Abstract
Background:
Numerous studies have attempted to determine the prognostic role of proliferating cell nuclear antigen (PCNA) expression in patients with osteosarcoma with no consistent conclusion. We performed this meta-analysis to systematically elucidate the association in a more precise manner.
The purpose of this meta-analysis is to determine the prognostic role of PCNA in patients with osteosarcoma.
Methods:
A systematic search of relevant studies was performed in 6 electronic databases including PubMed, Embase, Web of Science, Wanfang database, China National Knowledge Internet (CNKI) database, and Chinese Biological Medical (CBM) Database (up to March 1, 2016) with the following keywords: (PCNA OR proliferating cell nuclear antigen) AND (osteosarcoma OR osteogenic tumor). A manual search of references on relevant articles was also conducted by 2 investigators independently. We performed a comprehensive evaluation of the correlation between PCNA expression and overall survival (OS) or disease-free survival (DFS) by calculating relative ratios (RR) and their corresponding 95% confidence intervals (CI) using STATA software. A fixed- or random-effect model was chosen based on the between-study heterogeneity.
Results:
In total, 16 studies with 691 osteosarcoma patients were included in this meta-analysis. PCNA overexpression was found in approximately 57.31% of the patients with osteosarcoma. The meta-analysis suggested that PCNA overexpression in osteosarcoma patients is associated with low OS, but not significantly with DFS (RR = 1.82, 95% CI 1.53–2.18, P = .000; RR = 1.15, 95% CI 0.91–1.44, P = 0.234). Sensitivity analysis for OS and DFS showed no significant difference and the pooled RRs were stable when the included studies were removed one by one. Similar results were also obtained for subgroup analysis based on different follow-ups and cutoffs to determine PCNA expression.
Conclusion:
The findings from this meta-analysis indicate that PCNA overexpression is an effective biomarker for poor prognosis in patients with osteosarcoma for OS. Hence, more large-scale studies are still needed to further warrant this conclusion.
Keywords: disease-free survival, meta-analysis, osteosarcoma, overall survival, proliferating cell nuclear antigen, prognosis
1. Introduction
Osteosarcoma is the most common primary malignant bone tumor with higher mortality in teenagers.[1] Treatment of osteosarcoma has been improved greatly with the use of multiple chemotherapeutic agents before definitive resection of the primary tumor.[2] However, approximately 50% of the patients with osteosarcoma face multidrug resistance and poor clinical outcome,[3] with the 5-year overall relapse-free survival rate being 65%.[4,5] Although its occurrence and development are regulated by genetic factors,[6] the prognostic mechanism in osteosarcoma patients remains unclear. Thus, the identification of osteosarcoma prognostic markers and therapeutic targets is an urgent requirement.[7] Recently, several common markers[8,9] have been identified correlated with the metastasis and prognosis in osteosarcoma, and proliferating cell nuclear antigen (PCNA) is a promising marker among these.
Tumor antigens play an important role in tumor occurrence, development, and dispersal process, of which PCNA is the most important. In 1978, Miyachi et al[10] discovered antibodies against PCNA in the sera of patients with Cazenave lupus. PCNA is a cell cycle regulatory protein, and its expression increases significantly in the G1 phase, reaching a peak and decreases in the G2-M phase of cell cycle.[11] PCNA expression showed a periodic change with the replication of DNA phase change, which plays an important role in cellular transition from the G1 phase to S phase. PCNA has high expression in almost all tumor tissues because of its function. Therefore, numerous studies have reported that PCNA expression could accurately reflect the status of cell proliferation, and can be used as a biomarker for the diagnosis and prognosis of malignant tumors.[12,13]
Several studies have been performed to explore the association between PCNA expression status and prognosis in osteosarcoma. Some earlier studies showed that high PCNA expression was associated with poor prognosis in the overall survival (OS) of patients with osteosarcoma. However, several other studies suggested that it had no significance. Nevertheless, these results are so contradictory that the significance of overexpression for osteosarcoma prognosis is limited and unconvincing. Therefore, we conducted a meta-analysis to further investigate this prognostic value, and discussed the possibility of PCNA as a prognostic medical marker in osteosarcoma.
