior in Ada−/− mice, we initially took a pharmacological approach. Briefly, we treated Ada−/− mice for 2 weeks with PBS or four different antagonists specific for each type of adenosine receptor. Moreover, WT mice were treated with PBS as the control. First, we found that Ada−/− mice treated with PBS showed increased sensitivity to stimuli compared to the WT mice treated with PBS (Figure 1D–E). Moreover, among four specific adenosine receptor antagonists, only the ADORA2B-specific antagonist, PSB1115, significantly reduced mechanical and thermal hypersensitivity in the Ada−/− mice (Figure 1D–E), suggesting that ADORA2B is particularly important for adenosine-induced chronic pain in Ada−/− mice.
To validate our pharmacological findings, we generated Ada−/−/Adora2b−/− mice (Iriyama et al., 2015). As expected, the plasma adenosine levels were increased to a similar level in both Ada−/−/Adora2b−/− and Ada−/−/Adora2b−/+ mice without PEG-ADA treatment (Figure 1F). Supporting our pharmacological studies, genetic deletion of ADORA2B in Ada−/− mice significantly attenuated hypersensitivity (Figure 1G–H). The pharmacological and genetic evidence led us to conclude that ADORA2B is essential for pain behavior in response to persistently increased adenosine in the Ada−/− mice.
Adenosine-ADORA2B signaling produces hyperexcitability in sensory neurons of ADA-deficient mice
Hyperexcitable sensory neurons play a major role in driving many kinds of persistent pain (Basbaum et al., 2009; Gold and Gebhart, 2010; Walters, 2014). Supporting this notion, we found that the action potential threshold (rheobase) during prolonged depolarizing pulses was significantly reduced in the sensory neurons isolated from DRG of Ada−/− compared to Ada−/+ mice (Figure 1I). Treatment with PEG-ADA or PSB1115 normalized action potential threshold in DRG neurons of the Ada−/− mice (Figure 1I). These results show that a prolonged increase in adenosine-ADORA2B signaling enhances the excitability of primary sensory neurons and likely contributes to chronic pain in the Ada−/− mice.
Elevated circulating adenosine signaling via ADORA2B induces hypersensitivity of nociceptors and underlies chronic pain in sickle cell disease (SCD) mice
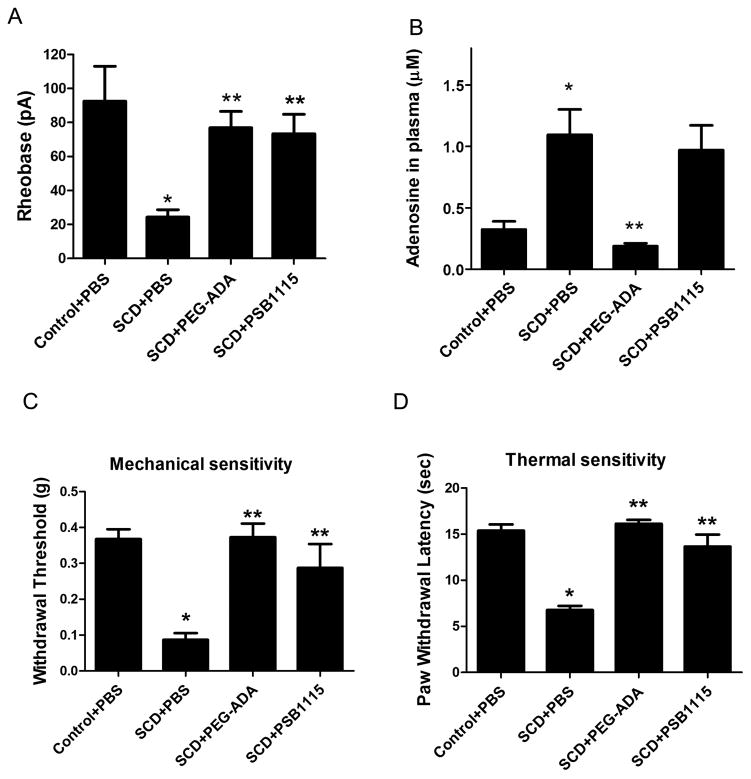
We have previously shown that elevated plasma adenosine in humans and mice with SCD contributes to sickling, the central pathogenic process of the disease, by activating ADORA2B on erythrocytes (Zhang et al., 2011). However, a role for ADORA2B signaling in SCD pain has not been addressed. Based on our findings that elevated adenosine contributes to chronic pain via activation of ADORA2B in Ada−/− mice, we hypothesized that elevated circulating adenosine may contribute to chronic pain in SCD by inducing hyperexcitability of nociceptive sensory neurons. To test this hypothesis, we took advantage of SCD mice, a well-accepted humanized mouse model that exhibits chronic pain like behavior. First, we found that action potential threshold (rheobase) was significantly reduced in DRG neurons isolated from SCD mice compared to control (Figure 2A). Next, we treated SCD mice with or without PEG-ADA or PSB1115 for 2 weeks to examine whether elevated adenosine-ADORA2B signaling is responsible for sensory neuron hyperexcitability. Similar to Ada−/− mice, we found that PEG-ADA treatment significantly reduced adenosine levels in the plasma of SCD mice (Figure 2B). Moreover, PEG-ADA or PSB1115 treatment normalized action potential threshold in sensory neurons of SCD mice (Figure 2A). As expected, the SCD mice displayed reflex hypersensitivity to both stimuli (Hillery et al., 2011). Importantly, both the plasma concentration of adenosine and the reflex hypersensitivity in SCD mice were significantly reduced by PEG-ADA (Figure 2C–D). PSB1115 treatment suppressed both mechanical and heat hypersensitivity without significantly changing the plasma adenosine level (Figure 2B–D). The similarity of the effects of PEG-ADA and PSB1115 on pain related electrophysiological and behavioral alterations in SCD and Ada−/− mice indicate a potentially general role for adenosine-ADORA2B signaling in chronic pain by inducing hyperexcitability in nociceptive sensory neurons.
Figure 2.
Adenosine-ADORA2B signaling contributes to sensory neuron hyperexcitability and reflex hypersensitivity in SCD mice.(A) Action potential threshold was lower in sensory neurons from SCD than control SCD−/+ mice (*P< 0.01, n=7). After in vivo treatment with PEG-ADA or PSB1115 the rheobase increased (**P< 0.01, n=7). PBS used as a control. (B)Plasma adenosine concentration was increased in SCD mice (*P< 0.01, n=10). In vivo treatment with PEG-ADA decreased the adenosine level (**P< 0.01, n=8). (C–D) SCD mice showed hypersensitivity compared to control mice (*P< 0.01, n=18). In vivo PEG-ADA or PSB1115 treatment reduced reflex hypersensitivity in SCD mice. (**P<0.01, n=18–22).
