Abstract
Rationale:
Currently available approaches to osteoporosis treatment include application of antiresorptive and anabolic agents influencing bone tissue metabolism. The aim of the study was to present bone mineral density (BMD) changes of lumbar spine in osteoporotic patient treated with bisphosphonates such as ibandronic acid and pamidronic acid, and beta-hydroxy-beta-methylbutyrate (HMB).
Patient concerns:
BMD and volumetric BMD (vBMD) of lumbar spine were measured during the 6 year observation period with the use of dual-energy X-ray absorptiometry (DEXA) and quantitative computed tomography (QCT).
Diagnoses:
The described case report of osteoporotic patient with family history of severe osteoporosis has shown site-dependent response of bone tissue to antiosteoporotic treatment with bisphosphonates.
Interventions and outcomes:
Twenty-five-month treatment with ibandronic acid improved proximal femur BMD with relatively poor effects on lumbar spine BMD. Over 15-month therapy with pamidronic acid was effective to improve lumbar spine BMD, while in the proximal femur the treatment was not effective. A total of 61-week long oral administration with calcium salt of HMB improved vBMD of lumbar spine in the trabecular and cortical bone compartments when monitored by QCT. Positive effects of nearly 2.5 year HMB treatment on BMD of lumbar spine and femur in the patient were also confirmed using DEXA method.
Lessons:
The results obtained indicate that HMB may be applied for the effective treatment of osteoporosis in humans. Further studies on wider human population are recommended to evaluate mechanisms influencing bone tissue metabolism by HMB.
Keywords: beta-hydroxy-beta-methylbutyrate, bisphosphonates, bone mineral density, dual-energy X-ray absorptiometry, osteoporosis, quantitative computed tomography
1. Introduction
Osteoporosis is the most common metabolic bone disease in humans. The first definition of osteoporosis formulated by Albright in 1941 has stated that osteoporosis is characterized by too little bone tissue in bone.[1] In 1994 year, World Health Organization (WHO) enhanced this definition stating that osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with consequent increase in bone fragility and susceptibility to fracture.[2] The definition provided by WHO associated decreased bone mass (determined by measurement of bone mineral density [BMD]) with microarchitectural deterioration of bone tissue and susceptibility to bone fractures. National Institute of Health and International Osteoporosis Foundation updated previous definitions in 2000 stating that osteoporosis is skeletal system disease characterized by decreased mechanical endurance of bones that increases fracture risk, connecting various risk factors with decreased mechanical endurance of bones and osteoporotic fractures incidence.[3,4]
Osteoporosis is diagnosed clinically when there is a presence of fragility fracture or BMD measured by bone densitometry that is less than or equal to 2.5 standard deviations below that of a young adult ethnic- and sex-matched reference population. The standard deviation value is described as T-score. T-score value between –1.0 and –2.5 indicates osteopenia, while T-score between +2.5 and –1.0 is considered to reflect normal bone mass status.[5] Z-score determined using bone densitometry shows the number of standard deviations of the measured BMD differing from the physiological range. The normative database is matched for age, gender, ethnicity, and body weight.[6,7] The determined Z-score reflects the difference from a demographically similar healthy population within physiological norm. Z-score value is usually less negative than T-score, especially with advancing age. Low Z-score value associated with low BMD indicates additional factors other than natural menopause and aging which have adversely affected skeletal system health.[6–8]
Currently available approaches to osteoporosis treatment include application of antiresorptive and anabolic agents influencing bone tissue metabolism. Antiresorptive drugs may be effective in restoring skeletal balance by reducing bone turnover at the tissue level and result in diminished osteoporotic fracture incidence.[9,10] Bisphosphonates are widely used antiresorptive drugs for osteoporosis treatment. Bisphosphonates restrain bone resorption via inhibition of osteoclast recruitment and differentiation and enhanced osteoclasts apoptosis which finally leads to reduction of fracture risk.[11] Beta-hydroxy-beta-methylbutyrate (HMB) administration was shown to induce anabolic effects on bone tissue metabolism in experimental animals improving BMD, geometrical properties, and mechanical strength of bones in axial and peripheral skeleton.[12–17] However, studies on effects of HMB on skeletal system quality in humans are strongly limited. Thus, the aim of the study was to present BMD changes of lumbar spine and femur in osteoporotic patient treated with bisphosphonates and HMB.
2. Materials and methods
All procedures performed in this study were in accordance with the institutional ethical standards required obligatory for Medical University in Lublin, Poland.
