Abstract
The United States has been a leader in biomedical science for decades, in large part because of the strategy used by the National Institutes of Health (NIH) to invest its budgetary portfolio. They identified talented young scientists from each generation and gave them the resources they needed to initiate and maintain strong research programs. However, recently this investment has become less diversified, with a larger fraction of grant dollars in the hands of a smaller fraction of researchers. This threatens the future of our field, as many productive early and midcareer scientists are facing having to close their labs. NIH and others have studied this problem, gathering data that suggest that over a certain level of funding to an individual investigator, there are diminishing returns in scientific output. Here I review these data and examine the issues that led NIH to propose and then reverse a cap on funding to individual investigators, the Grant Support Index. I consider other proposed solutions, and call on all in the field to examine whether the status quo is acceptable, and if not, urge them to propose and advocate for concrete alternatives.
It’s an exceptionally exciting time for basic biomedical science, as new tools drive exciting discoveries about the living world and offer new leads for treating disease. Some nations, like China, are embracing these possibilities and dramatically increasing research investment (Kristiansen, 2014; Conte et al., 2017). The United States has been a world leader in biomedical research for decades because of the thoughtful way the National Institutes of Health (NIH) invested its portfolio, identifying talented young scientists from each generation and giving them the resources they needed to initiate and maintain strong research programs. As the NIH budget increased in the early 2000s, talented young scientists began labs at universities all across the nation, further expanding the biomedical enterprise beyond the traditional research hubs in the Northeast and on the West Coast. However, for the past decade or more the budget of the NIH has been losing ground in inflation-adjusted dollars (FASEB, 2017). This means that each dollar invested takes on additional importance to ensure the best outcome for science and society.
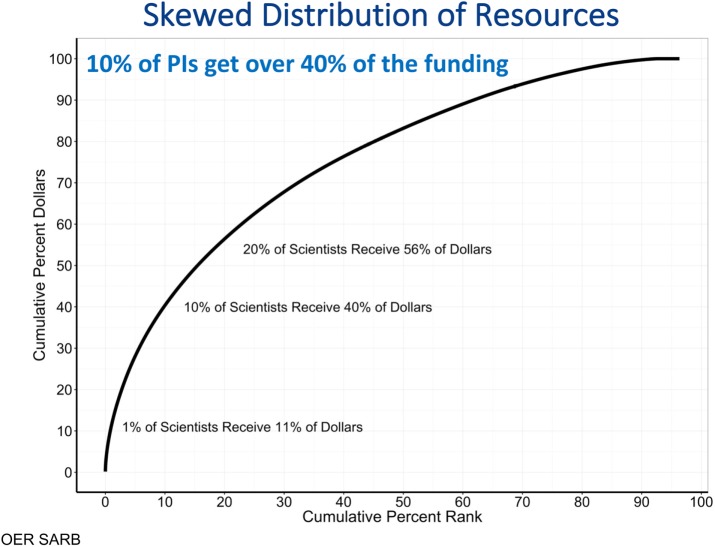
If you ask an investment advisor about the most sound and secure way to invest your funds, the first answer will be simple: don’t put all of your eggs in one basket. A diverse investment portfolio reduces risk and increases the likelihood that you’ll include in your investments those that pay off best. The analogy to basic science is striking—the story of CRISPR, for example, reminds us how difficult it can be to predict which labs will contribute to fundamental new discoveries. A look at NIH’s investment portfolio suggests that it is now taking a different strategy. Its research dollars are invested in a strikingly uneven way—1% of scientists now get 11% of NIH funding, 10% get 40% (Collins, 2017; Lorsch, 2017; Figure 1).
FIGURE 1:
NIH research portfolio investment is skewed toward a small fraction of the investigators funded. Source: Presentation by NIGMS Director Jon Lorsch to NIGMS Council, May 26, 2017 (Lorsch, 2017). These data reflect all research dollars, but analyses restricted to research project grants are similar: 1% of scientists get 11% of funds, 10% of scientists get 37% of funds, and 20% of scientists get 52% of funds.