2. Materials and methods
2.1. Data sources and search strategy
In total, 6 electronic databases including PubMed, Embase, Web of Science, Wanfang database, CBM (Chinese Biomedical Literature Database), and CNKI (China National Knowledge Infrastructure) were searched for all potential relevant articles published before March 1, 2016 without any language restrictions. The following search terms were adopted in the meta-analysis: (PCNA OR proliferating cell nuclear antigen) AND (osteosarcoma OR osteogenic tumor). A manual information retrieval was also conducted by 2 investigators (FL and XW) to recognize references of all potentially eligible studies.
2.2. Inclusion criteria
Studies were included if they fulfilled the following criteria: patients with osteosarcoma, providing enough data to calculate relative ratios (RRs) and their 95% confidence intervals (CIs), estimating the correlation between PCNA expression and OS or DFS, and case reports, systematic reviews, and letters were all excluded. All disagreements were resolved though discussion and consensus by 2 investigators (FL and XW). Only the most recent or complete study was enrolled if certain article was published duplicate.
2.3. Endpoints of interest
The OS and DFS of patients with osteosarcoma were the primary endpoints. No available data for DFS in other years were collected. Subgroup analyses were classified by PCNA expression status using cutoffs defined by respective studies.
2.4. Data extraction
All data were extracted from the 16 included studies, and were entered to a predesigned and standardized excel file independently by 2 investigators (FL and XW). These valuable details included the first author's family names in the original articles, year of publication, number of patients, gender, age, inclusion period, Enneking stage, method of evaluating PCNA, PCNA cutoff, follow-up, RR with 95% CI for OS and DFS at different years in osteosarcoma. Only the most recent or complete study was enrolled if a certain article was published in duplicate. All disagreements were resolved through discussion and consensus by 2 investigators (FL and XW).
2.5. Quality assessment
The methodological qualities of eligible studies were estimated using Newcastle-Ottawa Scale (NOS)[14] which considered 9 factors. A score of 1 was given to a study for each item. The quality scale ranged from a score of 0 to 9 and studies with high scores were considered as good reports. Studies with scores ≥7 were regarded as high-quality reports and the others were considered as low-quality reports.
2.6. Statistical analysis
The correlation between PCNA expression and prognosis of patients with osteosarcoma was evaluated by calculating the pooled RR and its 95% CI. The between-study heterogeneity of our meta-analysis was assessed using the I2 statistic, a quantitative measure describing inconsistency across studies from 0% to 100%.[15] When heterogeneity was significant (I2 > 50%), the potential sources of heterogeneity were identified by analyzing the methodological variability of the included studies or by omitting studies one by one to evaluate the impact of a single trial on the overall pooled estimate. If the heterogeneity across studies still existed, a random-effect model was used to calculate the pooled RR and its 95% CI. When the heterogeneity was low (I2 < 50%), a fixed-effect model was applied. Egger test was used to evaluate the possibility of publication bias. The software STATA, version 12.0 (StataCorp, CollegeStation, TX), was applied for statistical analysis, and P <.05 was regarded as not statistically significant.
2.7. Ethical statement
Our meta-analysis conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[16] Ethical approval or patient consent is not required for conducting this meta-analysis.
3. Results
3.1. Search results and study characteristics
In total, 2100 relevant articles were collected by screening the titles and abstracts, of which 2084 were excluded for duplicate publication and various reasons (reviews, letter, congress, case report, or irrelevant to this analysis). After all the full-texts of potentially relevant studies were downloaded, 43 articles were removed because they did not provide enough data to calculate the RRs and their 95% CIs. Finally, 16 studies[17–32] published between 1999 and 2011 with 691 patients (ranging 20–71 in each study, median 41.5) were selected for the meta-analysis. The detailed selection process for the included studies is presented as a flow diagram in Figure 1.
Figure 1.