2.1. Description of patient history, densitometric measurements, and antiosteoporotic treatment
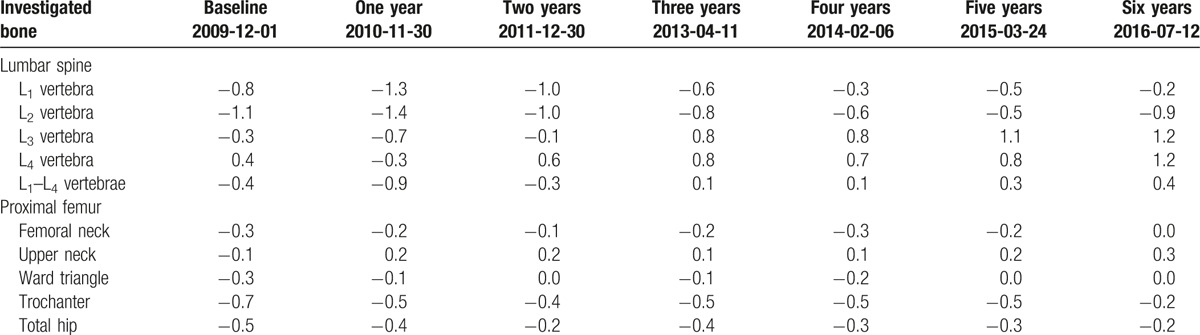
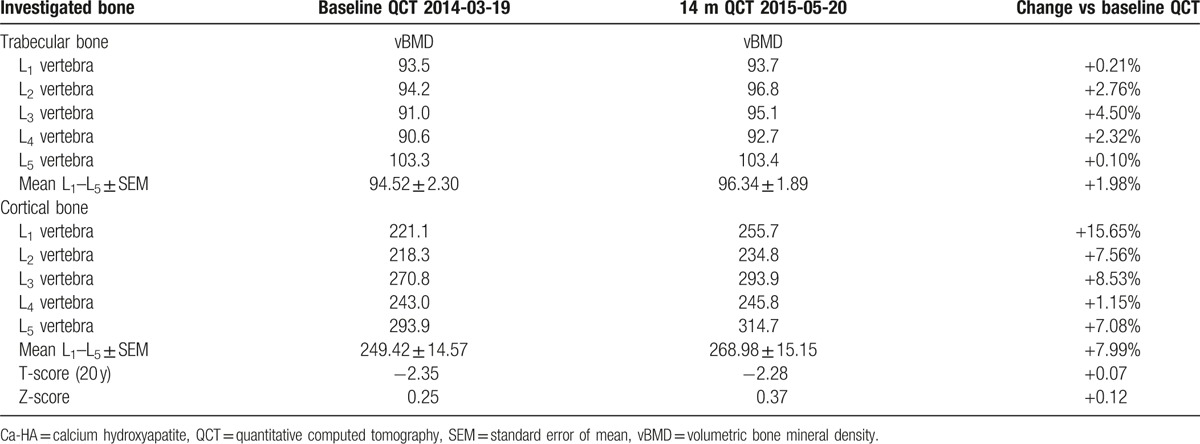
In December 2009 (baseline), 63-year woman with family history of severe osteoporosis was subjected to diagnostic densitometry of lumbar spine and proximal femur with the use of dual-energy X-ray absorptiometry (DEXA) method and Lunar Prodigy Advance apparatus (GE Healthcare Lunar, Europe). The patient was subjected to hormone replacement therapy for over 5 previous years. The patient has reported family history of severe osteoporosis in her father, 14 years older sister, and 9 years older brother before examination. The lowest values of BMD T-score were measured in the 2nd lumbar vertebra (L2) and Ward triangle (–2.8 and –2.3, respectively; Table 3). As the result of baseline densitometric examination, the patient was recommended to start antiresorptive treatment with bisphosphonates. Acidum ibandronicum (Bonviva, Roche Pharma AG, Germany) was taken orally once monthly in the dosage of 150 mg for over 2 years (25 months). After the 3rd DEXA examination in December 2011, the patient started therapy with acidum pamidronicum (Pamifos 90 mg per month intravenously, Vipharm SA, Poland). The patient was not diagnosed with any neoplastic disease concerning skeletal system or other tissues. The therapy with Pamifos lasted for 20 months until September 2013 when it was changed for calcium salt of beta-hydroxy-beta-methylbutyrate (CaHMB). To monitor metabolic response of the skeleton to the antiresorptive treatment, the patient was also subjected to DEXA examination in April 2013. The therapy with CaHMB (HMB Mega Caps 1250, Olimp Sport Nutrition, Poland) was performed at the dosage of 1250 mg per day orally. One CaHMB capsule consists of 1000 mg of pure HMB. The HMB capsule was taken during the diner each day and the treatment was continued until July 2016. During the HMB treatment course, 3 subsequent DEXA examinations (February 2014, March 2015, and July 2016) of the patient were performed. All the densitometric measurements with the DEXA method were performed in the same diagnostic laboratory using the same apparatus (Lunar Prodigy Advance, GE Healthcare Lunar, Europe). To monitor metabolic response of axial skeleton to the treatment with HMB, the patient was subjected to densitometric examination of lumbar spine in March 2014 with the use of quantitative computed tomography (QCT) method. SOMATOM EMOTION SIEMENS apparatus (Siemens, Erlangen, Germany) equipped with Somaris/5 VB10B software (version B10/2004A) and Osteo CT application package was used to determine the volumetric bone mineral density (vBMD) of the trabecular and cortical bone compartments in each lumbar vertebrae (L1–L5). Calcium hydroxyapatite (Ca-HA) density of trabecular bone was measured on cross-section of the vertebral body in the central part, while Ca-HA density of cortical bone was determined on the margins of the vertebral body, analogically for each vertebra. The results of the densitometric measurements were expressed in mg Ca-HA/mL. The lumbar spine was scanned together with the water- and bone-equivalent calibration phantom and the measuring scans were 10 mm thick and placed at 50% of the vertebral body length (Fig. 1). Moreover, T-score (20 years) and Z-score values were automatically determined. The following QCT examination of the patient was performed after 14 months (61 weeks) in May 2015. vBMD measurements of the lumbar spine were performed by the same radiologist and using the same equipment and software. Other medications and antiosteoporotic drugs were not used by this patient during the observation period between September 2014 and May 2015, and later. Independently from QCT measurements, densitometric measurement using DEXA method was performed in July 2016. Body weight and body mass index changes of the patient during the observation period are shown in Table 1.