All of us have seen the results at our own institutions and in the lives of colleagues in our fields. We have watched tightened grant funding threaten the careers of early and especially midcareer scientists as they attempt to obtain or to renew investigator-initiated research grants in competition with the more senior leaders of their fields. Most of us know productive scientists who are faced with closing their labs. This is discouraging many of our best trainees from pursuing a research career. Simply put, it is a crisis (Alberts et al., 2015; FASEB, 2015; Kimble et al., 2015).
When we concentrate our investment dollars in fewer labs and projects, we risk failing to fund young investigators with great promise but without the long track record or extensive scientific staff or resources of their senior, well-funded colleagues. Two examples from my own field illustrate this. In 1981 Princeton hired a new assistant professor named Eric Wieschaus, who had just finished 4 years of collaborative work at EMBL in a team of four, carrying out genetic screens in the fruit fly. At around the same time, a young assistant professor at MIT, Robert Horvitz, and his small group of graduate students were carrying out genetic screens in Caenorhabditis elegans. In our current system, it’s hard to know how their first NIH renewals would have fared if they were measured against well-funded labs with 25 or more postdocs and graduate students. But where would our fields be if they had not been able to continue the pathbreaking work that revolutionized developmental biology and led to the Nobel Prizes in Medicine and Physiology in 1995 and 2002?
I am on the Council of the National Institute of General Medical Sciences (NIGMS) and have watched that institute take steps to try to expand the pool of talented and productive researchers who have access to research grants. By taking measures like closing down some “big science” programs, they have made slow but steady progress, with a resulting increase in R01 success rates (Miklos and Lorsch, 2017). As part of this effort, NIGMS and the Office of the Director did an exceptionally thorough analysis of the distribution of research project grants across the scientific spectrum, and how productivity and scientific impact scale with the amount of grant funding possessed by a principal investigator (PI). I saw these data at our May 2017 NIGMS Council meeting, when Jon Lorsch gave a very compelling presentation. I would encourage everyone to review his slides (Lorsch, 2017).
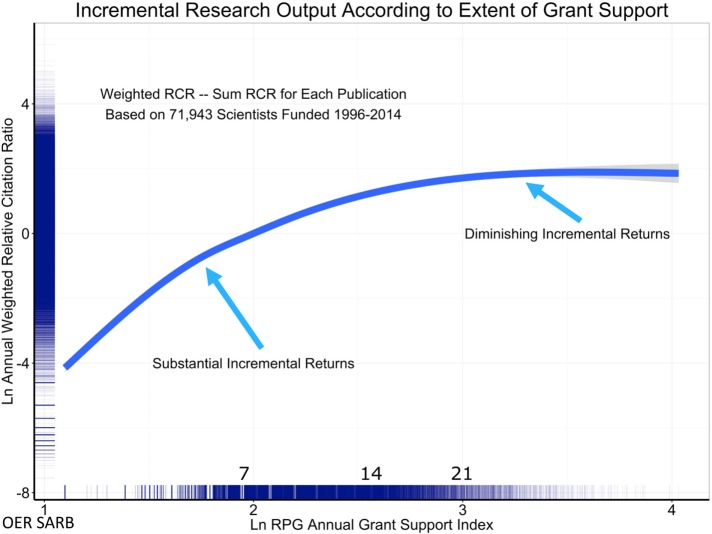
