Abstract
Rationale:
Mechanical ventilation of severe acute asthma is still considered a challenging issue, mainly because of the gas trapping phenomenon with the potential for life-threatening barotraumatic pulmonary complications.
Patient concerns:
Herein, we describe 2 consecutive cases of near-fatal asthma for whom the recommended protective mechanical ventilation approach using low tidal volume of 6 mL/kg and small levels of PEEP was rapidly compromised by giant pneumomediastinum with extensive subcutaneousemphysema.
Diagnoses:
Near fatal asthma.
Intervention:
A rescue therapeutic strategy combining extracorporeal CO2 removal membrane with ultra-protective extremely low tidal volume (3 mL/kg) ventilation was applied.
Outcomes:
Both patients survived hospital discharge.
Lessons:
These 2 cases indicate that ECCO2R associated with ultra-protective ventilation could be an alternative to surgery in case of life-threatening barotrauma occurring under mechanical ventilation.
Keywords: acute respiratory failure, asthma, extracorporeal life support, mechanical ventilation
1. Introduction
Extracorporeal life supports have exceptionally been used in status asthmaticus for almost 40 years.[1] In the last decade, a continuous venovenous extracorporeal CO2 removal (ECCO2R) membrane has been increasingly used for the most severe cases of acute asthma, despite no evidence that this life support modality is safer and less invasive than a classic arteriovenous extracorporeal membrane oxygenation.[2,3] In all these cases, the decision of ECCO2R implantation was generally motivated by an uncontrolled respiratory acidosis.[4] However, what initially makes mechanical ventilation of severe asthma challenging at the bedside is the issue of gas trapping and dynamic pulmonary hyperinflation with the subsequent risk of severe barotrauma, rather than the simple control of gas exchange.[5,6] As a direct catastrophic consequence of the incomplete lung emptying of acute asthma, several cases of giant pneumomediastinum causing cardiac tamponade have been reported.[7–9] When pneumothorax is present, chest tube insertion in the pleural space is the urgent recommended approach. But when there is no pneumothorax, things can get worse with positive pressure mechanical ventilation. In this dramatic scenario, surgical decompression of the mediastinum has been proposed as an aggressive risky therapeutic intervention.[7,8] We describe 2 consecutive cases of near-fatal asthma for whom a new rescue therapeutic strategy combining ECCO2R membrane with ultra-protective low-tidal volume ventilation was successfully applied to limit the risk of severe barotrauma during invasive mechanical ventilation. The successful medical management of these patients, without the resort to surgery, may present a new approach in the treatment of pulmonary barotrauma complicating severe status asthmaticus.
Ethical approval of the present manuscript was deemed unnecessary by our local ethics committee given that we just report here, retrospectively, how 2 patients were managed in our intensive care unit (ICU). Nevertheless, signed informed consents were obtained retrospectively by the patients for this publication.
2. Case record
Case 1: A 30-year-old obese female, with a body mass index of 33 kg/m2 and a known severe allergic asthma but few medical monitoring, was admitted through the emergency room for a severe asthma exacerbation. She has been a heavy smoker for 15 years and owned a dog despite a known allergy to it. On arrival, she presented widespread wheezes and extreme shortness of breath. The initial blood gas showed pH 7.29, PaO2 55 mm Hg and PaCO2 49 mm Hg. She failed to respond to nebulized salbutamol and ipratropium, to intravenous salbutamol and magnesium, and therefore was transferred to the ICU where noninvasive ventilation (NIV) was started. Despite this strategy, she presented a pronounced use of accessory respiratory muscles and rapidly required tracheal intubation. She was treated according to general principles of mechanical ventilation for severe acute asthma, namely low tidal volume (6 mL/kg of ideal body weight [IBW]), prolonged expiratory time, sedation, analgesia, and neuromuscular blocking.[5] But mechanical ventilation was rapidly complicated by an alarming giant subcutaneous emphysema of the cervicothoracic spaces the day after its initiation. Chest CT scan showed a large pneumomediastinum but no pneumothorax was identified. The extension of the pulmonary barotraumas despite the protective mechanical ventilation strategy prompts an escalation to ultra-protective mechanical ventilation with extremely low tidal volume of 3 mL/kgIBW. Anticipating the worsening of alveolar hypoventilation consecutive to these ventilator settings, the ECCO2R was implanted at the same time using a monocanula Novalung iLA Activve device. This device allows a blood flow from 0.5 to 4.5 L/min and an oxygen sweep up to 10 L/min. The right femoral vein was cannulated using a 22-Fr unique catheter allowing a venovenous blood flow at 1 L/min. The oxygen sweep was initially set at 1 L/min and the blood flow at 3 L/min. Due to relatively low extracorporeal blood flow, the Novalung device required anticoagulation with partial thromboplastin time objective between 60 and 90 seconds. Though intravenous heparin was started at 25000 UI per 24 hours, no adverse event such as bleeding or hematoma occurred. At day 6, the patient exhibited severe hypoxemia with PaO2/FiO2 <200 due to extensive gravitational atelectasis that are commonly encountered in such a critically ill obese patient in the supine position, especially during low-tidal volume mechanical ventilation and paralysis. To improve lung recruitment and gas exchange, the patient was placed in the prone position and the oxygen sweep flow was increased to 10 L/min even though the oxygenation effect of the ECCO2R remained limited. The subcutaneous emphysema and the pneumomediastinum decreased gradually as well as the sign of bronchospasm. The patient was extubated at day 10 and placed under NIV to prevent gravitational atelectasis with this obese patient. The ECCO2R cannula was removed on day 14 in the unit, requiring a simple compression for 5 to 10 minutes. None of the usual extracorporeal circulation adverse events such as hemolysis, thrombocytopenia, or gas embolism was observed during the ECCO2R device running period. The patient was transferred from ICU to the respiratory care unit at day 16 and was discharged from hospital at day 19. She quit smoking and got rid of her dog.