Table 3.
T-score values in lumbar vertebrae and proximal femur measured with the use of dual-energy X-ray absorptiometry (DEXA) method in the patient at the baseline and subsequent visits.
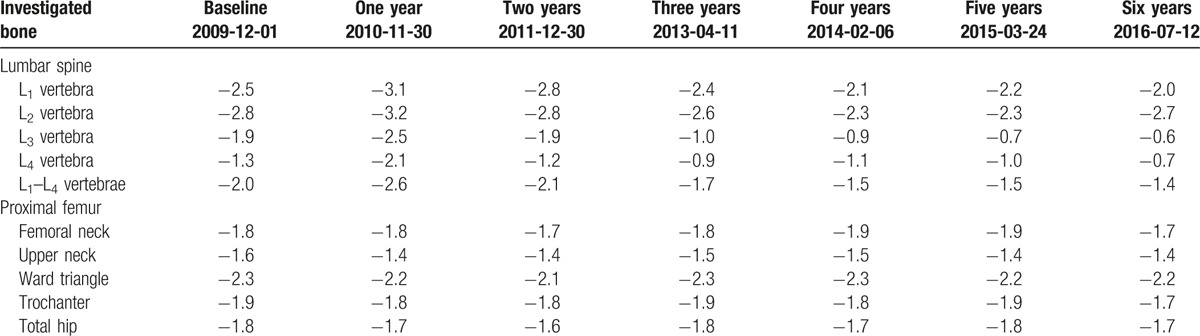
Figure 1.
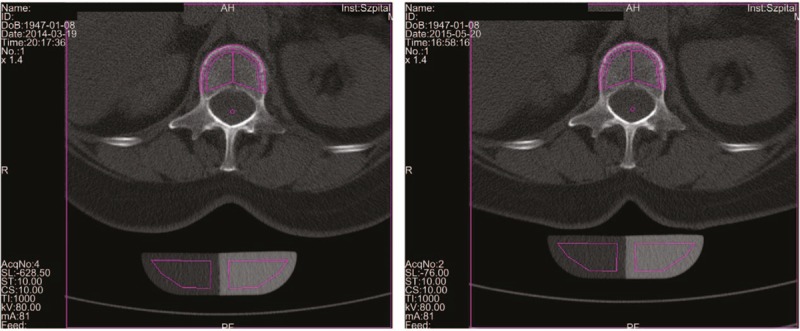
The measurements of vBMD of lumbar vertebrae (L1) in March 2014 (left panel) and after 14-month oral administration with calcium salt of beta-hydroxy-beta-methylbutyrate (right panel) in the patient. TbCa-HA was measured on cross-section of the vertebral body in its central part, while CbCa-HA was determined on the margins of the vertebral body of the cross-section. The results of the vBMD measurements were expressed in mg of Ca-HA/mL. Lumbar spine was scanned together with the water- and bone-equivalent calibration phantom. The measuring scans were 10 mm thick and placed at 50% of the vertebral body length. Ca-HA = calcium hydroxyapatite, CbCa-HA = calcium hydroxyapatite density of cortical bone, TbCa-HA = calcium hydroxyapatite density of trabecular bone, vBMD = volumetric bone mineral density.
Table 1.
Body weight and body mass index in the patient at the baseline and subsequent visits in densitometric laboratory.