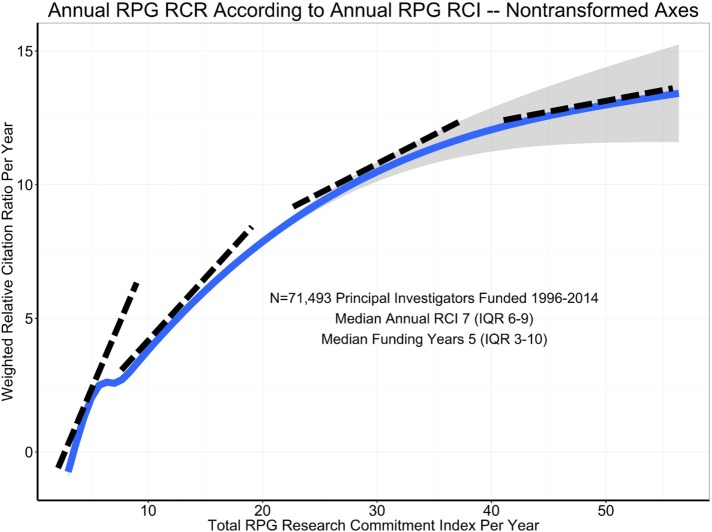
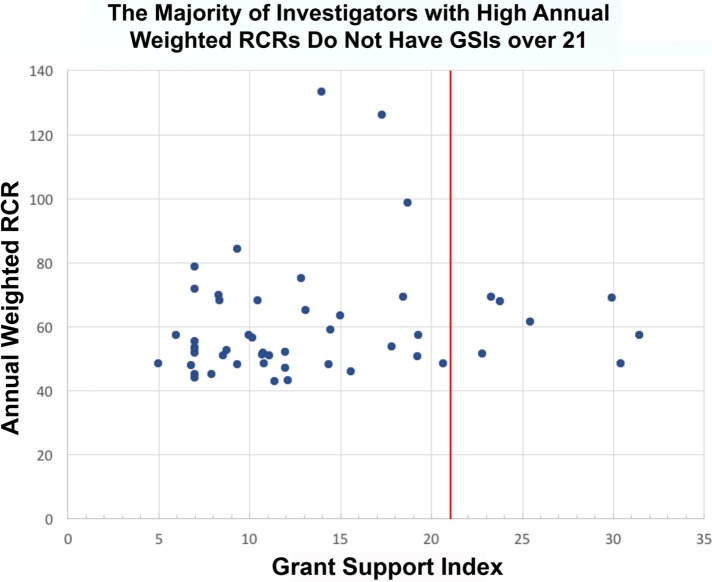
While no data source is perfect, the analyses were performed across many samples, using many different metrics (Lorsch, 2017). All the analyses tell a similar story. First, it is clear that impactful science costs money—a lab without grant funding cannot have an impact. Second, the data suggest that above a certain level of research project grant funding (R01 or equivalent), there are generally diminishing returns in scientific advances per unit dollar (Lauer et al., 2017; Lorsch, 2017; Figures 2 and 3). There has been much discussion of the statistical approaches used and what the curve really looks like at the far-right end, among the best-funded laboratories. With regard to this point, the NIH data fit well with that from several other published studies, all of which documented diminishing returns as funding levels increased (Fortin and Currie, 2013; Xie, 2014; Conti and Liu, 2015; Cook et al., 2015; Doyle et al., 2015; Lauer et al., 2015; Lorsch, 2015; Mongeon et al., 2016). Third, as noted above, it is impossible to predict from where the most important discoveries will emerge. The NIH analysis also addressed this aspect of research. Their data reveal that some of the most highly productive and highly cited scientists had only a single grant, and relatively few of the most highly productive were in the top 5% of funded researchers (Lorsch, 2017; Figure 4). This analysis strongly suggests that the best approach is to bet on the highest number of qualified investigators. Finally, the data reveal that highly funded labs are no more likely to produce trainees who go on to grant-funded careers and thus help drive forward biomedical science (Lorsch, 2017)—as Jessica Polka has pointed out (Polka, 2017), the human capital produced by NIH dollars is another important output to consider.
FIGURE 2:
Evidence supporting diminishing returns at the upper end of the funding spectrum. Association of the annual weighted Relative Citation Ratio (RCR) among all papers linked to a scientist’s grants with annual Grant Support Index (GSI). Owing to skewed distributions, both RCR and GSI values are natural log-transformed; the numbers inside the axes represent the raw, nontransformed values. An annual GSI value of 7 corresponds to approximately one R01 grant, while annual GSI values of 14 and 21 correspond to two and three R01 grants. Note the decreasing slope of the regression curve as annual GSI increases. Source: Presentation by NIGMS Director Jon Lorsch to NIGMS Council, May 26, 2017 (Lorsch, 2017).
