Supplemental Digital Content is available in the text
Keywords: after-hospital assessment, enhanced recovery after surgery, fast track, pancreaticoduodenectomy, propensity-score matching
Abstract
Enhanced recovery after surgery (ERAS) programs have been shown to decrease postoperative complications and hospital stay in pancreaticoduodenectomy. However, no studies concerned recovery after discharge except readmission. This study evaluated an ERAS program for pancreaticoduodenectomy from hospital to home.
A prospective ERAS cohort undergoing elective pancreaticoduodenectomy was compared with a retrospective control group in terms of postoperative complications and hospital stay, and home recovery after discharge. Propensity-score matching was used to balance their baselines.
Two groups of 31 patients with similar propensity scores were established. Postoperative morbidities were 18 of 31 and 26 of 31 in the ERAS and control groups, respectively (P = .06). Patients in the ERAS group suffered from fewer cardiovascular complications (3/31 vs 11/31; P = .04) and intestinal dysbacteriosis (4/31 vs 13/31; P = .04). Median postoperative hospital stay was shorter in the ERAS group (8 vs 16 days; P < .001). Although the 2 groups were similar in terms of sleep, defecation, vigor, performance status, and pain control in first month after discharge, patients in the ERAS group enjoyed better food intake recovery (18/31 vs 5/31 in first week, P = .002; 22/31 vs 9/31 in second week, P = .008; 23/31 vs 13/31 in fourth week, P = .01) and fewer weight loss (10/31 vs 19/31; P = .05). Multivariate analyses showed that both improvements were associated with no bowel preparation.
ERAS implementation in selected patients undergoing pancreaticoduodenectomy could promise better outcomes, not only in the hospital but also at home in the short term.
1. Introduction
Enhanced Recovery After Surgery (ERAS) program, also referred to as Fast-Track pathway, is an optimized framework of perioperative care that aims to improve safety and clinical outcomes.[1] It is a multimodal approach that involves close collaboration among surgeons, anesthesiologists, nurses, and other paramedics. Since it was initiated by Kehlet [2] in the 1990s for colonic surgery, ERAS programs have been adopted gradually by many other fields of surgery, including major gastrointestinal and liver surgery.[3,4] By reducing surgical stress, controlling postoperative pain, and promoting early oral diet and mobilization, ERAS programs can shorten the length of postoperative hospital stay (LOPH) and save medical costs without increasing postoperative morbidity, mortality, and readmission rate.[5]
Pancreaticoduodenectomy (PD) is currently the only curative treatment for periampullary malignancy and pancreatic cancer. However, it is also a high-risk abdominal surgery. In China, the LOPH remains about 20 days, even in high-volume centers.[6,7] Thus, there may be an opportunity to reduce LOPH by the introduction of ERAS.
Several studies have explored the feasibility and efficacy of ERAS in PD.[8,9] Evidence indicated that ERAS protocols implemented in PD shortened the LOPH without compromising postoperative outcomes. However, no studies provide data concerning the recovery after discharge, except for readmission. As we all know, the LOPH can still be sensitively influenced by many subjective factors, especially the acceptance or incentives for early discharge. Moreover, earlier discharge may lead to failure to document some delayed complications. Therefore, whether patients in the ERAS group are ready to go home physically and whether they will continue to recover as well as might in hospital are unknown. This is particularly important in China, where most patients go home after discharge and cannot get professional care. Thus, it would be useful to evaluate the recovery of these patients after discharge.
In the current study, we compared a consecutive cohort of patients undergoing elective PDs before and after implementation of ERAS with regard to postoperative outcomes in hospital and recovery at home. This is the first study evaluating in detail the impact of an enhanced recovery protocol for PD on after-hospital recovery. Our findings will help comprehensively assess the safety and efficacy of ERAS in PD.