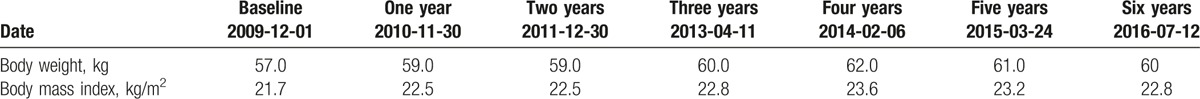
3. Results
3.1. Ibandronic acid treatment
Results of densitometric measurements of lumbar spine and proximal femur in patient at the baseline and after 1- and 2-year oral therapy with ibandronic acid are shown in Table 2. T-score and Z-score values corresponding to the BMD measurements are presented in Tables 3 and 4. Ibandronic acid treatment has not improved BMD values in L1–L4 that was slightly decreased at 2 time point measurements when compared to the baseline value. Similar results were observed for all single vertebrae, except for BMD measured after 2 years from baseline for L3 and L4 where 0.001 and 0.007 g/cm2 increases were observed. Except for the decline of BMD by 10% in upper femoral neck after 2 years from the baseline, all the other measurements in proximal femur 1 and 2 years from the baseline were increased as the consequence of ibandronic acid treatment. Total hip BMD increased by 0.005 and 0.020 g/cm2 after 1- and 2-year therapy with ibandronic acid, respectively.
Table 2.
BMD of lumbar vertebrae and proximal femur measured with the use of DEXA method in the patient at the baseline and subsequent visits.
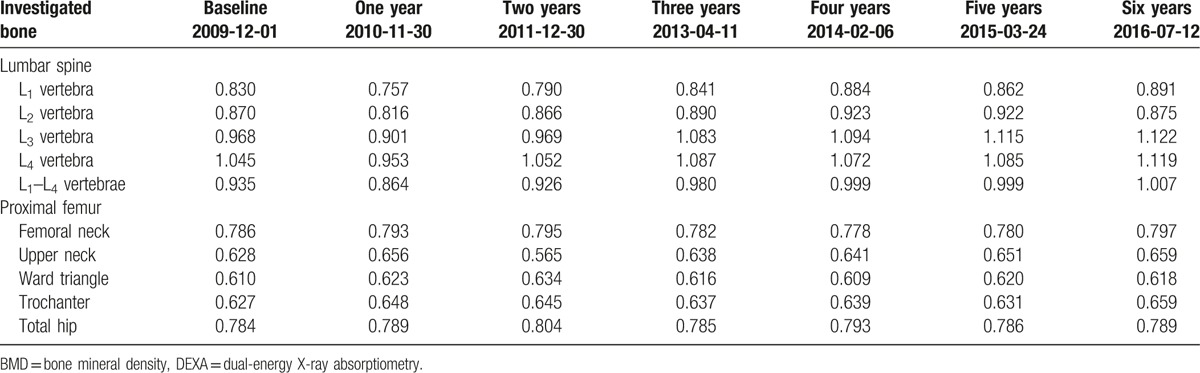
Table 4.
Z-score values in lumbar vertebrae and proximal femur measured with the use of dual-energy X-ray absorptiometry (DEXA) method in the patient at the baseline and subsequent visits.
3.2. Pamidronic acid treatment
Results of BMD measurements during pamidronic acid therapy are shown in Tables 2–4. In lumbar spine, BMD values have increased in all measures comparing time interval between December 2011 and April 2013, reaching 0.054 g/cm2 for L1–L4. As the consequence of nearly 16 months of the pamidronic acid treatment, proximal femur BMD measurements have shown the increase of 0.073 g/cm2 only in the upper femoral neck, while BMD values were declined in all the other regions of interest.
3.3. Beta-hydroxy-beta-methylbutyrate treatment
Results of BMD measurements in patient receiving HMB therapy are shown in Tables 2–4. BMD measurement in July 2016 with the use of DEXA method has shown positive change of BMD value in L1–L4 by 0.008 g/cm2 when compared to the values obtained in February 2014, even its slight decrease in L2. The measurements of BMD in proximal femur in this time interval have shown increased values in femoral neck (by 0.019 g/cm2), upper neck (by 0.018 g/cm2), Ward triangle (by 0.009 g/cm2), and trochanter (by 0.020 g/cm2) as the consequence of the treatment with HMB. Slight decrease by 0.004 g/cm2 of BMD values between February 2014 and July 2016 was obtained for total hip in the patient receiving HMB. Except for L2, T-score and Z-score values increased in all the evaluated lumbar vertebrae and regions of proximal femur (Tables 3 and 4).
Results of vBMD measurements with the use of QCT method for trabecular and cortical bone compartments in lumbar spine of the patient are shown in Table 5. As the consequence of 14 month therapy with HMB, TbCa-HA of lumbar vertebrae was increased within the range of 0.10% to 4.50%, reaching an average increase of 1.98% for L1–L5. vBMD values of the cortical bone compartment have increased for all lumbar vertebrae within the range of 1.15% to 15.65%, reaching an average increase of 7.99% for L1–L5. The increased values of T-score (20 years) and Z-score by 0.07 and 0.12 were also stated during the 14-month period of HMB therapy.