Case 2: A 27-year-old male with a known allergic asthma was admitted to the emergency room (ER) with an exacerbation of his disease for a few days. He had no history of severe asthmatic crisis. He was initially hospitalized in the emergency room care unit where he received nebulized salbutamol and ipratropium and parenteral corticosteroids and later intravenous salbutamol. Twenty-four hours after admission, he presented an increasing respiratory distress and was therefore transferred to the ICU, where he rapidly required tracheal intubation and mechanical ventilation. Quickly intravenous magnesium sulfate, ketamine, neuromuscular blocking, and protective ventilation were added to meet optimal treatment. Despite the maximalist management, the dynamic hyperinflation remained with a measured auto-PEEP of 22 cmH2O and the patient developed collaterally a left pneumothorax requiring pleural aspiration as well as a pneumomediastinum with large subcutaneous emphysema and cardiac tamponade calling for a hemodynamic support. A bronchoscopy was performed and confirmed the barotraumatic origin of the pneumothorax. The patient was placed under ultra-protective mechanical ventilation (4 mL/kgIBW) to prevent gas trapping and positive pressure ventilation-induced barotrauma. This was associated with worsening of the PaCO2 up to 90 mm Hg, which motivated the implantation of an ECCO2R membrane. A Novalung iLA AV shunt pumpless device (iLA, NovaLung GmbH, Hechingen, Germany) with arteriovenous flow was used and the left femoral vein and right artery were cannulated using ultrasound guidance, using respectively an 18-French and a 14-French catheters. Heparin was introduced at hypocoagulant doses to prevent thrombotic events. Cardiac flow remained sufficient, that is, superior to 2.5 L/min, and no inotropic agent such as dobutamine was needed to maintain an appropriate flow in the device.
The oxygen sweep was set at 1 L/min and the PaCO2 rapidly decreased despite the pursuit of ultra-protective mechanical ventilation and no increase of the oxygen sweep flow was needed. From this point on, the bronchospasm receded and so did the barotrauma, allowing the removal of the ECCO2R cannula and the pleural drain after 8 days in the unit. Thereafter, the patient was successfully extubated at day 9 and transferred in the adult respiratory care unit. He was discharged from hospital at day 12.
3. Discussion
According to the monoexponential equation describing lung emptying in a monocompartimental model of the lung, lung volume at expiration basically follows this relationship: V(t) = VEI.e(−t/τ) where V represents lung volume at any given time (t), VEI is end-inspiratory lung volume, and τ is the time constant of the respiratory system calculated as the product of respiratory system compliance by its resistance. Patients with obstructive respiratory disease exhibit higher resistances with a prolonged time constant promoting incomplete lung emptying at end expiration. This abnormal expiration curve generates significant air trapping with large end-expiratory lung volume and auto-PEEP, a well-known source of multiple cardiorespiratory complications especially during mechanical ventilation.[10] In the landmark physiological study of Tuxen et al published almost 30 years ago, the most important determinant of lung overinflation in mechanically ventilated asthmatics was end-inspiratory lung volume.[6] At each incremental level of PEEP, end-inspiratory lung volume increases with a subsequent dramatic rise in end-expiratory lung volume. At the final step of the protocol using very high tidal volume reaching 1000 mL with high level of external PEEP up to 15 cmH20, 5 out of 6 patients had developed lung overinflation-induced circulatory failure.[6] Positive pressure mechanical ventilation promotes life-threatening complications of status asthmaticus including pneumothorax, pneumomediastinum, giant subcutaneous emphysema, and circulatory shock. Despite the early use of a low-tidal volume (6 mL/kgIBW) protective mechanical ventilation with low level of applied PEEP in our 2 patients, this insufficient strategy was rapidly compromised by severe pulmonary barotrauma and uncontrolled respiratory acidosis. The use of ECCO2R allowed us to utterly reduce the tidal volume while managing the related increase in PaCO2 (Table 1). With this new strategy, the problematic barotrauma instantly stabilized and decreased progressively thanks to the ultralow tidal volume we used in these 2 patients. Another interesting point is that none of these 2 patients experienced any adverse events such as thrombosis or hemorrhage with this technique nor with the arteriovenous pumpless device nor with the monocanula Novalung ECCO2R.