FIGURE 3:
Evidence supporting diminishing returns at the upper end of the funding spectrum—non–log-transformed data. Source: Presentation by NIGMS Director Jon Lorsch to NIGMS Council, May 26, 2017 (Lorsch, 2017).
FIGURE 4:
The vast majority of the most highly cited investigators did not exceed the GSI funding cap. Top 50 most highly cited investigators plotted vs. their level of NIH support. An annual GSI value of 7 corresponds to approximately one R01 grant, while annual GSI values of 14 and 21 correspond to two and three R01 grants. More than half had the equivalent of two R01s or fewer while only seven of 50 exceeded the GSI cap (red line). Source: Presentation by NIGMS Director Jon Lorsch to NIGMS Council, May 26, 2017 (Lorsch, 2017).
Since this analysis was done, an independent study (Katz and Matter, 2017) added further information about the impact of funding inequity, revealing that “funding inequality has been rising since 1985, with a small segment of investigators and institutes getting an increasing proportion of funds, and that investigators who start in the top funding ranks tend to stay there (which results in stasis, or lack of mobility).” In simple terms, the system, as it is currently set up, helps ensure that the rich get richer and the rest suffer the consequences.
Given these data, how can we broaden the NIH’s investment portfolio, helping ensure that we do not lose talented investigators from the system whose work might power the next important breakthroughs? Without substantial increases in the NIH budget, money must be reallocated to fund a larger number of investigators. A number of influential groups and people have looked at these and similar data over the past few years. An impressive report from the University of Wisconsin–Madison Workshop on “Rescuing the US Biomedical Research Enterprise: Strategies and Pathways Ahead” noted, “At the same time, NIH awards have shifted towards senior investigators at the expense of junior investigators, and towards risk-averse projects, often with a translational focus. These shifts endanger the next generation of scientists, and they also endanger research in basic science, which has historically been the engine for groundbreaking discoveries. Our recommendations are designed to reverse these trends by redistributing funds to support both junior investigators and pioneering projects. That redistribution will be painful, especially for established senior investigators, but necessary to support the next generation and cutting edge research” (Kimble et al., 2015). Likewise, the FASEB Report “Sustaining Discovery in Biological and Medical Sciences: A Framework for Discussion” made a similar recommendation. “Limiting the amount of funding awarded to any individual scientist or laboratory would enable more people to be actively engaged in research. With more ‘hands at the bench,’ the number of ideas would increase, and this could expedite progress in many areas of science. Analyses produced by NIH as part of the call for suggestions on ‘Ways of Managing NIH Resources’ show that limiting a principal investigator’s total RPG support to $1 million would enable the funding of 2000 additional RPG awards at an average cost of $400,000″ (FASEB, 2015; numbers refer to direct costs). Finally, NIH asked community members to weigh in on this issue via an NIH Request for Information on “Optimizing Funding Policies and Other Strategies to Improve the Impact and Sustainability of Biomedical Research.” Among the most common responses were suggestions to cap the number of grants or the amount of funding available to a given PI (Lorsch, 2017).
In response to these data, NIH proposed a bold solution—the Grant Support Index (GSI; Collins, 2017). The GSI proposed to cap the number of concurrent grants NIH would provide to a single investigator—roughly three Research Project Grants per lab head. NIH calculated that this cap would affect only 3% of all investigators, and the funds freed up could fund 900 new grants for PIs who did not have other grant funding (Lorsch, 2017). They also listened to community feedback and tweaked the formula to exempt training grants and to encourage collaboration (Lauer, 2017; Lorsch, 2017). This change was bold, addressing a key issue head on. Not too surprisingly, there was pushback, the most strident and well-publicized of which seemed to be from a small number of very well-funded scientists who seem unwilling to relinquish their hold on a disproportionate amount of NIH funds. Some of their rhetoric was heated—one was quoted in the Boston Globe (Weisman, 2017) as saying, “If you have a sports team, you want Tom Brady on the field every time. You don’t want the second string or the third string.” For football fans (I’m not one, but so I was told), this comment was especially ironic as Tom Brady was the 199th overall pick in the sixth round of the NFL draft and warmed the bench his first year (Wikimedia, 2017). Undeterred, in May 2017 NIH seemed to be moving ahead with this program.