2. Material and methods
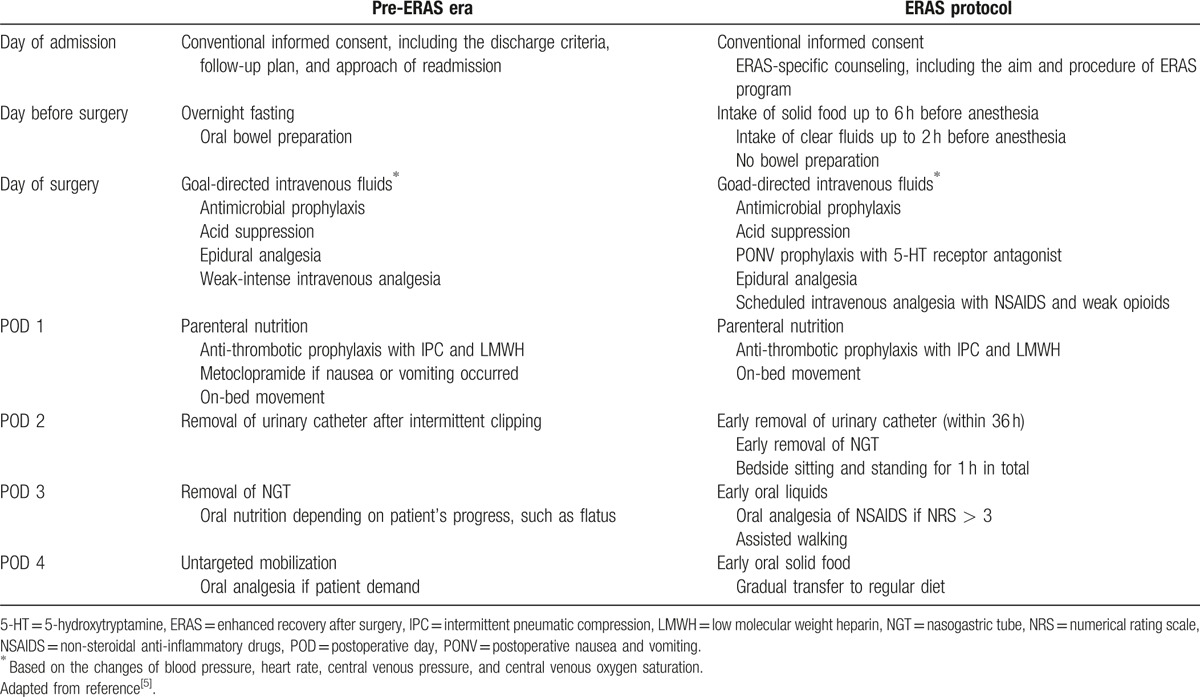
An ERAS protocol for PD with active contribution from the ERAS Society[5] was implemented in the Department of Hepatobiliary and Pancreatic Surgery, the Second Affiliated Hospital, Zhejiang University School of Medicine, in May 2014 (Table 1). The department is a drafter of ERAS guidelines for hepatobiliary and pancreatic surgery in China.
Table 1.
Perioperative care before and after introduction of the enhanced recovery after surgery protocol.
2.1. Study overview
A registered randomized clinical trial on PD was conducted in this center in 2012.[10] Correspondingly, an electronic database was developed for prospective data collection, including demographic information and perioperative data since then. To better evaluate postoperative outcomes, follow-ups were done systematically from June 2013 and were entered prospectively into the database.
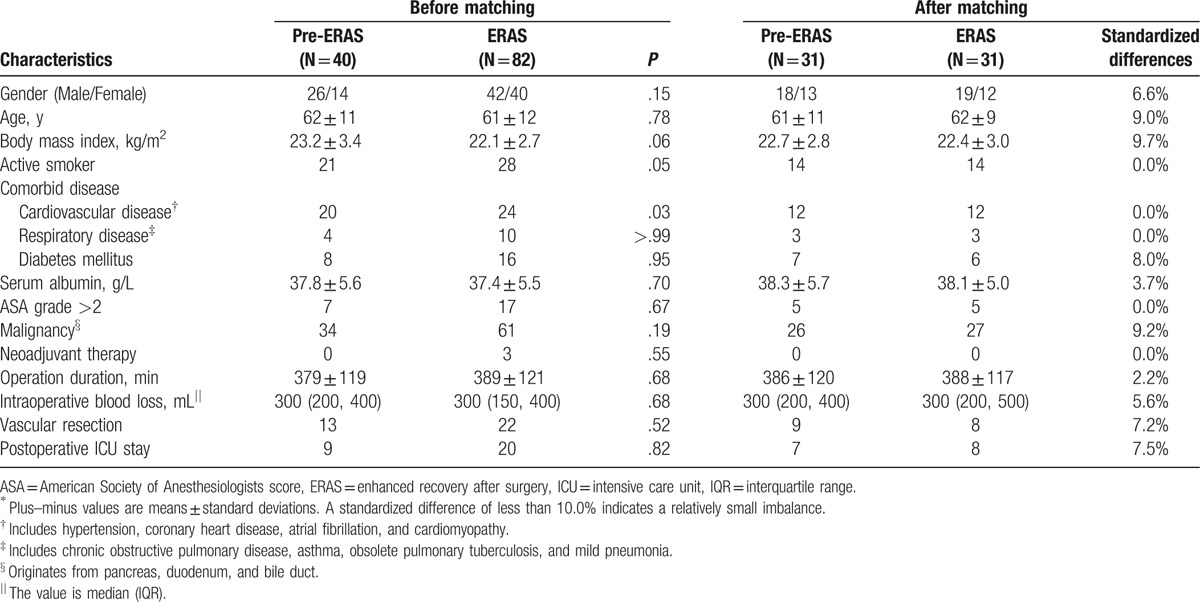
In the present study, patients undergoing elective PDs were included consecutively from June 2013 to November 2015. Those who stayed in the intensive care unit for more than 24 hours were unable to adhere to the protocol and were excluded. A prospective ERAS cohort (May 2014–November 2015) was compared with a retrospective control group (pre-ERAS, June 2013–April 2014) with regard to postoperative recovery, both in hospital and at home. The demographic information, perioperative data, and follow-ups were extracted from the database described above. The compliance with the ERAS protocol was recorded prospectively and was audited monthly by the chief resident in the ERAS group. But it was collected retrospectively in the pre-ERAS group. During the study period, key medical personnel, including surgeons and nurses, stayed the same. Given the imbalance in the baseline characteristics between the 2 groups (Table 2), propensity-score matching was used.