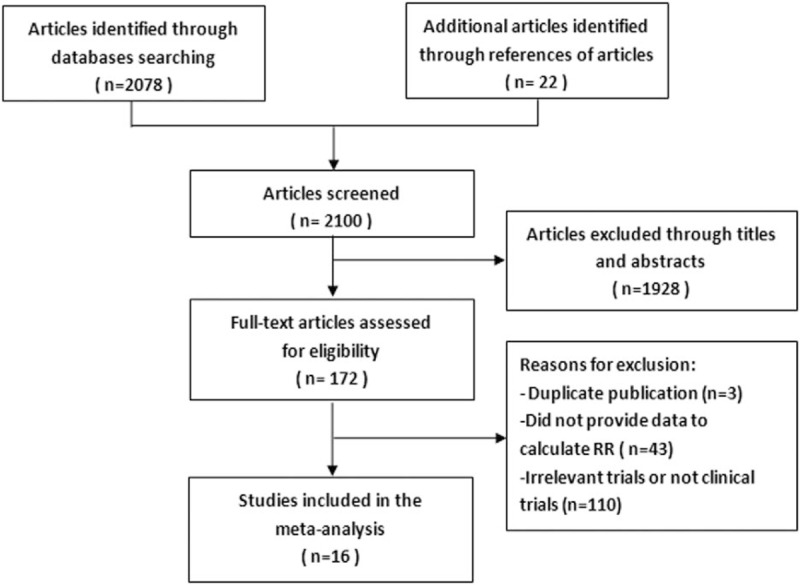
Selection process of eligible studies in the meta-analysis.
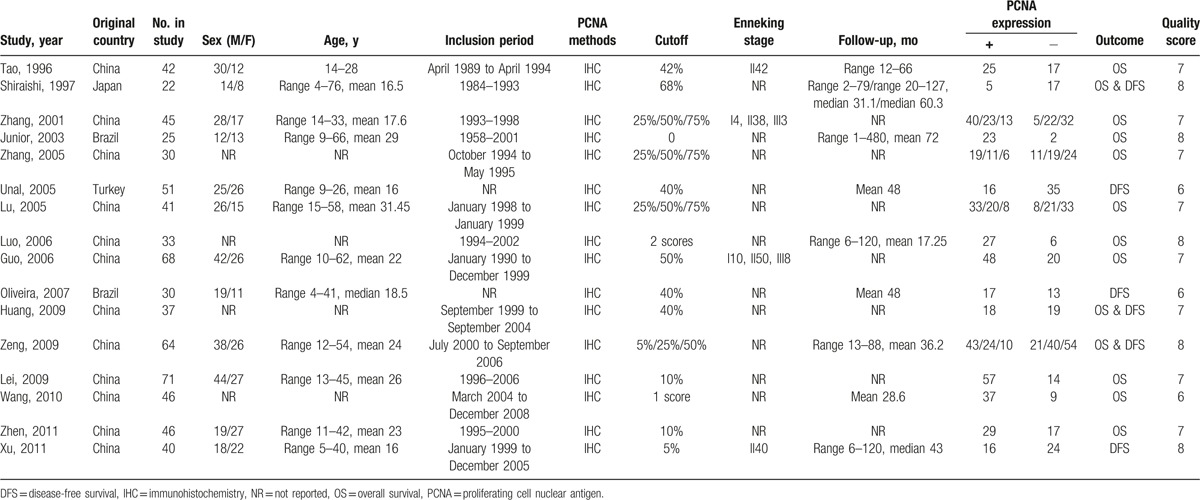
Among these, 5 studies were published in English[17–21] and 11 were published in Chinese.[22–32] The result of PCNA overexpression was found in approximately 57.31% of the osteosarcoma patients. Thirteen studies[17,18,21–30,32] reported the data of OS and 6 studies provided[17,19–21,29,31] the DFS of patients with osteosarcoma. Based on the NOS score, 5 studies got a score of 8, 8 studies achieved a score of 7, and 3 studies had a score of 6. The mean NOS score for these studies was 7.125 (range 6–8). The main characteristics of the 16 included studies are presented in Table 1.
Table 1.
Characteristics of enrolled studies in the meta-analysis.
3.2. Correlation between PCNA expression and OS
In total, 13 studies[17,18,21–30,32] explored the association between PCNA expression and OS with enough accessible data to calculate the RRs and 95% CI. The result showed that patients with high PCNA expression had a lower OS than those with low PCNA expression (RR = 1.82, 95% CI 1.53–2.18, P = .000) (Fig. 2). We adopted a fixed-effect model to pool the data and found no obvious between-study heterogeneity (I2 = 42.5%, P for heterogeneity = .052).