I was thus very disappointed to learn early in June 2017 that NIH abandoned the GSI before it even started (NIH Advisory Committee to the Director, 2017a). The tight timeline between its proposal and reversal meant that many scientists like me, who recognize the underlying problem, did not rapidly and vocally support the underlying idea, while at the same time pointing out needed tweaks. From the outside, it’s hard for me not to suspect that the feedback from a subset of very well-funded and powerful scientists threatened by this new approach tipped the balance. Importantly, the Advisory Committee (NIH Advisory Committee to the Director, 2017b) that made the decision to reverse the GSI did not represent the diversity of career stages affected by this critical decision. The reversal of the GSI policy sent a demoralizing message to many of us. I think if you ask your junior colleagues, whose voices were largely not taken into account in this discussion, you’ll find that the vast majority of them support some sort of funding limitations. My recent conversations with colleagues suggest a significant number of senior scientists also share these concerns. The almost 1500 people who have already signed a petition to NIH Director Francis Collins to reinstate a funding cap (Peifer, 2017) provide an indication of the breadth of this opinion.
As NIH considers solutions, it also has to consider a second major issue, which, in my mind, underlies the reluctance of some of my senior colleagues to embrace the GSI: the seemingly unsustainable growth of soft-money or largely soft-money faculty positions at medical schools and research institutes. This played an important role in the reaction of many to the GSI proposal, some of whom struggle to obtain enough grant support to cover more than 70% of their salaries. While I, in a College of Arts and Sciences position, have 75% salary support, many in medical schools are under severe pressure in this regard (Berg, 2015). Jon Lorsch of NIGMS has focused attention on this issue in discussions with the Association of American Medical Colleges. It also needs to be considered in any effort to diversify the research portfolio, as any solution to the soft-money problem will take time to implement.
My own goal is to see NIH develop a more diverse portfolio of NIH-funded research, opening up the field to a larger number of talented folks of all ages, while ensuring that taxpayer dollars are spent wisely. I think some mechanism that ensures that dollars are redistributed from the exceptionally well-funded few to support investigators who have no funding is essential. I continue to support the GSI, which provided a route forward. However, we could also consider other mechanisms to reach this goal. A dollar cap on funding similar to the NIGMS 750K policy (e.g., some have suggested $750K–1 million/investigator; Rosbash, 2016; Wahls, 2017) would also be a reasonable approach. Alternately, as Tom Pollard suggested in a recent post on the American Society for Cell Biology (ASCB) blog, we could consider some mechanism to limit lab size (Pollard, 2017)—this would also be a way forward. All solutions need to recognize that different sorts of science are more or less expensive, but a concrete mechanism of diversifying our research dollar investment to include more labs is, in my mind, essential.