Table 2.
Baseline characteristics before and after propensity-score matching∗.
The study received approval from the Medical Ethics Committee of the Second Affiliation Hospital of Zhejiang University School of Medicine.
2.2. Postoperative outcomes
Postoperative complications were documented and graded according to the Dindo–Clavien classification.[11] Pancreatic fistula, delayed gastric emptying (DGE), postpancreatectomy hemorrhage, biliary leakage, intra-abdominal infection, and pulmonary infection were defined in line with our previous study.[10] Impaired wound healing was diagnosed according to the definition by Kuppahally et al.[12] Intestinal dysbacteriosis was diagnosed on the basis of fecal culture of disturbed gut flora, where pathogenic bacteria such as Staphylococcus aureus and Candida albicans overgrew. Acute heart failure was diagnosed on the basis of an elevated level of serum amino-terminal pro-brain natriuretic peptide (<50 years, >450 pg/mL; 50–75 years, >900 pg/mL; >75 years, >1800 pg/mL).[13] Arrhythmia was diagnosed on the basis of electrocardiograph changes confirmed by a cardiologist. Pleural effusion was considered as a complication when it was detected by ultrasound or computed tomography scan, and was severe enough to cause clinical symptoms and require special treatments, such as thoracentesis. Infections of the central venous catheter and the urinary tract were diagnosed on the basis of clinical signs and positive culture results for bacteria.[14]
2.3. Discharge criteria
Patients were not allowed to leave hospital until they met the following criteria: good function of vital organs; no signs of infection; good pain control, with or without oral analgesics alone; tolerance for solid food; passage of stools; good movement with assistance; and good wound healing.
2.4. Follow-ups
Patients were followed up for at least 4 weeks and were surveyed by telephone on Day 7, Day 14, and Day 28 after discharge. At each time point, patients were asked about their food intake, sleep, defecation, vigor, performance status, pain control and weight changes, using a specially designed form. Food intake was staged into 3 grades according to its change before and after surgery. Patients with Grade A had similar daily food intake to that before surgery. Patients with Grade B had significantly reduced daily food intake, yet ate more than 50% of that before surgery. For those with Grade C, their daily food intake decreased to less than 50% of that before surgery. A patient was considered to have good recovery of food intake if he/she received more than one A scores in the 3 surveys. We documented patients’ complaints about sleep at the first 2 surveys and assessed their sleep qualities using Athens Insomnia Scale[15] at the third survey. A patient was considered to have satisfactory sleep if no complaints about sleep were reported at the first 2 surveys or if the score ranged from 0 to 3 at the third survey. Vigor was assessed using the Surgical Recovery Scale,[16] and fatigue was diagnosed if the score < 60. Performance status was scored as 0 to 5 using the Zubrod–Eastern Cooperative Oncology Group–World Health Organization (Zubrod–ECOG–WHO) scale.[17] Pain was assessed on the basis of a numerical rating scale.[18] A patient was considered having weight loss if his/her weight was reduced 1 kg or more than that at discharge. In addition, unplanned readmissions and death within 30 days after discharge were recorded. The follow-up was carried out thoroughly by the nursing team.
2.5. Statistical analysis
The propensity score was estimated using a nonparsimonious multivariable logistic-regression model with ERAS or pre-ERAS as the dependent variable and all the baseline characteristics outlined in Table 2 as covariates. Matching was performed using a 1:1 matching protocol without replacement (local algorithm), with a caliper width equal to 20% of the standard deviation of the logit of the propensity score. A standardized difference of less than 10.0% for a given covariate indicated a relatively small imbalance.[19] In the matched cohort, paired comparisons were performed using McNemar test for binary variables, Wilcoxon signed ranks test for ranked data, and a paired Student t test or Wilcoxon paired-sample test for continuous variables. For unpaired data, binary variables were compared using a Chi-square test or Fisher exact test, and continuous variables were compared using Student t test or Mann–Whitney U test. All possible variables were included for univariate analysis, and variables with P values < .1 were considered as candidates for multivariate analysis. To relieve the problem of multicollinearity, variables with a correlation coefficient > 0.8 were included exclusively. Multivariate analysis was performed using binary logistic regression. The selection of stepwise methods depended on C statistics and Hosmer–Lemeshow test. All statistical analyses were conducted using SPSS v23.0 (IBM Corp., Armonk, New York). All reported P values were 2-sided and a statistical significance was considered when P was no more than .05.