Upregulated TRPV1 gene expression and function in DRG neurons of both ADA-deficient and SCD mice are dependent on sustained elevated adenosine signaling via ADORA2B
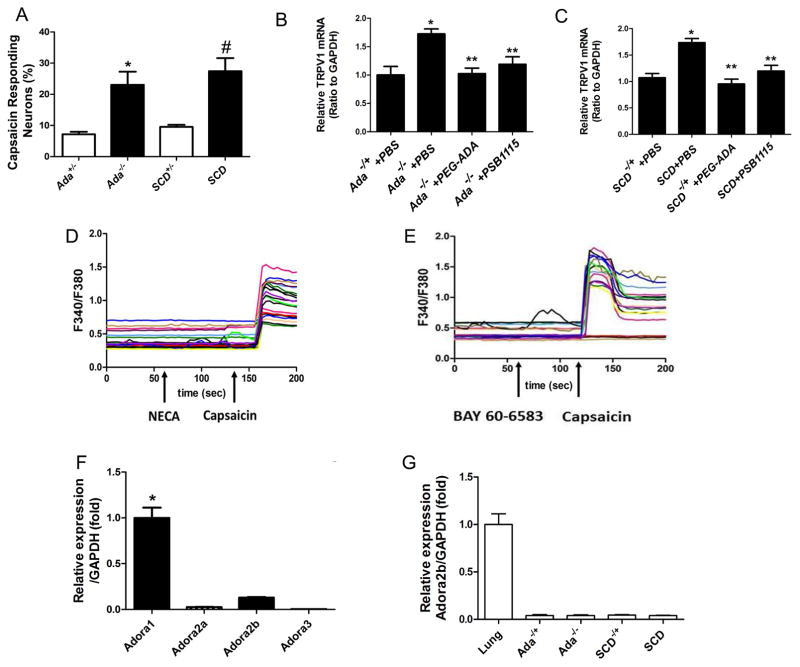
Enhanced transient receptor potential V1 (TRPV1) channel plays an important role in multiple forms of chronic pain including inflammatory pain models (Ji et al., 2002) and reflex hypersensitivity in SCD mice (Hillery et al., 2011). Because we found that adenosine signaling via ADORA2B contributed to neuronal hypersensitivity and subsequent chronic pain behavior in both Ada−/− and SCD mice, we hypothesized that sustained accumulated circulating adenosine, activating ADORA2B, likely underlies elevated TRPV1 activity. To test this possibility, we first determined whether adenosine-ADORA2B signaling enhances the sensitivity of isolated DRG neurons to a highly selective, plant-derived activator of TRPV1 channels, capsaicin. This was done by comparing the incidence of neurons responding with detectable intracellular Ca2+ transients when stimulated with low concentration of capsaicin (10 nM). DRG neurons isolated from Ada−/− or SCD mice showed a striking increase in the incidence of [Ca2+]i responses compared to DRG neurons isolated from control mice, which rarely responded to this low concentration of capsaicin (Figure 3A). These results show that a chemical activator of TRPV1 channels is particularly effective in activating DRG neurons in Ada−/− and SCD mice but not in control mice at such a low concentration.
Figure 3.
Adenosine-induced elevated TRPV1 gene expression, calcium influx and nociceptive hypersensitivity in Ada−/− and SCD mice are not directly mediated by activation of ADORA2B on DRG sensory neuronal cells. (A) The incidence of neurons with calcium responses to 10 nM capsaicin was higher in DRG neurons isolated from Ada−/− and SCD mice than DRG neurons from Ada−/+ or SCD−/+ (*P< 0.01, n=5–7) (#P<0.01). (B–C) TRPV1 gene expression was increased in DRG from Ada−/− and SCD mice compared to controls (*P<0.01, n=7–8) and the increased expression was attenuated by PEG-ADA or PSB1115 (**P<0.01, n=7–8). (D–E) Adenosine receptor agonists NECA and BAY 60-6583 failed to evoke calcium responses in DRG neurons. (F) Quantitative RT-PCR indicates that Adora1 is the major adenosine receptor expressed in DRG neurons of WT mice, with the other three adenosine receptors expressed at much lower levels (*P<0.05, n=3). (G) Expression of Adora2b mRNA in DRG isolated from Ada−/− and SCD mice. Lung tissue is a positive control.
One potential explanation for hypersensitivity to TRPV1 activators would be an increase in the number of TRPV1 channels expressed by DRG neurons. Thus, we determined if TRPV1 gene expression is elevated in DRG neurons of Ada−/− or SCD mice. We found that TRPV1 mRNA in DRGs was significantly elevated in both Ada−/− and SCD mice compared to the controls (Figure 3B). Moreover, the increased TRPV1 gene expression in DRG of Ada−/− or SCD mice was significantly reduced by lowering adenosine levels with PEG-ADA or by blocking ADORA2B with PSB1115 for 2 weeks (Figure 3B–C). These results demonstrate that prolonged adenosine activated ADORA2B signaling induces TRPV1 gene expression and promotes its function in nociceptors of both Ada−/− and SCD mice.
IL-6 functions downstream of ADORA2B signaling to promote sensory neuronal TRPV1 channel hyperexcitability and reflex hypersensitivity in Ada−/− and SCD mice
We next investigated whether ADORA2B signaling directly activates nociceptors of DRG sensory neurons by applying NECA, a non-metabolized adenosine analog, directly to (WT) mouse DRG neurons. Unexpectedly, NECA did not induce a measurable [Ca2+]i response (Figure 3D). Similarly, we did not observe a [Ca2+]i response upon treatment with the ADORA2B specific agonist, BAY 60-6583 (Figure 3E), although capsaicin induced a robust [Ca2+]i response in these cells (Figure 3D–E). Moreover, we found that Adora2b mRNA levels were much lower in DRG neurons isolated from controls than in the lung tissue as a positive control (Figure 3F). Furthermore, Adora2b mRNA was barely detectable in the DRGs of Ada−/− or SCD mice (Figure 3G). Therefore, both biochemical and functional observations suggest that enhanced TRPV1 expression and function in DRG neurons are not caused by acute and direct activation of ADORA2B in either Ada−/− or SCD mice.