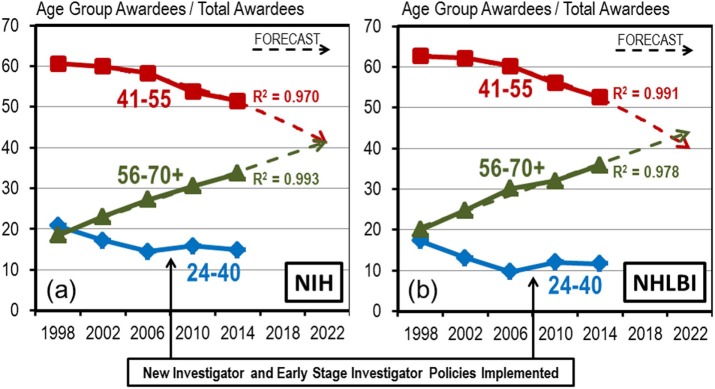
NIH, in abandoning the GSI, suggested a new initiative called the Next Generation Researcher Initiative (NGRI; NIH Office of Extramural Research, 2017). While initiatives like this to support early-career scientists are welcome, they cannot have the same impact if they do not put a cap at the top. Without this, we may be simply rearranging the deck chairs on the Titanic. Two critical differences make the NGRI much less effective than the GSI. First, no source was designated for the funds needed. About 70% of all NIH-funded researchers have a single grant (Rockey, 2011; Lauer et al., 2017). Since the NGRI lacks a funding cap at the top and the NIH budget is a zero-sum game, the NGRI may simply fund no-grant labs using dollars that would otherwise support a renewal to a lab with only a single grant. Thus the NGRI may not result in funding for a larger fraction of productive labs. Furthermore, the NGRI is limited to those with 10 years or fewer of NIH grant funding. NIH analysis suggests it is midcareer faculty who are struggling the most, as their fraction of the Research grant pie shrinks (Charette et al., 2016; Figure 5). New investigators currently benefit from some built-in advantages, at least at NIGMS, that help many of them get their first grant. It’s when they come in for a first, second, or third renewal that my colleagues are hitting the wall. The proposed NGRI excludes many of these midcareer scientists who are faced with shutting their labs.
FIGURE 5:
The fraction of NIH awardees who are midcareer investigators is declining. Source: Figure 4, Charette et al., 2016.
I thus would urge all in the research community to consider these issues, asking whether maintaining the status quo is acceptable, and if not, what solutions seem best. I hope these data stimulate a discussion among scientists at all career stages, including our trainees. Discussions are already underway at the ASCB and the Genetics Society of America, and the recent pieces published on the ASCB Post (ASCB Post Staff, 2017) end by soliciting views and feedback from all members. A committee commissioned by the National Academy of Sciences also asked for input on these issues (National Academy of Sciences, 2017). I personally hope that these discussions will lead the ASCB and other organizations like it to propose or support concrete proposals that will allow talented and productive junior investigators to start their labs and productive midcareer investigators to continue their work. This will require money, and since the NIH budget is likely not going to substantially increase, like the GSI these proposals also must provide clear guidance about which programs or investigators will receive fewer dollars in order for this effort to be successful. There are also routes for individuals to take action. NIH is open to community feedback—contact NIH Director Francis Collins and make your views known (collinsf@mail.nih.gov, copying Deputy Director for Extramural Research Michael.Lauer@nih.gov), and consider signing our petition (Peifer, 2017). Ask how NIH will address this issue and request concrete plans that include what programs will be reduced in order to broaden the NIH portfolio. Advocacy is also valuable, on this issue and others. Scientists of all ages can speak with their Congressional representatives about the value of basic and biomedical science and whether NIH should further diversify its investments.
Acknowledgments
I am very grateful to Jon Lorsch, Mike Lauer, and their many colleagues at NIGMS and the Office of Extramural Research whose data and analysis form much of the foundation of this piece. I am also very grateful to Prachee Avasthi, Mete Civelek, Melissa Wilson Sayres, Jessica Polka, and Gary McDowell for discussions that prompted this piece. I thank Bob Duronio, Kathy Green, Carol Ann McCormick, Doug Kellogg, and David Drubin for thoughtful and helpful comments on the manuscript. Work in the Peifer lab is supported by NIH R35 GM118096.