Table 5.
vBMD of trabelular and cortical bone compartments in lumbar spine (mg Ca-HA/mL) measured with the use of QCT in the patient in March 2014 and after following 14-mo therapy with beta-hydroxy-beta-methylbutyrate.
4. Discussion
Bisphosphonates are pyrophosphate analogs with high affinity for bone hydroxyapatite. Bisphosphonates bind directly to mineralized bone inducing blockage of the bone surface and preventing osteoclast-dependent bone resorption.[18–20] Bisphosphonates may also inhibit osteoclastic activity and reduce the lifespan of the osteoclasts.[21] Ibandronic acid and pamidronic acid belong to the 2nd-generation nitrogenous bisphosphonates group (aminobisphosphonates containing nitrogen in an alkyl chain) which is considered as more effective for the treatment of osteoporosis than 1st-generation nonnitrogenous bisphosphonates.[20] The performed densitometric measurements in this study enabled monitoring of the effectiveness of ibandronic acid and pamidronic acid administration in osteoporotic patient with family history of severe osteoporosis. Ibandronic acid was administered for 25 months and its effect on bone mineral density was differentiated depending on the examined skeletal site. In lumbar vertebrae (L1–L4), 7.6% and 1% decrease of BMD was observed after 1- and 2-year treatment since the basic densitometry. However, 1-year treatment with ibandronic acid increased BMD in all the investigated areas of proximal femur within the range of 0.6% to 4.5%. Similar increases of BMD were obtained in proximal femur regions after 2-year observation, except for the upper neck region where BMD was decreased by over 10% versus baseline value. The results of measurements of proximal femur BMD in the current study correspond to the results of meta-analysis of 34 studies on ibandronic acid treatment effectiveness in osteoporotic patients. It was shown that oral administration of ibandronate increased total hip BMD by 2.13% with the average duration of the ibandronate treatment 1.9 ± 1.06 years. However, the observed decrease of BMD values in lumbar spine in the current study seems to be opposite to the effects of ibandronate treatment reported in osteoporotic patients in the meta-analysis where 4.57% increase of lumbar spine BMD was reported.[22] In the other 24-month study on women suffering from postmenopausal osteoporosis, BMD has increased significantly relative to baseline in the group on continuous oral ibandronate therapy. The continuous ibandrnate therapy increased lumbar spine and total hip BMD by 5.64% and 3.35%, respectively.[23]
Pamidronic acid treatment is recommended for patients with cancer that cause osteolysis. It is recommended for the prevention of skeletal-related events in patients with advanced solid tumors such as breast and prostate cancers.[24,25] In this study, pamidronic acid treatment was recommended for the patient without any neoplastic disease concerning skeletal system and other tissues. Pamidronic acid administration in this study lasted for 20 months; however, DEXA examination was performed after nearly 16 months of the treatment. Similarly to ibandronic acid treatment, antiosteoporotic effects of pamidronic acid treatment were differentiated in lumbar spine and proximal femur. In all the examined lumbar vertebrae, BMD determined by DEXA method was improved within the range of 2.8% to 11.2% when compared to the previous measurement performed in December 2011. BMD measured for L1–L4 was increased by 5.8% as the consequence of the pamidronic acid treatment. However, except for the upper neck where 12.9% increase in BMD was observed, BMD values were decreased in the other examined regions of interest of proximal femur within the range of 1.3% to 2.9%. As shown in the previous study by Vis et al (2005),[26] intravenous administration of pamidronic acid at the dosage of 60 mg every 3 months was effective to improve BMD in lumbar spine and proximal femur. BMD values of the lumbar spine and hip increased significantly by 4.0% and 2.9% after 1-year pamidronate treatment. The effectiveness of pamidronate treatment was comparable to oral alendronate administration in patients suffering from osteoporosis. Moreover, intravenous infusion with pamidronate was suggested to be a therapeutic alternative for patients with gastrointestinal intolerance of oral bisphosphonates.[26] In the other 3-year study on patients suffering from postmenopausal osteoporosis, lumbar spine BMD was shown to be improved as the consequence of once monthly intravenous infusion of 60 mg of pamidronate. BMD was measured 3 times using DEXA in patients since baseline in 1-year intervals and the therapeutic effectiveness of pamidronate and alendronate was comparable.[27] Oral treatment with pamidronate (150 mg/day) in postmenopausal women increased lumbar spine BMD by 9.4%.[28] In the study on postmenopausal women and men with at least 1 vertebral fracture, an increase of 14.3% of BMD of the spine was observed as the consequence of 5-year oral treatment with pamidronate.[29] As opposite to the current study, the negative therapeutic effects of the treatment with both ibandronate and pamidronate on skeletal BMD were not reported in previous studies.