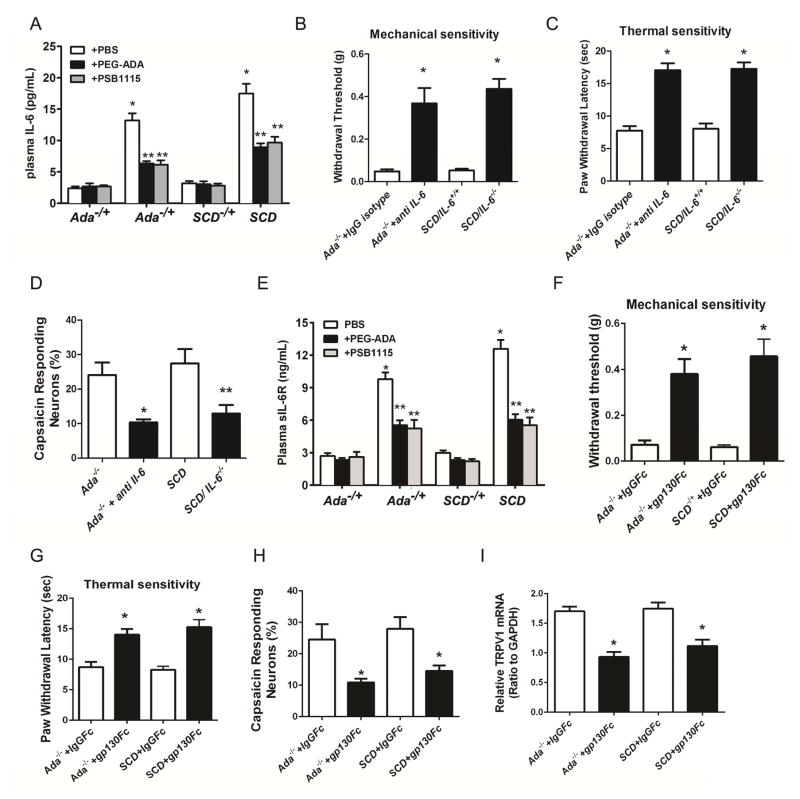
Elevated circulating IL-6 is associated with inflammation and chronic pain in both Ada−/− and SCD mice (Dai et al., 2011; Zhang et al., 2011) and IL-6 is involved in persistent inflammatory pain in humans and in preclinical models (Dina et al., 2011; Murphy et al., 1999; Nishimoto et al., 2009). It is possible that increased IL-6 is a common molecule functioning downstream of ADORA2B signaling underlying elevated circulating adenosine-induced nociceptor TRPV1 expression and excitability in Ada−/− and SCD mice. To test this possibility, we first measured circulating IL-6 in both Ada−/− and SCD mice treated with or without PEG-ADA or PSB1115 for 2 weeks. As expected, plasma IL-6 levels were significantly elevated in Ada−/− and SCD mice and PEG-ADA or PSB1115 treatment significantly reduced elevated circulating IL-6 in both Ada−/− and SCD mice (Figure 4A). These results indicate that prolonged exposure to high levels of adenosine enhances IL-6 release in both Ada−/− and SCD mice through ADORA2B signaling. We further investigated whether increased circulating IL-6 contributes to chronic pain in Ada−/− using an IL-6 neutralizing antibody. Strikingly, two week treatment with IL-6 neutralizing antibody significantly reduced both mechanical and thermal reflex hypersensitivity in Ada−/− mice (Figure 4B–C). Similarly, we found that genetic deletion of IL-6 in SCD mice significantly reduced reflex hypersensitivity in SCD mice (Figure 4B–C). Finally, the enhanced capsaicin responses in DRG neurons were significantly attenuated by IL-6 neutralizing antibody in Ada−/− mice or genetic deletion of IL-6 in SCD mice (Figure 4D). These studies identify IL-6 as a critical molecule functioning downstream of ADORA2B to promote TRPV1 function, sensory hypersensitivity, and pain-related behavior in two independent animal models.
Figure 4.
IL-6 trans-signaling via sIL-6R promotes reflex hypersensitivity and sensory neuron hyperexcitability in Ada−/− and SCD mice. (A) Plasma IL-6 levels were significantly elevated in Ada−/− and SCD mice compared with controls (*P<0.01) and the elevated IL-6 concentrations were decreased by in vivo treatment with PEG-ADA or PSB1115 (**P<0.01, n=8). (B–C) In vivo treatment with neutralizing IL-6 antibody in Ada−/− or genetic deletion of IL-6 in SCD mice reduced hypersensitivity (*P<0.01, n=12 in each case). (D)The incidence of DRG neurons responding to 10 nM capsaicin was reduced in Ada−/− mice treated with neutralizing IL-6 antibody and in SCD/IL-6−/− mice (*P<0.01, n=8 in each case). (E) Plasma sIL-6R levels were increased in Ada−/− and SCD mice (*P<0.01) and the elevated sIL-6R levels were reduced by PEG-ADA or PSB1115 treatment (**P<0.01, n=8). (F–G)Sgp130Fc treatment reduced reflex hypersensitivity in Ada−/− and SCD mice compared to those treated with isotype IgGFc (*P<0.01, n=10). (H) DRG neurons responding to 10 nM capsaicin was reduced in Ada−/− and SCD mice treated with sgp130Fc compared to mice treated with IgGFc (*P<0.01, n=6). (I) Sgp130Fc treatment reduced TRPV1 mRNA levels in DRG of Ada−/− and SCD mice compared to controls treated with isotype IgGFc (*P<0.01, n=6).
IL-6 trans-signaling via the sIL-6R induces sensory neuronal TRPV1 channel hyperexcitability and pain-related behavior in Ada−/− and SCD mice
We next investigated whether IL-6 by itself directly activates DRG sensory neurons, as suggested in other studies (Dubovy et al., 2013; Fang et al., 2015; Leskovar et al., 2000; Wei et al., 2013). Surprisingly, IL-6 did not induce obvious [Ca2+]i responses, unlike capsaicin in the same neurons (Figure S1A). Consistent with this finding, IL-6R mRNA expression levels were much lower in DRG neurons isolated from control, Ada−/− or SCD mice than in BMDCs as a positive control (Figure S1B). These result led us to conclude that IL-6 does not induce hyperexcitability by directly and acutely activating IL-6Rs on DRG neurons. Among all known cytokines, only IL-6 can activate cells lacking IL-6Rs by forming complex with sIL-6Rs that transactivates gp130, a ubiquitously expressed IL-6R cofactor. Given the unique feature of IL-6-sIL-6R trans-signaling, we immediately hypothesized that IL-6 trans-signaling via sIL-6R underlies ADORA2B-mediated neuronal hyperexcitability in both Ada−/− and SCD mice. To test this possibility, we first measured sIL-6R levels in Ada−/− and SCD mice treated with or without PEG-ADA or PSB1115. Similar to IL-6, we found that circulating sIL-6R levels were significantly elevated in both Ada−/− and SCD mice and the elevated levels were significantly reduced by PEG-ADA or PSB1115 treatment (Figure 4E). Next, we determined whether IL-6 could mediate sensory hypersensitivity in Ada−/− and SCD mice by IL-6 trans-activation of gp130 on DRG neurons by treating Ada−/− and SCD mice for 2 weeks with Fc isotype IgG or a specific fusion antibody to neutralize gp130. We found that reflex hypersensitivity to both stimuli were substantially reduced in both Ada−/− and SCD mice after gp130 neutralizing antibody treatment (Figure 4F–G). The proportion of DRG neurons from either Ada−/− or SCD mice that responded to 10 nM capsaicin was significantly reduced by treatment with the gp130 neutralizing antibody, which suppressed TRPV1 gene expression in DRG neurons (Figure 4H–I). Altogether, we demonstrated that IL-6 forms a complex with sIL-6R to transactivate gp130 which increases sensory neuronal TRPV1 expression and function and thereby contributes to persistent pain in both Ada−/− and SCD mice.
Bone marrow derived cells (BMDCs) are responsible for ADORA2B-mediated upregulation of TRPV1 expression and chronic pain in SCD mice
Plasma IL-6 and sIL-6R are produced primarily by circulating BMDCs and/or tissue resident immune cells (Xie et al., 2006). To determine which immune cells are responsible for ADORA2B-mediated elevation of plasma IL-6 and sIL-6R levels and subsequent chronic pain, we conducted BMT experiments. Specifically, we transplanted BM of SCD mice to irradiated WT or Adora2b−/− mice. Sixteen weeks post-BMT, flow cytometry was used to examine chimeras by quantifying blood CD45.1 and CD45.2 positive cells. As shown in Figure S2A, BMT from SCD to WT or Adora2b−/− mice was very successful with nearly 100% of circulating cells donor derived. Following successful BMT, we conducted mechanical and thermal behavioral assays. We found that WT mice transplanted with BM from SCD mice (SCD-WT group) displayed mechanical and thermal reflex hypersensitivity similar to the SCD mice (Figure S2B–C). Moreover, we observed that recipient Adora2b−/− mice transplanted with BM of SCD mice (SCD-Adora2b−/−) displayed behavioral hypersensitivity similar to SCD-WT mice (Figure S2B–C), indicating that deficiency of ADORA2B in peripheral tissues does not affect chronic pain-related behavior in SCD mice. We conclude that ADORA2B on BMDCs is essential for generating chronic pain in the SCD mice.