Abbreviations used:
- ASCB
American Society for Cell Biology
- GSI
Grant Support Index
- NGRI
Next Generation Researcher Initiative
- NIGMS
National Institute of General Medical Sciences
- NIH
National Institutes of Health
- RCR
Relative Citation Ratio
Footnotes
REFERENCES
- Alberts B, Kirschner MW, Tilghman S, Varmus H. Opinion: addressing systemic problems in the biomedical research enterprise. Proc Natl Acad Sci USA. 2015;112:1912–1913. doi: 10.1073/pnas.1500969112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASCB Post Staff. Opinions on the GSI. ASCB Post. 2017 Available at www.ascb.org/ascb-post/science-policy/opinions-on-the-gsi/ (accessed 24 August 2017) [Google Scholar]
- Berg JM. Percentages of faculty salary support at academic medical centers. Data Hound Blog. 2015 Available at http://datahound.scientopia.org/2015/06/17/percentages-of-faculty-salary-support-at-academic-medical-centers/ (accessed 24 August 2017) [Google Scholar]
- Charette MF, Oh YS, Maric-Bilkan C, Scott LL, Wu CC, Eblen M, Pearson K, Tolunay HE, Galis ZS. Shifting demographics among research project grant awardees at the National Heart, Lung, and Blood Institute (NHLBI) PLoS One. 2016;11:e0168511. doi: 10.1371/journal.pone.0168511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS. New NIH approach to grant funding aimed at optimizing stewardship of taxpayer dollars. 2017. Available at www.nih.gov/about-nih/who-we-are/nih-director/statements/new-nih-approach-grant-funding-aimed-optimizing-stewardship-taxpayer-dollars (accessed 24 August 2017)
- Conte ML, Liu J, Schnell S, Omary MB. Globalization and changing trends of biomedical research output. JCI Insight. 2017;2:e95206. doi: 10.1172/jci.insight.95206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti A, Liu CC. Bringing the lab back in: personnel composition and scientific output at the MIT Department of Biology. Res Policy. 2015;44:1633–1644. [Google Scholar]
- Cook I, Grange S, Eyre-Walker A. Research groups: how big should they be. Peer J. 2015;3:e989. doi: 10.7717/peerj.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JM, Quinn K, Bodenstein YA, Wu CO, Danthi N, Lauer MS. Association of percentile ranking with citation impact and productivity in a large cohort of de novo NIMH-funded R01 grants. Mol Psychiatr. 2015;20:1030–1036. doi: 10.1038/mp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FASEB. Report: sustaining discovery in bviological and medical sciences: a discussion framework. 2015. Available at http://faseb.org/Science-Policy-and-Advocacy/Science-Policy-and-Research-Issues/Sustaining-Discovery.aspx (accessed 24 August 2017)
- FASEB. NIH Research Funding Trends. 2017. Available at http://faseb.org/Science-Policy-and-Advocacy/Federal-Funding-Data/NIH-Research-Funding-Trends.aspx (accessed 24 August 2017)
- Fortin JM, Currie DJ. Big science vs. little science: how scientific impact scales with funding. PLoS One. 2013;8:e65263. doi: 10.1371/journal.pone.0065263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz Y, Matter U. On the biomedical elite: inequality and stasis in scientific knowledge production. 2017. Available at https://dash.harvard.edu/bitstream/handle/1/33373356/BKC_Report_KatzMatter2017.pdf (accessed 24 August 2017)
- Kimble J, Bement WM, Chang Q, Cox BL, Drinkwater NR, Gourse RL, Hoskins AA, Huttenlocher A, Kreeger PK, Lambert PF, et al. Strategies from UW-Madison for rescuing biomedical research in the US. eLife. 2015;4:e09305. doi: 10.7554/eLife.09305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen C. America is losing biomedical research leadership to Asia. NIH Fogarty International Center News. 2014 Available at www.fic.nih.gov/News/GlobalHealthMatters/january-february-2014/Pages/spending-investment-biomedical-research-development.aspx (accessed 24 August 2017) [Google Scholar]
- Lauer MS. Following up on your feedback on how to strengthen the biomedical research workforce. Extramural Nexus Open Mike Blog. 2017 Available at https://nexus.od.nih.gov/all/2017/06/05/following-up-on-feedback-on-strengthening-biomedical-research-workforce/ (accessed 24 August 2017) [Google Scholar]