HMB is a metabolite of the essential amino acid leucine, and it is produced from alpha-ketoisocaproate by enzyme alpha-ketoisocaproate-dioxygenase. Experimental studies have suggested HMB to be the bioactive metabolite of leucine responsible for inhibiting proteolysis and for modulating protein turnover in vitro and in vivo.[30,31] Dietary administration with HMB was shown to induce numerous beneficial effects including increased lean body mass and muscle strength, stimulation of lipolytic processes and reduction of fat mass, anticatabolic, and anabolic activities including inhibition of protein degradation and stimulation of protein synthesis in skeletal muscles, as well as collagen synthesis and hydroxyproline formation improvement.[32,33] Studies in humans showing results of dietary administration with HMB on skeletal system properties are strongly limited. There is only one 12-week nutritional study performed in 8 men and 12 women (mean age 54 years) administered orally with calcium salt of HMB at the daily dosage of 3 g. It was shown that the treatment of rheumatoid cachexia with HMB (3 g of calcium salt), glutamine (14 g), and arginine (14 g) has improved whole body BMC of the patients by 5.9 g versus baseline measurements, while in the controls receiving isocaloric and isonitrogenous placebo whole-body BMC was decreased by 1.5 g. However, 3-month trial was relatively short to obtain significant metabolic response of bone tissue in whole skeleton in patients at a mean age of 54 years.[34] In this study, for the 1st time positive effects of 61-week oral administration with HMB on lumbar spine vBMD in osteoporotic patient were documented. The dosage used in the current study was one third of the daily dose used in the trial described by Marcora et al (2005); however, its duration was more than 5 times longer. Moreover, the patient in this study received calcium salt of HMB without an additional administration of glutamine and arginine.
The positive effects of HMB administration on vBMD in lumbar vertebrae were differentiated depending on bone tissue compartment. Higher increase of vBMD (L1–L5) reaching nearly 8% was observed in cortical bone in comparison to the trabecular bone compartment where nearly 2% increase was observed. Both these results prove that dietary administration with HMB in humans improves vBMD. It should also be highlighted that no side effects were reported by the patient during the whole HMB treatment course. Moreover, after approximately 12 months of the therapy with HMB the patient reported lack of previously experienced low back pain feeling. As opposed to the study performed by Marcora et al (2005) in which DEXA method was used for BMD and BMC determination, in the current study vBMD was measured using both QCT and DEXA techniques. Positive effects of nearly 2.5 year HMB treatment on BMD of lumbar spine and femur in the patient were also confirmed. Except for L2 and total hip, BMD, T-score, and Z-score values increased in all the evaluated lumbar vertebrae and regions of proximal femur. The DEXA method provides combined results of BMD measurement in trabecular and cortical bone (expressed in g/cm2), while QCT allows separate volumetric analysis of trabecular and cortical bone density (expressed in g/cm3), independent of one another. The advantage resulting from this methodological approach is that the measurements of vBMD were performed independently for both the trabecular and cortical bone compartments of the axial skeleton. In contrast to DEXA method where bone size may affect BMD value, vBMD measurement with the use of QCT method provides results nondependent on bone size. Moreover, vBMD can be easy measured with the use of QCT without potential overestimating errors resulting from surrounding soft tissues volume, as well as possible osteoarthritic and osteophytic changes which can not be eliminated performing DEXA analysis. It is worth to underline that the number of degenerative and osteophytic changes increases in patients with advanced age.[35–37]
The positive effect of HMB administration on vBMD in the described osteoporotic patient is in accordance to the previous studies on animals at the stages of systemic growth and osteopenia induction. Both prenatal and neonatal administrations with HMB in pigs and sheep have increased vBMD of the trabecular and cortical bone compartments and mechanical endurance of bones in peripheral (femur) and axial (lumbar spine) skeleton. These effects were associated with increased concentration of growth hormone, insulin-like growth factor I, and serum bone formation markers.[13,14] In the other study on pigs, HMB administration (0.05 g/kg of body weight/day – per os) throughout 7 months was effective to reduce the development of severe osteopenia induced by fundectomy performed on 40th day of life. HMB increased significantly in the fundectomized pigs mean vBMD (MvBMD), vBMD of trabecular bone, Ca-HA density of trabecular bone, Ca-HA density of cortical bone, BMD, BMC, ultimate force, ultimate stress, Young's modulus, stiffness, and work to the ultimate force point in lumbar vertebrae. The antiosteopenic effect of HMB administration was associated with improved amino acid metabolism and higher plasma concentration of valine, leucine, threonine, methionine, tyrosine, tryptophan, and arginine.[15] Similar antiosteopenic effects were observed in studies on ovariectomized rats with established osteopenia. Daily administration with water solution of CaHMB (1.9 g/L of drinking water administered ad libitum) throughout 2 months was effective to reverse osteopenia of femur and lumbar vertebrae (L2–L4). BMD and mechanical properties of femur and lumbar spine were improved in ovariectomized and HMB-treated rats in comparison to the ovariectomized controls and the values obtained were comparable to those in sham-operated group.[12]
5. Conclusions
In conclusion, the described case report of osteoporotic patient with family history of severe osteoporosis has shown site-dependent response of bone tissue to antiosteoporotic treatment with bisphosphonates. It may be summarized that ibandronic acid treatment improved proximal femur BMD with relatively poor effects on lumbar spine BMD. Pamidronic acid therapy was effective to improve lumbar spine BMD, while in the proximal femur the treatment was not effective to improve BMD. A total of 61-week long oral administration with calcium salt of HMB in the patient improved vBMD of lumbar spine in the trabecular and cortical bone compartments indicating that HMB may be applied for the effective treatment of osteoporosis in humans. Positive effects of nearly 2.5 year HMB treatment on BMD of lumbar spine and femur in the patient was also confirmed using DEXA method. However, further studies on wider human population are recommended to evaluate mechanisms influencing bone tissue metabolism by HMB. It should also be explained whether exist relationships between HMB dosage and the response of skeletal system to the treatment.