3. Results
A total of 122 eligible patients undergoing elective PDs were identified, of whom 40 were included in the pre-ERAS group and 82 were included in the ERAS group (Table 2). No patients underwent pylori-preserving PD. Before matching, the 2 groups were imbalanced in terms of body mass index, active smoking, and comorbid cardiovascular disease. After propensity-score matching, 2 groups containing 31 patients in each were established. The standardized differences were less than 10.0% for all variables. All the subsequent analyses were based on the matched groups unless indicated otherwise.
3.1. Protocol compliance
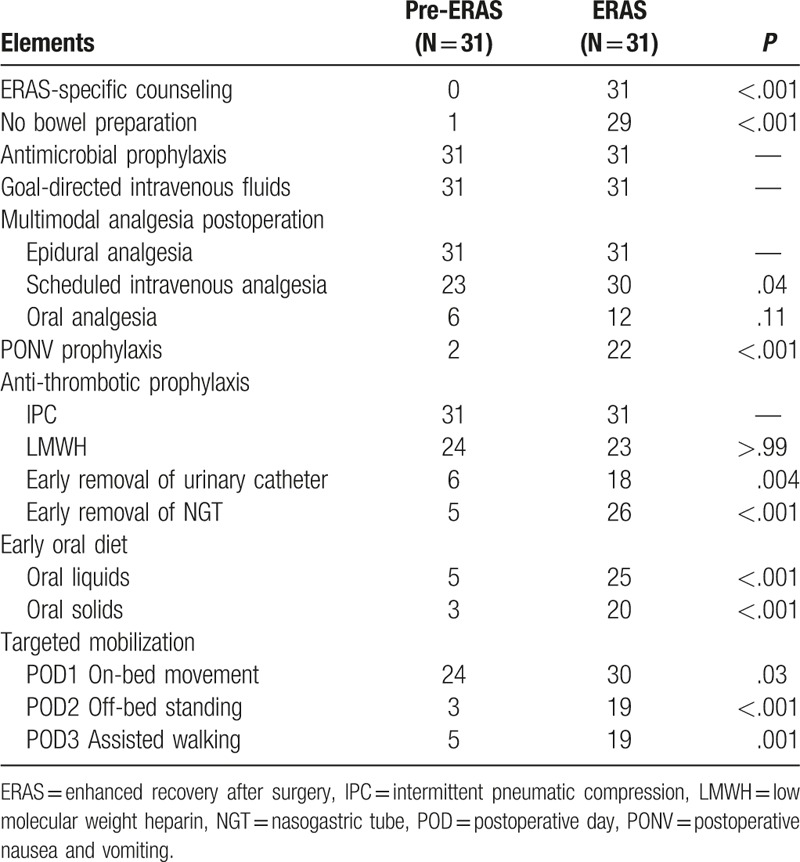
The degree of adherence to core protocol elements was summarized in Table 3. In general, mean compliance with these factors was significantly improved after ERAS implementation (43.8% vs 81.2%; P = .002).
Table 3.
Compliance with core ERAS protocol elements.
3.2. Postoperative outcomes in hospital
Compared with those in the pre-ERAS group, patients in the ERAS group tended to suffer from fewer (26/31 vs 18/31; P = .06) and minor (P = .06) complications (Table 4). The overall incidence of cardiovascular complications, including acute heart failure (6/31 vs 3/31; P = .51), arrhythmia (4/31 vs 1/31; P = .38), and uncontrollable hypertension (1/31 vs 0/31; P > .99), was significantly lower in the ERAS group (11/31 vs 3/31; P = .04). It should be noted that all the acute heart failure cases were mild, even asymptomatic. Besides, the incidence of intestinal dysbacteriosis was also significantly lower in the ERAS group (13/31 vs 4/31; P = .04).
Table 4.
Postoperative outcomes in hospital.