- Lauer MS, Danthi NS, Kaltman J, Wu C. Predicting productivity returns on investment: thirty years of peer review, grant funding, and publication of highly cited papers at the National Heart, Lung, and Blood Institute. Circ Res. 2015;117:239–243. doi: 10.1161/CIRCRESAHA.115.306830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer MS, Roychowdhury D, Patel K, Walsh R, Pearson K. Marginal returns and levels of research grant support among scientists supported by the National Institutes of Health. 2017. Available at www.biorxiv.org/content/early/2017/05/29/142554 (accessed 24 August 2017)
- Lorsch JR. Maximizing the return on taxpayers’ investments in fundamental biomedical research. Mol Biol Cell. 2015;26:1578–1582. doi: 10.1091/mbc.E14-06-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsch JR. National Advisory General Medical Sciences Council Presentation, May 26, 2017. 2017. Available at https://drive.google.com/file/d/0B-TGzynElXw4NlQ2QWZJejRYZmM/view?usp=sharing (accessed 24 August 2017)
- Miklos A, Lorsch JR. Stable success rates and other funding trends in fiscal year 2016. NIGMS Feedback Loop Blog. 2017. Available at https://loop.nigms.nih.gov/2017/03/stable-success-rates-and-other-funding-trends-in-fiscal-year-2016/ (accessed 24 August 2017)
- Mongeon P, Brodeur C, Beaudry C, Lariviere V. Concentration of research funding leads to decreasing marginal returns. Res Eval. 2016;25:396–404. [Google Scholar]
- NAS. National Academies of Sciences, Engineering and Medicine Committee on the Next Generation Researchers Initiative [note this is NOT the same as the NIH NGRI although both have the same name] Dear Colleague Letter. 2017. Available at http://sites.nationalacademies.org/pga/bhew/nextgeneration/pga_180813 (accessed 24 August 2017)
- NIH Advisory Committee to the Director. Videocast of June meeting. 2017a. Available at https://videocast.nih.gov/summary.asp?Live=23678&bhcp=1. Of particular note is the critique by an audience member (4:11:54-4:23:38) (accessed 24 August 2017)
- NIH Advisory Committee to the Director. ACD Membership Roster. 2017b. Available at https://acd.od.nih.gov/members.html (accessed 24 August 2017)
- NIH Office of Extramural Research. Next Generation Researchers Initiative. 2017. Available at https://grants.nih.gov/ngri.htm (accessed 24 August 2017)
- Peifer M. Cap NIH funding for individual investigators to save the future of biomedical science. Change.org Petition. 2017. Available at www.change.org/p/dr-collins-cap-nih-funding-for-individual-investigators-to-save-the-future-of-biomedical-science (accessed 24 August 2017)
- Polka JK. GSI: Opinion by Jessica Polka. ASCB Post. 2017 Available at www.ascb.org/ascb-post/science-policy/opinion-jessica-polka/ (accessed 24 August 2017) [Google Scholar]
- Pollard TD. GSI: Opinion by Tom Pollard. ASCB Post. 2017 Available at www.ascb.org/ascb-post/science-policy/opinion-tom-pollard/ (accessed 24 August 2017) [Google Scholar]
- Rockey S. Update on myth busting: number of grants per investigator. Extramural Nexus Rock Talk Blog. 2011 Available at https://nexus.od.nih.gov/all/2011/05/13/update-on-myth-busting-number-of-grants-per-investigator/ (accessed 24 August 2017) [Google Scholar]
- Rosbash M. Point of view: five suggestions for substantial NIH reforms. eLife. 2016;5:e22471. doi: 10.7554/eLife.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahls WP. NIH’s ineffective funding policies. Science. 2017;356:1132–1133. doi: 10.1126/science.aan6504. [DOI] [PubMed] [Google Scholar]
- Weisman R. Scientists worry about plan to cap individual labs’ federal funding. Boston Globe Article about the GSI. 2017. Available at www.bostonglobe.com/business/2017/06/07/local-scientists-worry-about-nih-proposed-cap-funding-for-individual-labs/8qv6ydg4xBT32Y4a7LkVfN/story.html (accessed 24 August 2017)
- Wikimedia. Tom Brady. Wikipedia. 2017. Available at https://en.wikipedia.org/wiki/Tom_Brady (accessed 24 August 2017)
- Xie Y. ‘‘Undemocracy’’: inequalities in science. Science. 2014;344:809–810. doi: 10.1126/science.1252743. [DOI] [PMC free article] [PubMed] [Google Scholar]