Next, we performed patch clamp studies on DRG neurons isolated from both SCD-WT and SCD-Adora2b−/− mice to directly assess nociceptor hyperexcitability. We found that sensory neurons isolated from SCD-Adora2b−/− mice showed excitability similar to SCD-WT (Figure S2D). Additionally, we found that TRPV1 mRNA levels were similar among these mice (Figure S2E). These studies provide in vivo evidence that elevated adenosine-ADORA2B signaling in circulating BMDCs contributes to the upregulation of TRPV1 gene expression and chronic pain in SCD mice.
ADORA2B in myeloid cells contributes to hypersensitivity in a chronic inflammatory pain model
Myeloid cells maintain the innate immune system and may have complex involvement in chronic inflammatory pain (Abbadie et al., 2003; Moalem and Tracey, 2006; Willemen et al., 2014). To determine the functional role of ADORA2B on myeloid cells in chronic inflammatory pain, we injected a hind-paw with CFA, a well-established mouse model of chronic inflammatory pain, in control mice (Lyz-Cre) and mice in which ADORA2B was knocked out specifically in myeloid cells (Adora2bf/f-Lyz-Cre). Lyz-Cre mice displayed more severe paw edema than Adora2bf/f-Lyz-Cre mice (Figure S3). Moreover, Adora2bf/f-Lyz-Cre mice showed CFA-induced reflex hypersensitivity similar to that of Lyz-Cre mice during the acute phase (Figure 5A–B), the Adora2bf/f-Lyz-Cre mice displayed significantly less reflex hypersensitivity compared to the controls during the delayed phase (Figure 5C–D), suggesting that genetic ablation of ADORA2B on myeloid cells reduces CFA-induced chronic inflammatory pain. No difference of basal levels of circulating adenosine and IL-6 were observed between Adora2bf/f-Lyz-Cre and Lyz-Cre mice (Figure S4). We found that CFA-induced elevation of plasma adenosine levels, which reached a peak within 2 hours post-injection and remained elevated at 6 hours in both control Lyz-Cre and Adora2bf/f-Lyz-Cre mice (Figure 5E). We found that CFA increased IL-6 levels in Lyz-Cre mice within 6 hours and remained elevated for 24 hours (Figure 5F). In contrast, genetic ablation of myeloid cell specific ADORA2B significantly attenuated plasma IL-6 levels compared to control mice 6 and 24 hours after CFA injection (Figure 5F). Additionally, we found that TRPV1 mRNA levels of isolated DRG neurons were not significantly different between Lyz-Cre and Adora2bf/f-Lyz-Cre mice without CFA injection (Figure 5G). However, TRPV1 mRNA levels were significantly induced in DRG neurons by CFA in the control mice on day 2 up to day 7, while its induction in DRGs isolated from the Adora2bf/f-Lyz-Cre mice on 2 and 7 days after CFA injection were significantly decreased when compared to Lyz-Cre mice (Figure 5G). Finally, we confirmed that nociceptor excitability was significantly lower in isolated sensory neurons from Adora2bf/f-Lyz-Cre mice than in neurons from control mice 6 hours and 7 days following CFA injection (Figure 5H). These findings provide strong evidence that ADORA2B on myeloid cells is required for IL-6 production in response to elevated levels of circulating adenosine, along with increased TRPV1 gene expression and associated hyperexcitability of nociceptors in this model of chronic inflammatory pain.
Figure 5.
ADORA2B on myeloid cells is required for reflex hypersensitivity and sensory neuron hyperexcitability induced in a CFA model of inflammatory pain. (A–D) Intraplantar injection of CFA induced similar hypersensitivity for the first 3 hours in Lyz-Cre and Adora2bf/f/Lyz-Cre mice. From 6 hours to 7 days, CFA induced less mechanical and heat hypersensitivity in Adora2bf/f/Lyz-Cre mice compared to Lyz-Cre mice (*P<0.01, n=6). (E) CFA injection induced similar changes in plasma adenosine concentration in Lyz-Cre and Adora2bf/f/Lyz-Cre mice (*P<0.05, n=3). (F) CFA injection induced less plasma IL-6 in Adora2bf/f/Lyz-Cre mice compared to Lyz-Cre mice (*P<0.05, n=3). (G) TRPV1 mRNA levels in DRG neurons in CFA-injected Adora2bf/f/Lyz-Cre and Lyz-Cre mice at different time points (*P<0.05, n=3). (H) Action potential thresholds in sensory neurons from Adora2bf/f/Lyz-Cre mice were significantly higher than sensory neurons from Lyz-Cre mice at 6 hours and 7 days post CFA injection (*P<0.05, n=3).
IL-6 trans-signaling via the sIL-6R directly induces TRPV1 gene expression in DRG neurons in a STAT3-dependent manner
STAT3 is a key signaling molecule downstream of IL-6R activation. When STAT3 is phosphorylated, it translocate to the nuclei and regulate gene transcription. STAT3 might contribute to the induction of TRPV1 gene expression in primary nociceptors by the IL-6 trans-signaling pathway. To test the direct involvement of sIL-6R and gp130 in the induction of TRPV1 mRNA in nociceptors, we isolated DRG neurons from WT mice and treated them with IL-6, sIL-6R, or their combination, either in the presence or absence of gp130 neutralizing antibody. After 30-minute treatment, cells stained positive for pSTAT3 were slightly but not significantly increased in DRG neurons treated with IL-6 alone (Figure 6A), consistent with our finding of very low expression of IL-6R in DRG neurons (Figure S1A). Similarly, sIL-6R alone had no significant effect. Strikingly, co-administration of IL-6 and sIL-6R significantly increased pSTAT3 staining in DRG neuron nuclei. As expected, addition of gp130 neutralizing antibody prevented the increase in pSTAT3 nuclear staining stimulated by IL-6 and sIL-6R (Figure 6A). These results indicate that IL-6 trans-signaling can activate pSTAT3 via sIL-6R to activate gp130 in DRG neurons.
Figure 6.
IL-6 complexes with sIL-6R to induce TRPV1 gene expression in a STAT3-dependent manner in DRG neurons. (A) Representative immunofluorescence staining of pSTAT3 in DRGs isolated form WT mice treated with control buffer, IL-6, sIL-6R, IL-6+sIL-6R, IL-6+sIL-6R+gp130Fc, IL-6+sIL-6R+IgGFc. Right panel: the fraction of DRG cells stained with pSTAT3 (*P<0.05, **P<0.05, n=6), scale bar 50μm. (B) TRPV1 mRNA levels were increased in DRG cells isolated from WT mice treated for 24 hours with IL-6+sIL-6R together but not separately. TRPVI mRNA levels were reduced by pretreatment with gp130Fc or Statics, a pSTAT3 inhibitor (*P<0.05, **P<0.01).