Figure 2.
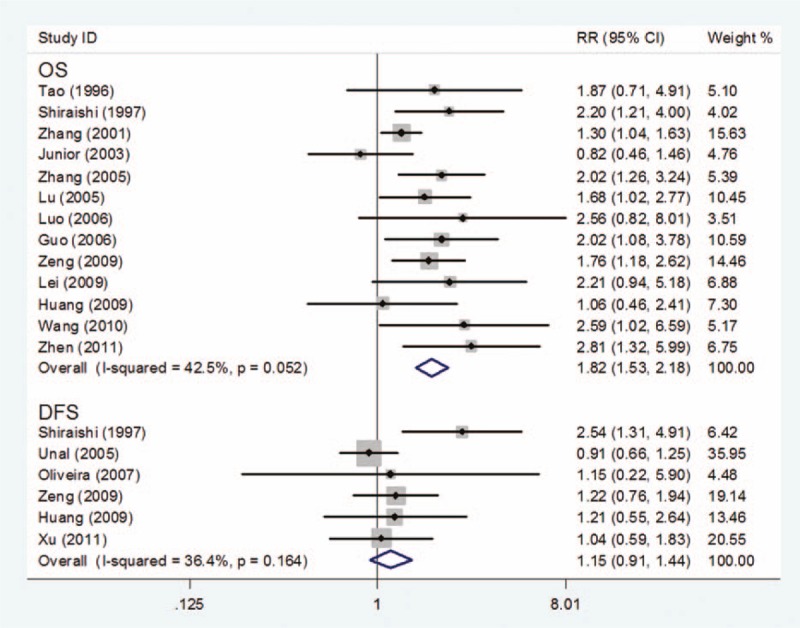
Forest plot of the association of PCNA expression and OS or DFS in osteosarcoma. DFS = disease-free survival, PCNA = proliferating cell nuclear antigen, OS = overall survival.
3.3. Correlation between PCNA expression and DFS
Six studies[17,19–21,29,31] explored the association between PCNA expression and DFS with enough accessible data to calculate the RRs and 95% CI. Meta-analysis of these 6 studies showed no statistical association between PCNA overexpression and DFS of osteosarcoma (RR = 1.15, 95% CI 0.91–1.44, P = .234) (Fig. 3). A fixed-effect model was used for statistical analysis, and no significant heterogeneity was found (I2 = 36.4%, P for heterogeneity = .164).
Figure 3.
Survival outcome at different years by PCNA expression. PCNA = proliferating cell nuclear antigen.
3.4. Subgroup analysis based on different follow-ups
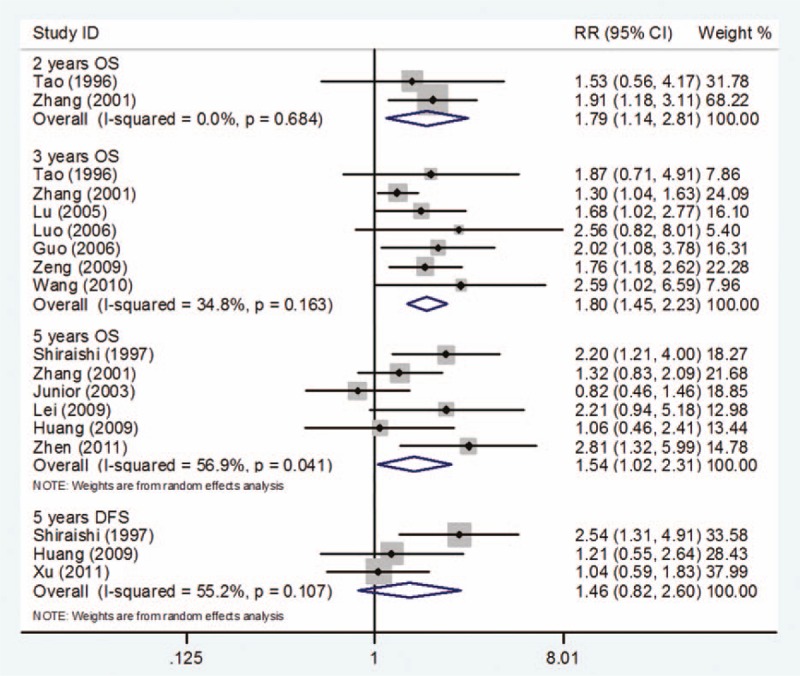
3.4.1. Two-year OS
Only 2 studies[22,23] were searched and included in this subgroup analysis for 2-years OS. Compared with low PCNA expression, high PCNA expression was associated with a poor prognosis of osteosarcoma (RR = 1.79, 95% CI 1.14–2.81, P = .011). There was no evidence of statistically significant heterogeneity with a fixed-effect model (I2 = 0%, P for heterogeneity = .684) (Fig. 3).