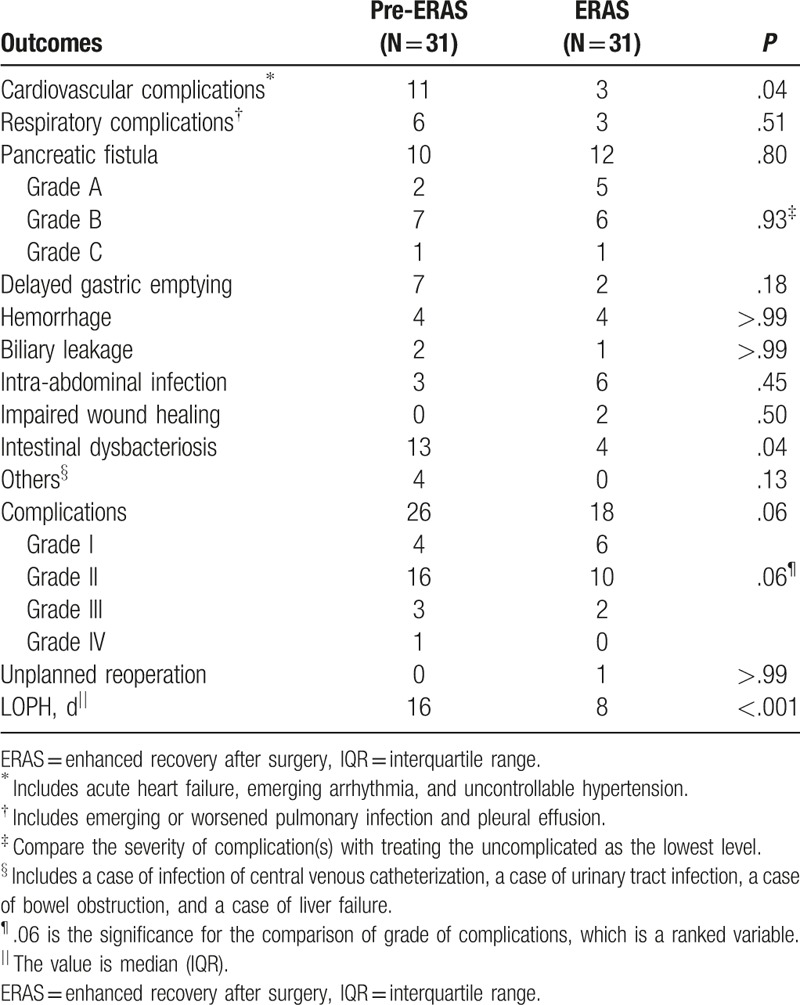
Two patients in the pre-ERAS group and one in the ERAS group underwent endoscopic intervention because of DGE. A patient in the pre-ERAS group underwent thoracentesis because of massive pleural effusion. A patient in the ERAS group underwent unplanned reoperation because of a severe pancreatic fistula. Liver failure occurred in 1 patient in the pre-ERAS group (Grade IV).
Median (interquartile range, IQR) LOPH was largely shortened from 16 (12–24) days to 8 (7–17) days after the implementation of the ERAS program (P < .001).
3.3. Home recovery
All patients went home after discharge and no patients were lost to follow-up within 30 days after discharge. During this period, no patients died. However, 2 patients in the ERAS group were readmitted (P = .50): 1 on second day after discharge because of severe infection, and the other in second week because of severe abdominal pain.
Generally, most patients achieved acceptable home recovery in the first month after discharge (Fig. 1). Notably, the proportion of patients with Grade A in food intake kept higher in the ERAS group (5/31 vs 18/31 in first week, P = .002; 9/31 vs 22/31 in second week, P = .008; 13/31 vs 23/31 in fourth week, P = .01). Furthermore, weight changes were evaluated at the last survey. Three data in the pre-ERAS group and two data in the ERAS group were unavailable. However, both intention-to-treat analysis with the worst-case scenario (19/31 vs 10/31; P = .05) and per-protocol analysis (18/26 vs 7/26; P = .007) showed that the proportion of patients suffering weight loss was lower in the ERAS group.
Figure 1.
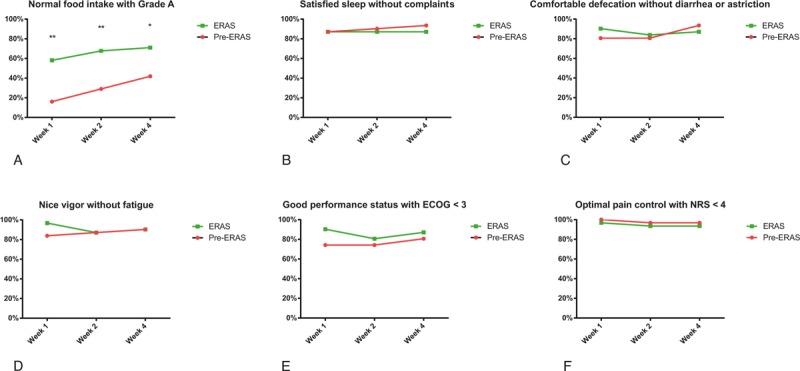
Home recovery in the first 4 weeks after discharge. Patients readmitted were treated as the worst cases in the analysis. ECOG = Eastern Cooperative Oncology Group score, ERAS = enhanced recovery after surgery, NRS = numerical rating scale. Compared with the pre-ERAS group: ∗P < .05; †P < .01.