Next, we assessed the effects of sIL-6R/IL-6-mediated trans-signaling via pSTAT3 on TRPV1 gene expression in DRG neurons. After 24 hour treatment of dissociated DRG neurons, we found that TRPV1 gene expression was significantly induced by combined IL-6 and sIL-6R, but not by IL-6 or sIL-6R alone (Figure 6B). Moreover, a gp130-neutralizing antibody or specific inhibitor of pSTAT3 (Statics), significantly suppressed IL-6/sIL-6-induced TRPV1 gene expression in dissociated DRG neurons (Figure 6B). These results show that sIL-6R/IL-6 transactivate gp130 in DRG neurons to induce TRPV1 gene expression in a pSTAT3-dependent manner.
Discussion
Here we report that sustained elevated circulating adenosine signaling via ADORA2B is required for chronic pain behavior and neuronal hypersensitivity in three independent animal models including: Ada−/−, SCD, and CFA-injected mice. Surprisingly, adenosine-dependent pain behavior did not involve direct and acute actions of ADORA2B on primary nociceptors. Instead, elevated adenosine required ADORA2B signaling in myeloid cells to stimulate sensory hyperexcitability and hypersensitivity through IL-6 and sIL-6R, and consequent trans-activation of gp130, which stimulates a STAT3 dependent enhancement of TRPV1 gene expression in DRG neurons. These findings reveal an unexpectedly important role for prolonged adenosine-ADORA2B signaling in several models of chronic pain, and show that these adenosine effects depend upon IL-6 signaling from myeloid cells to transactivate sensory neurons.
Adenosine is a signaling molecule induced under hypoxia and energy depletion and involved in multiple physiological and pathological conditions (Eckle et al., 2014; Eltzschig and Carmeliet, 2011; Mi et al., 2008; Sun et al., 2006; Zhang et al., 2011). Importantly, acute and prolonged effects of adenosine on injured or stressed tissues often differ dramatically, with brief exposure reducing inflammation and prolonged exposure promoting inflammation (Dai et al., 2011; Iriyama et al., 2015; Karmouty-Quintana et al., 2012; Reutershan et al., 2009; Schingnitz et al., 2010; Zhou et al., 2011). A diversity of preclinical models of pain have illustrated the anti-nociceptive properties of ADORA1 activation (Korboukh et al., 2012; Sowa et al., 2010). In contrast, the role of ADORA2A in pain has been ambiguous with some studies showing anti-nociceptive and others showing pro-nociceptive roles. Moreover, there have been fewer reports regarding the effects of ADORA2B signaling in nociception, but in general the observations are consistent with a peripheral pro-nociceptive role (Abo-Salem et al., 2004; Bastia et al., 2002; By et al., 2011; Li et al., 2010). Adenosine either promotes or inhibits nociception depending on the site of action and the specific receptor involved (Sawynok and Liu, 2003; Sawynok et al., 1998). Moreover, the reports linking adenosine signaling and pain have varied according to the preclinical model used for testing (Sawynok, 2015). Of note, in contrast to the analgesic effect of local intrathecal activation of ADORA2B by BAY60-60583 reported by Loram et al.(Loram et al., 2013), other early reports showed that IP injection of ADORA2B antagonists reduces pain induced by diphenyl diselenide (Savegnago et al., 2008) and an acute pain model (Abo-Salem et al., 2004). However, molecular mechanisms and cell type underlying ADORA2B antagonism in chronic inflammatory pain remained unclear prior to our current studies. Supporting early pharmacological studies showing analgesic effects of IP injection of ADORA2B antagonists (Abo-Salem et al., 2004; Savegnago et al., 2008), we consistently showed that IP injection of an ADORA2B antagonist is analgesic in two independent models of chronic pain including Ada−/− and SCD mice. Consistent with pharmacological evidence, we provided genetic evidence revealing that prolonged accumulation of systemic adenosine in chronic inflammatory pain by activating ADORA2B signaling on myeloid cells in both SCD and CFA-injected mice, a chronic inflammatory pain model. Mechanistically, we further revealed myeloid cell ADORA2B-mediated IL-6 trans-signaling is a previously unrecognized pathway underlying chronic inflammatory pain by inducing TRPVI gene expression in neuronal cells of the DRG in a pSTAT3 dependent manner. Altogether, we revealed that sustained elevated circulating adenosine signaling via ADORA2B on myeloid cells mediated an IL-6 trans-signaling cascade that is a mechanism underlying chronic inflammatory pain, which is different from neuropathic models.
Notably, circulating adenosine is chronically elevated and contributes to sickling in SCD patients and mice (Zhang et al., 2011), a humanized model of SCD that exhibits chronic pain by activating ADORA2B. Moreover, early studies showed that ADORA2A activation in iNK cells contributes to reduced pulmonary damage in SCD (Wallace and Linden, 2010). However, a role for adenosine signaling in chronic pain in SCD had not been recognized until we discovered that Ada−/− mice display chronic pain. As we found with Ada−/− and SCD mice in which adenosine levels were lowered by PEG-ADA treatment or ADORA2B signaling was inhibited by a specific receptor antagonist, showed significant reduction of pain behavior and a reversal in DRG neurons of both electrical hyperexcitability and hypersensitivity to capsaicin. These effects were paralleled by a reversal of enhanced TRPV1 gene expression found in DRG neurons taken from both Ada−/− and SCD mice. These findings are consistent with the earlier demonstration that pharmacological inhibition of TRPV1 blocked hypersensitivity of mechanically evoked reflexes in SCD mice (Hillery et al., 2011). Moreover, early studies demonstrated that elevated adenosine signaling via ADORA2B on erythrocytes stimulated 2,3-BPG production, sickle hemoglobin deoxygenation and subsequent sickling (Sun et al., 2006; Zhang et al., 2011). Thus, prolonged adenosine-ADORA2B signaling in SCD mice has at least two important effects: induction of sickling by increasing 2,3-BPG levels in erythrocytes and induction of a state of thermal and mechanical hypersensitivity in nociceptors that involves upregulation of TRPV1 expression and function. Overall, we have demonstrated the sustained elevated circulating adenosine is a common factor contributing to chronic pain by activating ADORA2B in Ada−/− mice independent of sickling and in SCD mice with sickling. Our findings of the critical role of sustained elevated circulating adenosine signaling via ADORA2B in chronic pain is significant because it immediately suggests lowering persistently elevated adenosine by PEG-ADA or interfering with ADORA2B activation are potential mechanistic-based therapies for chronic pain. In particular, PEG-ADA is an FDA-approved drug that has been used to treat ADA-deficient humans for more than thirty years. Our preclinical evidence indicates that lowering adenosine by PEG-ADA in SCD is a promising and relatively safe approach to treat both sickling and chronic pain.