3.4.2. Three-year OS
In total, 7 studies[22,23,25–27,29,30] provided data for OS at 3 years. Meta-analysis of these 7 studies suggested that PCNA expression was obviously associated with a lower OS rate in patients with osteosarcoma (RR = 1.80, 95% CI 1.45–2.23; P = .000). No evidence of statistically significant heterogeneity (I2 = 34.8%, P for heterogeneity = .163) was found between the studies (Fig. 3).
3.4.3. Five-year OS and DFS
Six studies[17,18,21,23,28,32] provided data for OS at 5 years. Osteosarcoma patients with low PCNA expression were found to have higher OS than those with high PCNA expression (RR = 1.54, 95% CI 1.02–2.31; P = .038). However, the heterogeneity of between-study was found when a fixed-model was used (I2 = 56.9%, P for heterogeneity = .041), and so, a random-effect model was chosen (Fig. 3).
Only 3 studies[17,21,31] reported DFS information of 5 years. Meta-analysis of these 3 studies showed that PCNA expression status did not influence the 5-year DFS (RR = 1.46, 95% CI 0.82–2.60; P = .194). Evidence of between-study heterogeneity was found (I2 = 55.2%, P for heterogeneity = .142) when a fixed-effect model was used (Fig. 3).
3.5. Subgroup analysis based on different cutoffs to determine PCNA expression
Differences between subgroups were assessed according to cutoffs for defining PCNA expression.
3.5.1. Cutoff >5% in DFS
Only 2 studies[29,31] provided data for DFS with cutoff > 5% for determining PCNA expression. The results suggested that PCNA expression was not associated with DFS in patients with osteosarcoma (RR = 1.13, 95% CI 0.78–1.62; P = 0.527). No evidence of statistically significant heterogeneity (I-square = 0.0%, P for heterogeneity = 0.663) was found between studies (Fig. 4).
Figure 4.
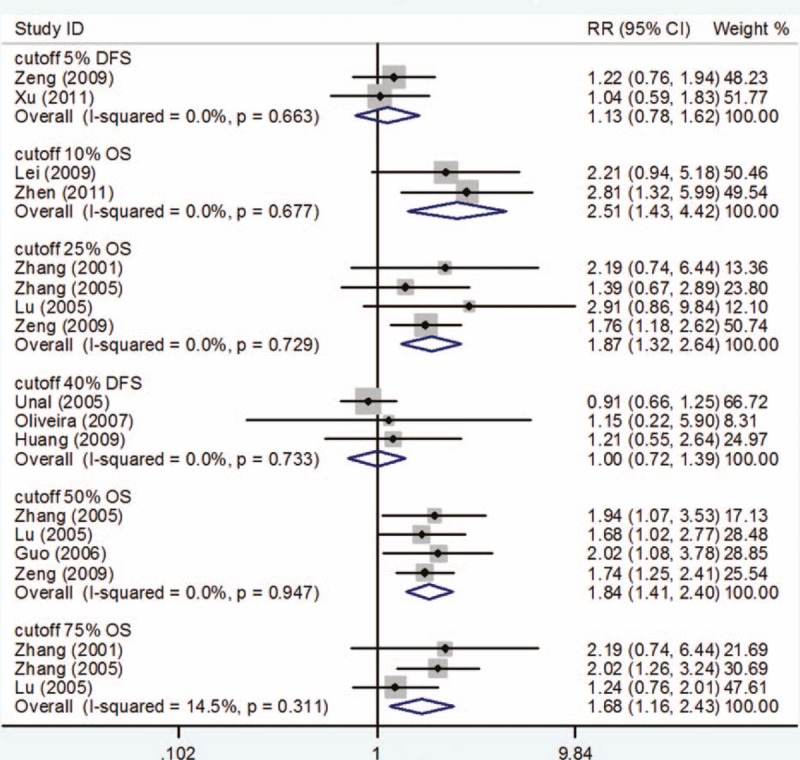
Survival outcome on different cutoffs to determine PCNA positivity. PCNA = proliferating cell nuclear antigen.