3.4. Factors influencing postoperative recovery
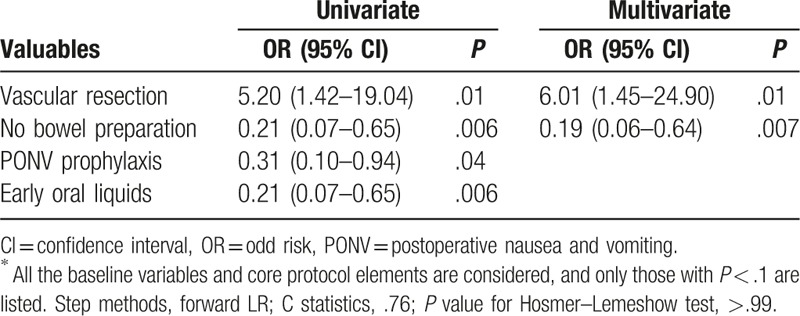
To explore the factors influencing postoperative recovery, all the baseline variables and core protocol elements were considered. The univariate analyses for cardiovascular complications and intestinal dysbacteriosis were summarized in Tables S1 and S2 (See Supplementary Material). And, multivariate regression analyses were performed for good recovery of food intake after discharge and weight loss (Tables 5 and 6). No bowel preparation was suggested as an independent protective factor for both food intake recovery [odd risk (OR) = 4.43; 95% confidence interval (CI): 1.41–13.97; P = .01] and weight loss (OR = 0.19; 95% CI: 0.06–0.64; P = .007).
Table 5.
Uni- and multivariate analyses for good recovery of food intake after discharge∗.
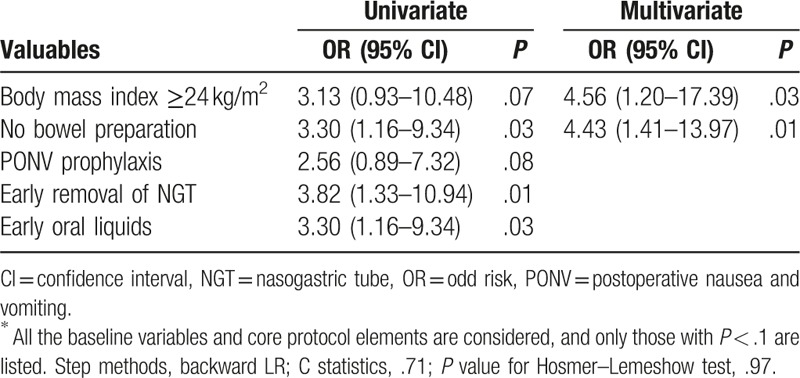
Table 6.
Uni- and multivariate analyses for weight loss∗.
4. Discussion
In this before–after cohort of patients undergoing elective PDs, we observed a reduced incidence of postoperative complications, especially cardiovascular complications and intestinal dysbacteriosis, and a shortened LOPH in hospital; an improved recovery of food intake and a decreased rate of weight loss postdischarge after implementation of an ERAS protocol. No bowel preparation was associated with better recovery of food intake and prevention of weight loss. Short-term outcomes, both in hospital and at home, suggested that the ERAS protocol could promise a better and faster recovery after surgery. As far as we know, this is the first study to evaluate comprehensively the impact of an ERAS protocol on patients undergoing elective PDs from hospital to home.
Postoperative outcomes in hospital were documented in detail. The overall morbidity tended to be reduced, in keeping with previous studies.[20,21] Particularly, cardiovascular complications were found significantly reduced after implementing ERAS, which had been hardly ever reported in previous studies on ERAS for PD. This reduction possibly resulted from that mild heart failure was considered as a complication in the present study. Although it was mild, even asymptomatic, and could be handled easily by a reduction of fluid infusion and diuretics in most cases or combined with digitalis in some cases, it is a valuable indicator of cardiac protection and restoration. Thus, it is worthy of consideration. Univariate analysis indicated that no bowel preparation, postoperative nausea and vomiting prophylaxis, early oral intake, and early and scheduled mobilization were associated with prevention of postoperative cardiovascular events. Previous studies in the field of colorectal surgery demonstrated that mechanical bowel preparation could lead to dehydration and electrolyte imbalance, especially the loss of calcium and potassium, and increasing the risk of cardiovascular events, particularly in the elderly.[22,23] Early oral intake has been shown to be feasible and safe for PD patients.[24] It could help reduce intravenous fluids and achieve a better fluids balance, which was believed critical to improve clinical outcomes including reducing cardiovascular events.[25] Extended bed rest was recognized as a risk factor for unwanted cardiovascular effects a long time ago.[26]
Intestinal dysbacteriosis was also significantly reduced after ERAS implementation and was not reported in the previous studies. Mounting evidence showed that establishing and maintaining beneficial interactions between the host and its associated microbiota were key requirements for host health.[27,28] A common and direct result of gut microbiota disturbance in clinical practice is severe diarrhea. Therefore, it is essential to take it into consideration. In addition to avoidance of mechanical destruction to gut microbiota, such as mechanical bowel preparation, and early resumption of gut microenvironment, an optimal pain control might also contribute to prevention of intestinal dysbacteriosis.[29]
The incidences of other complications and readmission were similar between groups in line with preceding studies,[8] suggesting the safety of ERAS in PD. A study with 252 patients in each group reported reduced DGEs after implementation of a similar ERAS protocol.[30] In our study, DGE was also reduced in the ERAS group, but not significantly. This was possibly a consequence of limited sample size.