A particularly important finding was that pain-related behavior induced by prolonged elevation of adenosine levels were not exerted by direct action of adenosine on sensory neurons. Instead, adenosine-induced pain behavior was produced by activating ADORA2B in myeloid cells, possibly including microglial cells. Although microglia are well recognized to promote local inflammation and chronic pain (Basbaum et al., 2009; McMahon et al., 2015), emerging evidence indicates that increased peripheral inflammatory responses contribute to chronic pain (Chiu et al., 2012; McMahon et al., 2015). To determine the role of myeloid cells in chronic inflammatory pain, we conducted genetic studies showing that CFA-induced pain was significantly reduced in Adora2bf/f/Lyz-Cre compared to Lyz-Cre mice. Lyz-Cre is highly expressed by all myeloid cells, including microglia (Clausen et al., 1999). It is possible that ADORA2B on microglia may contribute to CFA-induced pain. To further assess whether BMDCs play an important role in chronic pain in SCD mice, we transplanted BMDCs from SCD mice into irradiated WT and Adora2b−/− mice. We found no significant difference in pain behavior between irradiated WT and Adora2b−/− mice transplanted with SCD BM. Several studies show the specific infiltration of monocyte-derived macrophages under pathological conditions (Larochelle et al., 2015) including multiple sclerosis, experimental autoimmune encephalomyelitis or irradiation prior to BMT. It is possible that ADORA2B positive SCD mouse BMDCs of the myeloid lineage may infiltrate the CNS after irradiation, differentiate into ADORA2B positive glia and contribute to the pain behavior observed in the Adora2b−/− recipient mice. Although our current experimental results do not allow us to clearly distinguish between circulating monocytes and microglia, we have provided considerable genetic and pharmacological evidence that elevated circulating adenosine signaling via myeloid cell ADORA2B (including microglial cells) contributes to chronic inflammatory pain.
How does adenosine-ADORA2B signaling in BMDCs lead to persistent hypersensitivity in nociceptors? Both Ada−/− and SCD mice exhibited elevated plasma levels of IL-6 and sIL-6R that depended upon prolonged elevated blood adenosine-mediated ADORA2B activation on myeloid cells. Moreover, neutralizing antibodies to IL-6 or gp130 blocked reflex hypersensitivity, capsaicin responses and TRPV1 upregulation. Combined with the lack of detectable IL-6R expression or physiological responses to IL-6 in isolated DRG neurons, these results indicate that an IL-6/sIL-6R complex transactivates gp130 on sensory neurons to induce TRPV1 gene expression and sensory neuron hypersensitivity in both conditions. In addition to a role for myeloid cells, as shown here, mast cell activation has been shown to promote pain-related inflammatory neuropathy in SCD mice (Vincent et al., 2013). Interestingly, elevated adenosine is known to activate mast cells and lead to trachea constriction and respiratory obstruction in Ada−/− mice (Zhong et al., 2001). Moreover, ADORA2B activation can directly induce IL-8 secretion from cultured human mast cells (Feoktistov and Biaggioni, 1995). Adenosine signaling via ADORA2B may promote pain by mast cell activation. Nevertheless, we have provided both genetic and pharmacological evidence that prolonged elevated adenosine signaling via ADORA2B on myeloid cells induces circulating IL-6-sIL-6R complex to transactivate gp130 on neuronal cells resulting in increased TRPV1 gene expression/function and chronic pain in both Ada−/− and SCD mice.
The role of adenosine signaling and IL-6 trans-signaling in nociception in both Ada−/− and SCD mice is further validated in another widely used inflammatory pain model, CFA injection. We demonstrated that CFA injection caused elevated plasma adenosine and IL-6 levels. Statistically significant elevation of adenosine lasted at least 6 but less than 48 hours, which is much briefer than the chronic elevation produced in Ada−/− and SCD mice. Because extracellular adenosine is degraded very rapidly and rarely remains elevated in plasma for more than a few minutes, more than 6 hour elevated adenosine with a single injection of CFA would represent an unusually prolonged exposure of blood-borne cells to this signaling molecule. Selective genetic ablation of ADORA2B on myeloid cells significantly attenuated the CFA-induced increase of plasma IL-6, as well as reflex hypersensitivity, sensory neuron hyperexcitability, and upregulation of TRPV1 gene expression in these neurons. Notably, our finding is strongly supported by earlier studies showing plasma IL-6 induces pain-related behavior by forming a complex with sIL-6R, which transactivates gp130 on sensory neurons (Baastrup et al., 2014; Boettger et al., 2010). However, the specific cell type and signaling cascade leading to activation of IL-6-sIL-6R trans-signaling in chronic pain remained unidentified. Here using three independent chronic pain mouse models, we demonstrate that persistently elevated circulating adenosine activates ADORA2B on myeloid cells, leading to IL-6 and sIL-6R trans-activation of gp130 on DRG neurons, resulting in increased TRPV1 gene expression, and hypersensitivity and chronic pain. Supporting the in vivo findings in both Ada−/−, SCD and CFA-injected mice, we further demonstrated that IL-6 trans-signaling via sIL-6R activates gp130, resulting in the translocalization of pSTAT3 to nucleus, and the induction of TRPV1 gene expression in isolated DRG neuronal cells from WT mice.
Although DRG neurons contain no IL-6R, they can respond to IL-6 trans-signaling via gp130 activation and contributing to chronic pain by inducing TRPV1 in DRG neurons in a pSTAT3 dependent manner (Figure 6C).
In summary, our studies lead to a working model for the induction of persistent pain in which activation of ADORA2B on myeloid cells by prolonged exposure to adenosine in the blood causes the release of IL-6 from these cells, transactivation of gp130 on nociceptive sensory neurons by a sIL-6R/IL-6 complex, and a consequent pSTAT3-mediated increase in the nociceptive function of sensory neurons that involves enhanced TRPV1 gene expression. These mechanistic findings suggest therapeutic approaches for treating chronic pain including PEG-ADA (an FDA approved drug), ADORA2B specific antagonists, and inhibiting IL-6-sIL-6R transactivation of gp130 in nociceptive sensory neurons.
Materials and Methods
Additional methods in supplementary material Animals
Ada −/− mice were generated and genotyped as previously described (Blackburn et al., 2000). Mice homozygous for the null Ada allele were designated Ada−/−, whereas mice heterozygous for the null Ada allele were designated as Ada−/+. Ada−/−/Adora2b−/− mice were generated by mating Ada−/− mice with Adora2b−/−. Berkeley SCD transgenic mice were purchased from The Jackson Laboratory. All studies were reviewed and approved by the University of Texas Health Science Center at Houston Animal Welfare Committee.
Pharmacological applications in mice including treatment with PEG-ADA, adenosine receptor antagonists, neutralizing gp130Fc antibody and humanized IL-6 neutralizing antibody
PEG-ADA was generated by the covalent modification of purified bovine ADA with activated PEG as described previously (Blackburn et al., 2000). Ada−/− mice were maintained on high-dose (HD) enzyme therapy at 5U/wk until 8 wks to maintain viability (Mi et al., 2008). The Ada−/− mice were divided into five groups, each receiving a specific adenosine receptor antagonist, except for controls, that received PBS. For IL-6 antibody studies, mice were divided into two groups. The first was injected IP with neutralizing IL-6 antibody or sgp130Fc (100 μg per day; R&D Systems) for 2 weeks (Le et al., 2014). The second group was injected daily with PBS or IgG Fc for 2 weeks. SCD mice at 8–14 weeks of age were divided into two groups, followed the same procedures for Ada−/− mice, except for the lower dosage of PEG-ADA (2.5U per week), PSB1115 (200 μg per day; Tocris Bioscience) or sgp130Fc (100 μg per day) for 2 weeks.