Table 1.
Respiratory mechanics and gas exchange of the 2 asthmatic subjects according to the ventilator settings at ECCO2R initiation (H0), after 1 h of ECCO2R (H1), and at the time of weaning from mechanical ventilation.
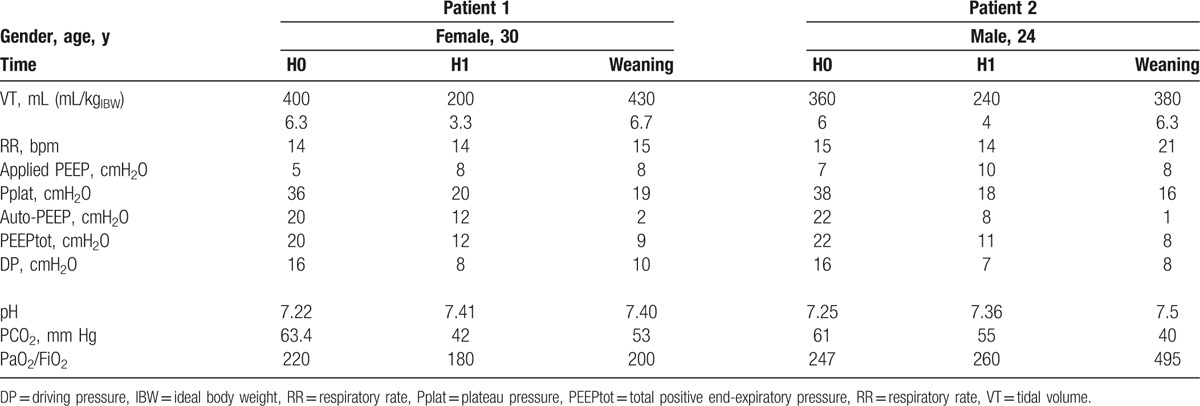
In conclusion, besides the growing place of ECCO2R in critically ill patients, this rescue technique seems particularly interesting in the case of asthmatic patients with life-threatening pulmonary barotrauma despite the early resort to low-tidal volume (6 mL/kgIBW) protective mechanical ventilation. Herein, we report one of the first uses of ECCO2R in 2 cases of uncontrolled pulmonary barotrauma complicating mechanical ventilation in acute near-fatal asthma. ECCO2R membrane implantation allowed the use of ultra-protective mechanical ventilation with a drastic reduction in tidal volume (3 mL/kgIBW) and minute ventilation, immediately limiting lung overinflation and gas trapping, and secondarily resolving the barotrauma.
Footnotes
Abbreviations: ECCO2R = extracorporeal CO2 removal, IBW = ideal body weight, ICU = intensive care unit, NIV = noninvasive ventilation.
The authors report no conflicts of interest.
References
- [1].MacDonnell KF, Moon HS, Sekar TS, et al. Extracorporeal membrane oxygenator support in a case of severe status asthmaticus. Ann Thorac Surg 1981;31:171–5. [DOI] [PubMed] [Google Scholar]
- [2].Brenner K, Abrams D, Agerstrand C, et al. Extracorporeal carbon dioxide removal for refractory status asthmaticus: experience in distinct exacerbation phenotypes. Perfusion 2014;29:26–8. [DOI] [PubMed] [Google Scholar]
- [3].Jung C, Luaten A, Pfeifer R, et al. Pumpless extracorporeal lung assist for the treatment of severe, refractory status asthmaticus. J Asthma 2011;48:111–3. [DOI] [PubMed] [Google Scholar]
- [4].Elliot SC, Paramasivam K, Oram J, et al. Pumpless extracorporeal carbon dioxide removal for life-threatening asthma. Crit Care Med 2007;35:945–8. [DOI] [PubMed] [Google Scholar]
- [5].Leatherman J. Mechanical ventilation for severe asthma. Chest 2015;147:1671–80. [DOI] [PubMed] [Google Scholar]
- [6].Tuxen D. Detrimental effects of positive end-expiratory pressure during controlled mechanical ventilation of patients with severe airflow obstruction. Am Rev Respir Dis 1989;140:5–9. [DOI] [PubMed] [Google Scholar]
- [7].McNicholl B. Pneumomediastinum and subcutaneous emphysema in status asthmaticus, requiring surgical decompression. Arch Dis Child 1960;35:389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shennib HF, Barkun AN, Matouk E, et al. Surgical decompression of a tension pneumomediastinum. A ventilatory complication of status asthmaticus. Chest 1988;93:1301–2. [DOI] [PubMed] [Google Scholar]
- [9].Jones MG, Rae W, Lwin AA, et al. Pneumomediastinum leading to respiratory compromise as a complication of acute severe asthma. Am J Respir Crit Care Med 2013;187:e5–6. [DOI] [PubMed] [Google Scholar]
- [10].Berlin D. Hemodynamic consequences of auto-PEEP. J Intensive Care Med 2014;29:81–6. [DOI] [PubMed] [Google Scholar]