It was reported that median LOPH for PD in most high-volume centers in United States is 7 to 11 days without implementing ERAS.[31] However, median LOPH continues to plateau at a median of about 20 days even at high-volume hospitals in China.[6,7] It is partially due to several cultural issues, including the acceptance for early discharge, the lack of discharge disposition other than home, and the availability of home care nursing. On the contrary, the big gap indicates that a big improvement can be achieved by changing traditional concept and refining conventional care. This is crucial to China where medical resource is scarce. An ERAS pathway can be a solution.
Short-term home recovery was also surveyed. Patients in the ERAS group enjoyed much better recovery of food intake and suffered fewer weight loss. Statistically, both the improvements were associated with no bowel preparation and early oral intake. Remarkably, multivariate regression analyses implied that no bowel preparation was an independent protective factor for both food intake recovery and weight loss. Previously, a large retrospective analysis of 200 consecutive patients undergoing PD found no benefits of mechanical bowel preparation before surgery in postoperative complications and hospital stay, and the 12-month survival rates.[32] Mechanical bowel preparation should probably be avoided or limited in the context of PD, especially in patients needing vascular resection, based on current evidence.
Several limitations of our study should be considered. First, like all before–after studies, experience obtained during the study could have an impact on postoperative outcomes. However, currently, no randomized controlled trials comparing ERAS programs with traditional care in pancreatic surgery have been reported. This is probably because the ERAS program is a multimodal approach involving various interventions and professionals, and several protocol elements of an ERAS program have already become a standard practice.[8] A before–after study may be the most practicable and effective approach. Second, limited to the follow-up data available, the sample size was relatively small. But the 2 groups were robustly matched and all the data concerning outcomes were prospectively collected. Third, as no blinding was performed and patients in the ERAS group were informed that they were undergoing an ERAS program, the Hawthorne effect was inevitable.
In conclusion, in this before–after cohort of patients undergoing elective PDs, patients in the ERAS group experienced shorter postoperative hospital stay, fewer cardiovascular complications and intestinal dysbacteriosis in hospital, and better recovery of food intake and fewer weight loss at home in the short term than those in the pre-ERAS group. The long-term influence of ERAS on these patients is under investigation.
Acknowledgments
We thank QZ for his suggestion on data analysis and manuscript organization, and MZ and JW for data verification and data input. These contributors are from our department. We appreciate all the colleagues in our center for their significant supports.
Supplementary Material
Footnotes
Abbreviations: 5-HT = 5-hydroxytryptamine, ASA = American Society of Anesthesiologists score, CI = confidence interval, ECOG = Eastern Cooperative Oncology Group, ERAS = enhanced recovery after surgery, ICU = intensive care unit, IPC = intermittent pneumatic compression, IQR = interquartile range, LMWH = low molecular weight heparin, LOPH = length of postoperative hospital stay, NGT = nasogastric tube, NRS = numerical rating scale, NSAIDS = nonsteroidal anti-inflammatory drugs, OR = odd risk, PD = pancreaticoduodenectomy, POD = postoperative day, PONV = postoperative nausea and vomiting, WHO = World Health Organization.
Funding: This study was funded by the National High Technology Research and Development Program of China (SS2015AA020405), the Training Program of Key Program of National Natural Science Foundation of China (91442115), the Key Innovative Team for the Diagnosis and Treatment of Pancreatic Cancer of Zhejiang Province (2013TD06), the Key Program of National Natural Science Foundation of China (81530079), and the Key Research and Development Project of Zhejiang Province (2015C03G2010148).
The authors declared no conflict of interest.
Supplemental Digital Content is available for this article.