3.5.2. Cutoff >10% in OS
Only 2 studies[28,32] reported data for OS with cutoff >10% for determination of PCNA expression. Compared with low PCNA expression, high PCNA expression was associated with poor prognosis for OS (RR = 2.51, 95% CI 1.43–4.42; P = .001). P for heterogeneity was .677 with I2 = 0.0% and no between-study heterogeneity was found (Fig. 4).
3.5.3. Cutoff >25% in OS
With a cutoff >25% for determining PCNA expression, there were 4 studies[23–25,29] that assessed the association between PCNA and OS. Meta-analysis of these 4 studies showed that high PCNA expression was associated with a statistically significantly poor prognosis for OS (RR = 1.87, 95% CI 1.32–2.64; P = .000). No evidence of statistically significant heterogeneity (I2 = 0.0%, P for heterogeneity = .729) was found between studies (Fig. 4).
3.5.4. Cutoff >40% in DFS
Only 3 studies[19–21] provided data for DFS with a cutoff >40% for determining PCNA expression. The result showed that PCNA expression had no correlation with DFS of osteosarcoma (RR = 1.00, 95% CI 0.72–1.39; P = .998). No evidence of statistically significant heterogeneity (I2 = 0.0%, P for heterogeneity = .733) was found between studies (Fig. 4).
3.5.5. Cutoff >50% in OS
In total, 4 studies[24,25,27,29] reported data for OS with a cutoff >50% for determining PCNA expression. Compared with low PCNA expression, high PCNA expression was correlated with a poor prognosis in OS of osteosarcoma (RR = 1.84, 95% CI 1.41–2.40; P = .000). No evidence of statistically significant heterogeneity (I2 = 0.0%, P for heterogeneity = .947) was found between studies (Fig. 4).
3.5.6. Cutoff >75% in OS
Three studies[23–25] provided valuable information for OS with cutoff >75% for determination of PCNA expression. Meta-analysis of these 3 studies showed no statistical association between PCNA expression and OS of osteosarcoma (RR = 1.68, 95% CI 1.16–2.43; P = .006). No evidence of statistically significant heterogeneity (I2 = 14.5%, P for heterogeneity = .311) was found between studies (Fig. 4).
3.6. Sensitivity analyses and publication bias
Sensitivity analyses showed no statistical significance with the result, remaining highly consistent when each study was removed one by one (Fig. 5). Publication bias evaluated by Egger test showed no statistical significance (P = .075 and P = .330, respectively) (Fig. 6).
Figure 5.