Footnotes
Abbreviations: BMI = body mass index, BMD = bone mineral density, Ca-HA = calcium hydroxyapatite, CaHMB = calcium salt of beta-hydroxy-beta-methylbutyrate, CbCa-HA = calcium hydroxyapatite density of cortical bone, DEXA = dual-energy X-ray absorptiometry, HMB = beta-hydroxy-beta-methylbutyrate, IOF = International Osteoporosis Foundation, NIH = National Institute of Health, QCT = quantitative computed tomography, TbCa-HA = calcium hydroxyapatite density of trabecular bone, vBMD = volumetric bone mineral density, WHO = World Health Organization.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Albright F, Smith PH, Richardson AM. Postmenopausal osteoporosis; its clinical features. JAMA 1941;116:2465–74. [Google Scholar]
- [2].Anonymous. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 1993;94:646–50. [DOI] [PubMed] [Google Scholar]
- [3].Anonymous. Osteoporosis prevention, diagnosis and therapy. NIH Consens Statement 2000;17:1–45. [PubMed] [Google Scholar]
- [4].Czerwiński E, Badurski JE, Marcinowska-Suchowierska E, et al. Current understanding of osteoporosis according to the position of the World Health Organization (WHO) and International Osteoporosis Foundation. Ortop Traumatol Rehabil 2007;9:337–56. [PubMed] [Google Scholar]
- [5].Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol 2014;142:155–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA 2002;288:1889–97. [DOI] [PubMed] [Google Scholar]
- [7].McKiernan FE, Berg RL, Linneman JG. The utility of BMD Z-score diagnostic thresholds for secondary causes of osteoporosis. Osteoporos Int 2011;22:1069–77. [DOI] [PubMed] [Google Scholar]
- [8].NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785–95. [DOI] [PubMed] [Google Scholar]
- [9].Recker R, Lappe J, Davies KM, et al. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res 2004;19:1628–33. [DOI] [PubMed] [Google Scholar]
- [10].Seeman E, Delmas PD. Bone quality – the material and structural basis of bone strength and fragility. N Engl J Med 2006;354:2250–61. [DOI] [PubMed] [Google Scholar]
- [11].Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone 1999;25:97–106. [DOI] [PubMed] [Google Scholar]
- [12].Bieńko M, Radzki RP, Kapica M, et al. Influence of beta-hydroxy-beta-methylbutyrate (HMB) on the structural strength of bones. Med Weter 2006;62:963–5. [Google Scholar]
- [13].Tatara MR, Śliwa E, Krupski W. Prenatal programming of skeletal development in the offspring: effects of maternal treatment with beta-hydroxy-beta-methylbutyrate (HMB) on femur properties in pigs at slaughter age. Bone 2007;40:1615–22. [DOI] [PubMed] [Google Scholar]
- [14].Tatara MR. Neonatal programming of skeletal development in sheep is mediated by somatotrophic axis function. Exp Physiol 2008;93:763–72. [DOI] [PubMed] [Google Scholar]
- [15].Tatara MR, Śliwa E, Krupski W, et al. 3-Hydroxy-3-methylbutyrate administration diminishes fundectomy-induced osteopenia of the lumbar spine in pigs. Nutrition 2008;24:753–60. [DOI] [PubMed] [Google Scholar]
- [16].Tatara MR. Effect of β-hydroxy-β-methylbutyrate (HMB) administration on volumetric bone mineral density, and morphometric and mechanical properties of tibia in male turkeys. J Anim Physiol Anim Nutr 2009;93:669–77. [DOI] [PubMed] [Google Scholar]
- [17].Tatara MR, Krupski W, Tymczyna B, et al. Effects of combined maternal administration with alpha-ketoglutarate (AKG) and β-hydroxy-β-methylbutyrate (HMB) on prenatal programming of skeletal properties in the offspring. Nutr Metab 2012;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dunford JE, Thompson K, Coxon FP, et al. Structure-activity relationships for inhibition of farnesyl disphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 2001;296:235–42. [PubMed] [Google Scholar]
- [19].Russell R. Determinants of structure-function relationships among bisphosphonates. Bone 2007;40:S21–5. [Google Scholar]