Mechanical and Thermal hypersensitivity
All animals were acclimated to the behavioral testing apparatus. Each mouse was placed in a clear Plexiglas testing chamber (8 × 8 × 12 cm) and acclimated prior to all experiments. To measure mechanical sensitivity, animals were placed on an elevated wire grid and the left hind-paw was probed with calibrated von Frey filaments (0.02–0.4g bending forces, North Coast). To measure heat hypersensitivity, mice were placed on a glass plate (Plantar Analgesia meter; IITC, Woodland Hills, CA, USA), and a radiant heat source was applied to the plantar surface of a hind-paw. The tests and data analysis were conducted in a double-blind fashion.
Neuronal excitability tests
DRGs were isolated from 3–6 mice per group. Depending on the number of mice per group, we recorded an optimal number of around 36–60 neurons for patch clamp studies. About 80% of the series resistance was compensated electronically. Membrane potentials were recorded using an Axopatch 200B amplifier (Axon Instruments, Burlingame, CA) in response to series of 500 ms current injections (except where noted). Signals were digitized with a Digidata 1440A A/D converter at 10 kHz, filtered at 2 kHz, and analyzed using Clampfit 10 software (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
All data are expressed as mean ± SEM. Data were analyzed for statistical significance using GraphPad Prism 4 software (GraphPad Software). Student’s t tests (paired or unpaired as appropriate) were applied in two-group analysis. Differences among the means of multiple groups were compared by the one-way analysis of variance (ANOVA). Moreover, two-way ANOVA was used for difference among the means of multiple groups for Fig. 4A & E, and two-way repeat measures ANOVA was used for difference among the means of multiple groups at different time points for Fig. 5A–F, followed by a Tukey’s multiple comparisons test. A value of P< 0.05 was considered significant and was the threshold to reject the null hypothesis.
Supplementary Material
Highlights.
Adenosine (Ado) deaminase deficient mice displayed chronic pain
Excess Ado signaling via ADORA2B promotes chronic pain in three distinct models
ADORA2B activation induces immunoneuronal interaction and promotes chronic pain
IL-6 trans-signaling underlies ADORA2B-induced prolonged hypersensitive nociception
Acknowledgments
This work was supported by National Institute of Health Grants HL113574 (to Y.X.), DK083559 (to Y.X.), HL119549 (to M.R.B., E.K.H., Y.X.), and American Heart Association Grant 12IRG9150001 (to Y.X.), and by China National Science Foundation 81228004 (to Y.X.).
Footnotes
Disclosures
None.
Author Contribution
X. H. carried out pain behavior measurement in all of the mice; isolation of DRG neurons for patch clamp, calcium influx and mRNA analysis; M.A. conducted mRNA analysis, genotyping, behavior measurement and organized the graphs and manuscript; J.L., O.I.Z. and M.M. conducted patch clamp analysis; K.Q.S. and A.H. genotyped Ada−/− mice and measured adenosine concentration in the mice; T.L., provided expertise in IL-6-sIL-6R trans-signaling; Y.J.Z., conducted BMT; H.Y.W. & Y.E.W. genotyped SCD mice; S.S.Z. and H. L. conducted chimerism analysis in BMT mice; O. M. P. provided his expertise on patch clamp and electrophysiology; H.K.E. provided Adora2bf/f-LyzCre mice; M.R.B. provided Ada−/−/Adora2b−/− mice; R.E.K. provided Ada−/− mice and proofread the manuscript; D. H., provided expertise in pain; E.T.W. & H. Z.H. provided expertise in pain and helped organized manuscript; Y.X. was the principle investigator of the study, oversaw the overall design of experiments and interpretation of all results, the writing and organization of the manuscript including text and figures and did final editing of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A, Muller CE. Antinociceptive effects of novel A2B adenosine receptor antagonists. The Journal of pharmacology and experimental therapeutics. 2004;308:358–366. doi: 10.1124/jpet.103.056036. [DOI] [PubMed] [Google Scholar]
- Aley KO, Green PG, Levine JD. Opioid and adenosine peripheral antinociception are subject to tolerance and withdrawal. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:8031–8038. doi: 10.1523/JNEUROSCI.15-12-08031.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baastrup C, Finnerup NB, Rice AS, Jensen TS, Yezierski RP. ‘Inhibition of IL-6 signaling: a novel therapeutic approach to treating spinal cord injury pain’ by Guptarak et al. Pain. 2014;155:197–198. doi: 10.1016/j.pain.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn MR, Aldrich M, Volmer JB, Chen W, Zhong H, Kelly S, Hershfield MS, Datta SK, Kellems RE. The use of enzyme therapy to regulate the metabolic and phenotypic consequences of adenosine deaminase deficiency in mice. Differential impact on pulmonary and immunologic abnormalities. J Biol Chem. 2000;275:32114–32121. doi: 10.1074/jbc.M005153200. [DOI] [PubMed] [Google Scholar]
- Boettger MK, Leuchtweis J, Kummel D, Gajda M, Brauer R, Schaible HG. Differential effects of locally and systemically administered soluble glycoprotein 130 on pain and inflammation in experimental arthritis. Arthritis Res Ther. 2010;12:R140. doi: 10.1186/ar3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nature neuroscience. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic research. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y. A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J Am Soc Nephrol. 2011;22:890–901. doi: 10.1681/ASN.2010080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Levine JD, Green PG. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. European journal of pain. 2011;15:796–800. doi: 10.1016/j.ejpain.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovy P, Brazda V, Klusakova I, Hradilova-Svizenska I. Bilateral elevation of interleukin-6 protein and mRNA in both lumbar and cervical dorsal root ganglia following unilateral chronic compression injury of the sciatic nerve. J Neuroinflammation. 2013;10:55. doi: 10.1186/1742-2094-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T, Kewley EM, Brodsky KS, Tak E, Bonney S, Gobel M, Anderson D, Glover LE, Riegel AK, Colgan SP, et al. Identification of hypoxia-inducible factor HIF-1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. J Immunol. 2014;192:1249–1256. doi: 10.4049/jimmunol.1100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. The New England journal of medicine. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Faigle M, Knapp S, Karhausen J, Ibla J, Rosenberger P, Odegard KC, Laussen PC, Thompson LF, Colgan SP. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood. 2006;108:1602–1610. doi: 10.1182/blood-2006-02-001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. The Journal of experimental medicine. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Kong LY, Cai J, Li S, Liu XD, Han JS, Xing GG. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. Pain. 2015;156:1124–1144. doi: 10.1097/j.pain.0000000000000158. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J Clin Invest. 1995;96:1979–1986. doi: 10.1172/JCI118245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, Pankow JS, Ravid K, Fredholm B, Hedrick CC, Rich SS, et al. Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes. 2011;60:669–679. doi: 10.2337/db10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, APIJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gereau RWt, Sluka KA, Maixner W, Savage SR, Price TJ, Murinson BB, Sullivan MD, Fillingim RB. A pain research agenda for the 21st century. The journal of pain : official journal of the American Pain Society. 2014;15:1203–1214. doi: 10.1016/j.jpain.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nature medicine. 2010;16:1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DS, McGee SJ. Pain as a global public health priority. BMC public health. 2011;11:770. doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nature neuroscience. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield MS. PEG-ADA: an alternative to haploidentical bone marrow transplantation and an adjunct to gene therapy for adenosine deaminase deficiency. Human mutation. 1995;5:107–112. doi: 10.1002/humu.1380050202. [DOI] [PubMed] [Google Scholar]