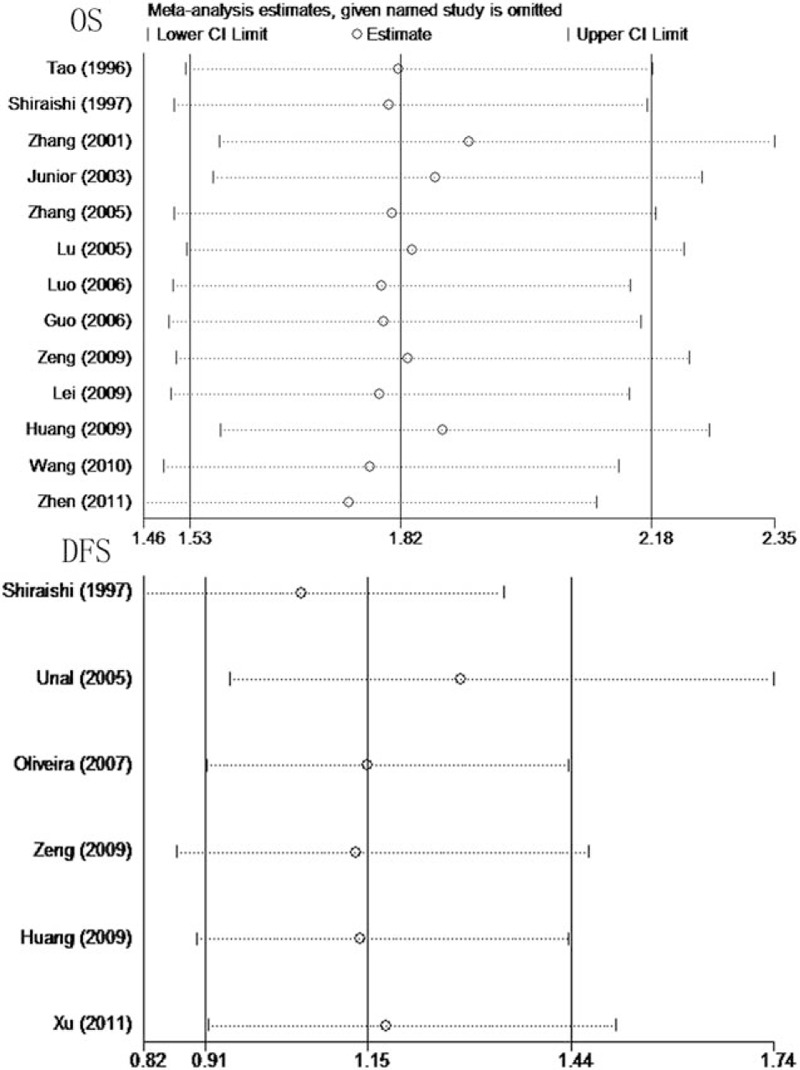
Results of sensitivity analyses for OS and DFS in osteosarcoma. DFS = disease-free survival, OS = overall survival.
Figure 6.
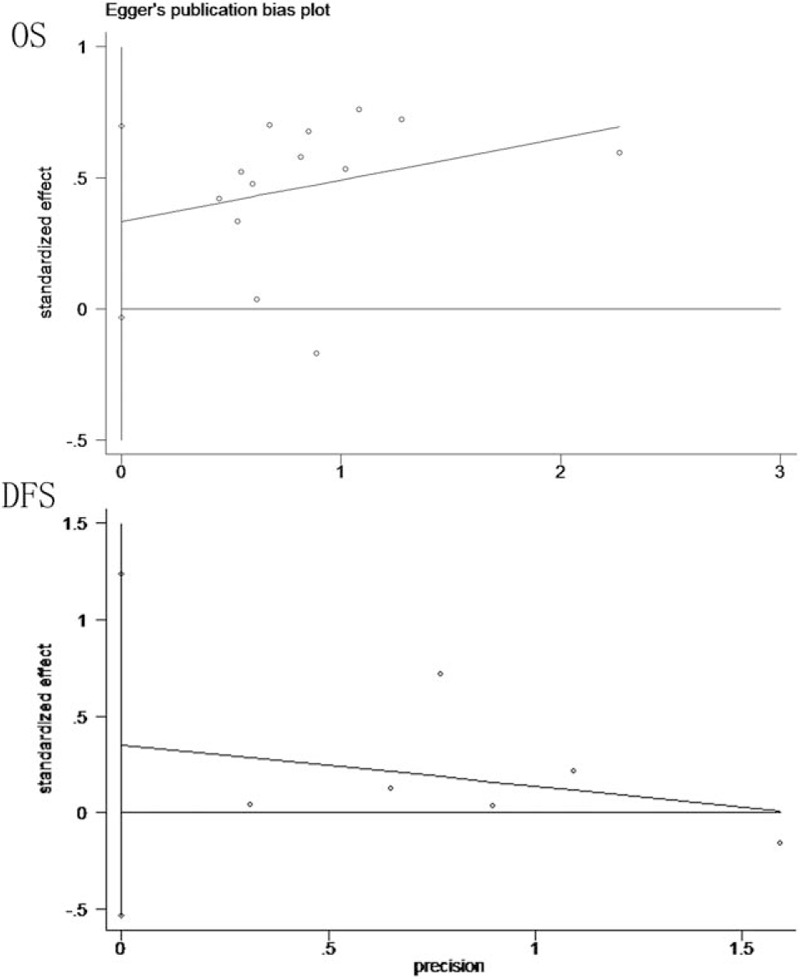
Egger publication bias plot for OS and DFS in osteosarcoma. DFS = disease-free survival, OS = overall survival.