- [20].Demontiero O, Duque G. Once-yearly zoledronic acid in hip fracture prevention. Clin Interv Aging 2009;4:153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest 1996;97:2692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hou Y, Gu K, Xu C, et al. Dose-effectiveness relationships determining the efficacy of ibandronate for management of osteoporosis: a meta-analysis. Medicine 2015;94:e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Riis BJ, Ise J, von Stein T, et al. Ibandronate: a comparison of oral daily dosing versus intermittent dosing in postmenopausal osteoporosis. J Bone Miner Res 2001;16:1871–8. [DOI] [PubMed] [Google Scholar]
- [24].Clézardin P, Ebetino FH, Fournier PG. Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Res 2005;65:4971–4. [DOI] [PubMed] [Google Scholar]
- [25].Spence MM, Hui RL, Chan J, et al. Risk of skeletal-related events in patients with advanced prostate cancer treated with pamidronate or zoledronic acid. Ann Pharmacother 2010;44:1384–8. [DOI] [PubMed] [Google Scholar]
- [26].Vis M, Bultink IE, Dijkmans BA, et al. The effect of intravenous pamidronate versus oral alendronate on bone mineral density in patients with osteoporosis. Osteoporos Int 2005;16:1432–5. [DOI] [PubMed] [Google Scholar]
- [27].Heijckmann AC, Juttmann JR, Wolffenbuttel BH. Intravenous pamidronate compared with oral alendronate for the treatment of postmenopausal osteoporosis. Neth J Med 2002;60:315–9. [PubMed] [Google Scholar]
- [28].Reid IR, Wattie DJ, Evans MC, et al. Continuous therapy with pamidronate, a potent bisphosphonate, in postmenopausal osteoporosis. J Clin Endocrinol Metab 1994;79:1595–9. [DOI] [PubMed] [Google Scholar]
- [29].Brumsen C, Papapoulos SE, Lips P, et al. Daily oral pamidronate in women and men with osteoporosis: a 3-year randomized placebo-controlled clinical trial with a 2-year open extension. J Bone Miner Res 2002;17:1057–64. [DOI] [PubMed] [Google Scholar]
- [30].Nissen S, Abumrad NN. Nutritional role of the leucine metabolite β-hydroxy-β-methylbutyrate (HMB). J Nutr Biochem 1997;8:300–11. [Google Scholar]
- [31].Tatara MR, Krupski W, Majer-Dziedzic B. Watson RR, Mahadevan D. Novel and current approaches to dietary and non-dietary bone metabolism regulation. Handbook of nutrition and diet in therapy of bone diseases. Human Health Handbooks. Vol. 13. The Netherlands: Wageningen Academic Publishers; 2016. 129–90. [Google Scholar]
- [32].Williams J, Abumrad N, Barbul A. Effect of a specialized amino acid mixture on human collagen deposition. Ann Surg 2002;236:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Szcześniak KA, Ostaszewski P, Fuller JC, Jr, et al. Dietary supplementation of β-hydroxy-β-methylbutyrate in animals – a review. J Anim Physiol Anim Nutr 2015;99:405–17. [DOI] [PubMed] [Google Scholar]
- [34].Marcora S, Lemmey A, Maddison P. Dietary treatment of rheumatoid cachexia with beta-hydroxy-beta-methylbutyrate, glutamine and arginine: a randomised controlled trial. Clin Nutr 2005;24:442–54. [DOI] [PubMed] [Google Scholar]
- [35].Lochmüller EM, Bürklein D, Kuhn V, et al. Mechanical strength of the thoracolumbar spine in the elderly: prediction from in situ dual-energy X-ray absorptiometry, quantitative computed tomography (QCT), upper and lower limb peripheral QCT, and quantitative ultrasound. Bone 2002;31:77–84. [DOI] [PubMed] [Google Scholar]
- [36].Krupski W, Tatara MR, Nogalski A, et al. Degenerative changes of vertebrae and intervertebral discs, and narrowing of the intervertebral foramens of the lumbar spine in physical workers with low back pain. Polish J Environ Stud 2007;16(5C):290–3. [Google Scholar]
- [37].Krupski W, Tatara MR. Degenerative changes of vertebrae, intervertebral discs, and narrowing of the intervertebral foramens of the cervical spine in physical workers with neck pain. J Pre-Clin Clin Res 2011;5:60–2. [Google Scholar]