References
- [1].Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189–98. [DOI] [PubMed] [Google Scholar]
- [2].Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Brit J Anaesth 1997;78:606–17. [DOI] [PubMed] [Google Scholar]
- [3].Dorcaratto D, Grande L, Pera M. Enhanced recovery in gastrointestinal surgery: upper gastrointestinal surgery. Digest Surg 2013;30:70–8. [DOI] [PubMed] [Google Scholar]
- [4].Van Dam R, Hendry P, Coolsen M, et al. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg 2008;95:969–75. [DOI] [PubMed] [Google Scholar]
- [5].Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr 2012;31:817–30. [DOI] [PubMed] [Google Scholar]
- [6].Ke S, Ding XM, Gao J, et al. A prospective, randomized trial of Roux-en-Y reconstruction with isolated pancreatic drainage versus conventional loop reconstruction after pancreaticoduodenectomy. Surgery 2013;153:743–52. [DOI] [PubMed] [Google Scholar]
- [7].Huang X, Liang B, Zhao XQ, et al. The effects of different preoperative biliary drainage methods on complications following pancreaticoduodenectomy. Medicine (Baltimore) 2015;94:e723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coolsen MM, van Dam RM, van der Wilt AA, et al. Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg 2013;37:1909–18. [DOI] [PubMed] [Google Scholar]
- [9].Kagedan DJ, Ahmed M, Devitt KS, et al. Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford) 2015;17:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bai X, Zhang Q, Gao S, et al. Duct-to-mucosa vs invagination for pancreaticojejunostomy after pancreaticoduodenectomy: a prospective, randomized controlled trial from a single surgeon. J Am Coll Surg 2016;222:10–8. [DOI] [PubMed] [Google Scholar]
- [11].Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kuppahally S, Al-Khaldi A, Weisshaar D, et al. Wound healing complications with de novo sirolimus versus mycophenolate mofetil-based regimen in cardiac transplant recipients. Am J Transplant 2006;6(5 Pt 1):986–92. [DOI] [PubMed] [Google Scholar]
- [13].Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients. Eur Heart J 2006;27:330–7. [DOI] [PubMed] [Google Scholar]
- [14].Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–32. [DOI] [PubMed] [Google Scholar]
- [15].Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res 2000;48:555–60. [DOI] [PubMed] [Google Scholar]
- [16].Paddison JS, Sammour T, Kahokehr A, et al. Development and validation of the Surgical Recovery Scale (SRS). J Surg Res 2011;167:e85–91. [DOI] [PubMed] [Google Scholar]
- [17].Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- [18].MacCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Saint Louis, MO: Mosby; 1989. [Google Scholar]
- [19].Bangalore S, Guo Y, Samadashvili Z, et al. Everolimus-eluting stents or bypass surgery for multivessel coronary disease. N Engl J Med 2015;372:1213–22. [DOI] [PubMed] [Google Scholar]
- [20].Hilal MA, Di Fabio F, Badran A, et al. Implementation of enhanced recovery programme after pancreatoduodenectomy: a single-centre UK pilot study. Pancreatology 2013;13:58–62. [DOI] [PubMed] [Google Scholar]
- [21].Joliat GR, Labgaa I, Petermann D, et al. Cost-benefit analysis of an enhanced recovery protocol for pancreaticoduodenectomy. Br J Surg 2015;102:1676–83. [DOI] [PubMed] [Google Scholar]
- [22].Holte K, Nielsen KG, Madsen JL, et al. Physiologic effects of bowel preparation. Dis Colon Rectum 2004;47:1397–402. [DOI] [PubMed] [Google Scholar]
- [23].Jung B, Påhlman L, Nyström PO, et al. Multicentre randomized clinical trial of mechanical bowel preparation in elective colonic resection. Br J Surg 2007;94:689–95. [DOI] [PubMed] [Google Scholar]
- [24].Lassen K, Kjaeve J, Fetveit T, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg 2008;247:721–9. [DOI] [PubMed] [Google Scholar]
- [25].Tambyraja LA, Sengupta F, MacGregor BA, et al. Patterns and clinical outcomes associated with routine intravenous sodium and fluid administration after colorectal resection. World J Surg 2004;28:1046–52. [DOI] [PubMed] [Google Scholar]
- [26].Convertino VA. Cardiovascular consequences of bed rest: effect on maximal oxygen uptake. Med Sci Sports Exerc 1997;29:191–6. [DOI] [PubMed] [Google Scholar]
- [27].Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242–9. [DOI] [PubMed] [Google Scholar]
- [28].Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 2013;11:227–38. [DOI] [PubMed] [Google Scholar]
- [29].Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009;6:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Balzano G, Zerbi A, Braga M, et al. Fast-track recovery programme after pancreatico-duodenectomy reduces delayed gastric emptying. Br J Surg 2008;95:1387–93. [DOI] [PubMed] [Google Scholar]
- [31].Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One 2013;8:e59260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lavu H, Kennedy EP, Mazo R, et al. Preoperative mechanical bowel preparation does not offer a benefit for patients who undergo pancreaticoduodenectomy. Surgery 2010;148:278–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