- Hershfield MS. New insights into adenosine-receptor-mediated immunosuppression and the role of adenosine in causing the immunodeficiency associated with adenosine deaminase deficiency. European journal of immunology. 2005;35:25–30. doi: 10.1002/eji.200425738. [DOI] [PubMed] [Google Scholar]
- Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118:3376–3383. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriyama T, Sun K, Parchim NF, Li J, Zhao C, Song A, Hart LA, Blackwell SC, Sibai BM, Chan LN, et al. Elevated placental adenosine signaling contributes to the pathogenesis of preeclampsia. Circulation. 2015;131:730–741. doi: 10.1161/CIRCULATIONAHA.114.013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nature reviews Drug discovery. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmouty-Quintana H, Zhong H, Acero L, Weng T, Melicoff E, West JD, Hemnes A, Grenz A, Eltzschig HK, Blackwell TS, et al. The A2B adenosine receptor modulates pulmonary hypertension associated with interstitial lung disease. FASEB J. 2012;26:2546–2557. doi: 10.1096/fj.11-200907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korboukh I, Hull-Ryde EA, Rittiner JE, Randhawa AS, Coleman J, Fitzpatrick BJ, Setola V, Janzen WP, Frye SV, Zylka MJ, et al. Orally active adenosine A(1) receptor agonists with antinociceptive effects in mice. Journal of medicinal chemistry. 2012;55:6467–6477. doi: 10.1021/jm3004834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle A, Bellavance MA, Michaud JP, Rivest S. Bone marrow-derived macrophages and the CNS: An update on the use of experimental chimeric mouse models and bone marrow transplantation in neurological disorders. Biochimica et biophysica acta. 2015 doi: 10.1016/j.bbadis.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Le TT, Karmouty-Quintana H, Melicoff E, Le TT, Weng T, Chen NY, Pedroza M, Zhou Y, Davies J, Philip K, et al. Blockade of IL-6 Trans signaling attenuates pulmonary fibrosis. J Immunol. 2014;193:3755–3768. doi: 10.4049/jimmunol.1302470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskovar A, Moriarty LJ, Turek JJ, Schoenlein IA, Borgens RB. The macrophage in acute neural injury: changes in cell numbers over time and levels of cytokine production in mammalian central and peripheral nervous systems. The Journal of experimental biology. 2000;203:1783–1795. doi: 10.1242/jeb.203.12.1783. [DOI] [PubMed] [Google Scholar]
- Li L, Hao JX, Fredholm BB, Schulte G, Wiesenfeld-Hallin Z, Xu XJ. Peripheral adenosine A2A receptors are involved in carrageenan-induced mechanical hyperalgesia in mice. Neuroscience. 2010;170:923–928. doi: 10.1016/j.neuroscience.2010.07.045. [DOI] [PubMed] [Google Scholar]
- Loram LC, Taylor FR, Strand KA, Harrison JA, Rzasalynn R, Sholar P, Rieger J, Maier SF, Watkins LR. Intrathecal injection of adenosine 2A receptor agonists reversed neuropathic allodynia through protein kinase (PK)A/PKC signaling. Brain, behavior, and immunity. 2013;33:112–122. doi: 10.1016/j.bbi.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, La Russa F, Bennett DL. Crosstalk between the nociceptive and immune systems in host defence and disease. Nature reviews Neuroscience. 2015;16:389–402. doi: 10.1038/nrn3946. [DOI] [PubMed] [Google Scholar]
- Mi T, Abbasi S, Zhang H, Uray K, Chunn JL, Xia LW, Molina JG, Weisbrodt NW, Kellems RE, Blackburn MR, et al. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest. 2008;118:1491–1501. doi: 10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain research reviews. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Murphy PG, Ramer MS, Borthwick L, Gauldie J, Richardson PM, Bisby MA. Endogenous interleukin-6 contributes to hypersensitivity to cutaneous stimuli and changes in neuropeptides associated with chronic nerve constriction in mice. The European journal of neuroscience. 1999;11:2243–2253. doi: 10.1046/j.1460-9568.1999.00641.x. [DOI] [PubMed] [Google Scholar]
- Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Annals of the rheumatic diseases. 2009;68:1580–1584. doi: 10.1136/ard.2008.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- Savegnago L, Jesse CR, Nogueira CW. Caffeine and a selective adenosine A(2B) receptor antagonist but not imidazoline receptor antagonists modulate antinociception induced by diphenyl diselenide in mice. Neuroscience letters. 2008;436:120–123. doi: 10.1016/j.neulet.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Sawynok J. Adenosine receptor targets for pain. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Progress in neurobiology. 2003;69:313–340. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A, Poon A. Peripheral antinociceptive effect of an adenosine kinase inhibitor, with augmentation by an adenosine deaminase inhibitor, in the rat formalin test. Pain. 1998;74:75–81. doi: 10.1016/S0304-3959(97)00153-X. [DOI] [PubMed] [Google Scholar]
- Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184:5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa NA, Voss MK, Zylka MJ. Recombinant ecto-5′-nucleotidase (CD73) has long lasting antinociceptive effects that are dependent on adenosine A1 receptor activation. Mol Pain. 2010;6:20. doi: 10.1186/1744-8069-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Direct cutaneous hyperalgesia induced by adenosine. Neuroscience. 1990;38:757–762. doi: 10.1016/0306-4522(90)90068-f. [DOI] [PubMed] [Google Scholar]
- Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122:1853–1862. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KL, Linden J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 2010;116:5010–5020. doi: 10.1182/blood-2010-06-290643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET. Neuroinflammatory contributions to pain after SCI: roles for central glial mechanisms and nociceptor-mediated host defense. Experimental neurology. 2014;258:48–61. doi: 10.1016/j.expneurol.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Wei XH, Na XD, Liao GJ, Chen QY, Cui Y, Chen FY, Li YY, Zang Y, Liu XG. The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Experimental neurology. 2013;241:159–168. doi: 10.1016/j.expneurol.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Willemen HL, Eijkelkamp N, Garza Carbajal A, Wang H, Mack M, Zijlstra J, Heijnen CJ, Kavelaars A. Monocytes/Macrophages control resolution of transient inflammatory pain. The journal of pain : official journal of the American Pain Society. 2014;15:496–506. doi: 10.1016/j.jpain.2014.01.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WP, Hao JX, Halldner L, Lovdahl C, DeLander GE, Wiesenfeld-Hallin Z, Fredholm BB, Xu XJ. Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain. 2005;113:395–404. doi: 10.1016/j.pain.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–822. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV, et al. Detrimental effects of adenosine signaling in sickle cell disease. Nature medicine. 2011;17:79–86. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xia Y. Adenosine signaling in normal and sickle erythrocytes and beyond. Microbes Infect. 2012;14:863–873. doi: 10.1016/j.micinf.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Chunn JL, Volmer JB, Fozard JR, Blackburn MR. Adenosine-mediated mast cell degranulation in adenosine deaminase-deficient mice. The Journal of pharmacology and experimental therapeutics. 2001;298:433–440. [PubMed] [Google Scholar]
- Zhou Y, Schneider DJ, Morschl E, Song L, Pedroza M, Karmouty-Quintana H, Le T, Sun CX, Blackburn MR. Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury. J Immunol. 2011;186:1097–1106. doi: 10.4049/jimmunol.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.