4. Discussion
Osteosarcoma, often occurring in the pediatric age group, is the most common primary malignant bone tumor, and is the second leading cause of cancer-related death in teenagers.[33,34] With improvement of treatment with numerous methods, including surgical resection of primary and tumors distant metastases, radiotherapy, and chemotherapy, the OS rate of osteosarcoma has been significantly improved in the last decade, however, the prognosis for osteosarcoma patients is still poor with low OS. Currently, the ability to predict osteosarcoma prognosis is limited. Identifying the level of malignancy in osteosarcoma is of great importance for clinical decision making. It is important to discover new and reliable markers to predict the prognosis of osteosarcoma. Therefore, increasing attention has been given to explore better prognostic markers and find the best therapeutic targets to improve the accuracy of first diagnosis and identify the prognosis of osteosarcoma.
Multiple studies have attempted to explore the relationship between PCNA expression and OS or DFS in osteosarcoma but the sample size of these studies was small, which weakened the reliability of their result. Currently, the issue remains controversial and whether the result is consistent in subgroup analyses is not known. Systematic review and meta-analysis is a quantitative statistical approach that collects all possible traits of similar studies to resolve controversial issues with appropriate quantitative measures of the average effect, and has been widely used to evaluate multiple biomarkers.[22,23] To the best of our knowledge, no meta-analysis has been performed to resolve this unanswered question by pooling the available data of relevant studies. Therefore, a meta-analysis including high quality studies was needed to comprehensively evaluate the association of PCNA expression and prognosis of osteosarcoma patients. Thus, to assess this association more precisely, we comprehensively researched all relevant published studies to conduct a meta-analysis.
Tumor antigens play an important role in tumor occurrence, development, and metastasis and among these, PCNA is one of the most important. Mathews et al[12] recognized the PCNA and Waseem et al[35] isolated and identified 11 kinds of anti-PCNA antibodies, of which PC-10 (Mo-Ab) is the most widely used. PCNA is a cell cycle regulatory protein, whose expression shows a periodic change with the DNA replication phase, which plays an important role in the cell transition from the G1 to S phase. As a co-factor for DNA polymerase, PCNA regulates the synthesis of the leading and lagging strands, which is essential for DNA duplication.[36,37] Welkoborsky et al[38] reported that PCNA expression is closely tied to the tumor prognosis, which can reflect the biological characteristics of the tumor cells. Based on a study of 49 osteosarcoma cases, Lopes suggested[18] that PCNA expression was significantly associated with clinical stage, histological grade, and poor prognosis of osteosarcoma, which could evaluate tumor cell proliferation, and predict its biological behavior and prognosis.
In our investigation, 16 studies with 691 patients were selected for a meta-analysis. Meta-analysis of 13 studies showed that patients with high PCNA expression had a lower OS than those with low PCNA expression (RR = 1.82, 95% CI 1.53–2.18, P = .000).
In 1997, Shiraishi et al[17] first performed immunohistochemical staining of osteosarcoma specimens with an anti-PCNA monoclonal antibody to describe utility of the PCNA labeling index for predicting prognosis in osteosarcoma.
The PCNA labeling index, p53 expression and p53 labeling index in immunohistochemical stained specimens were simple and feasible indicators of prognosis in osteosarcoma. In our study, 6 studies provided valuable information to estimate the prognostic role of PCNA expression in patients with osteosarcoma at disease-free survival (DFS). Meta-analysis of these 6 studies showed no statistical association between PCNA expression and DFS of osteosarcoma (RR = 1.15, 95% CI 0.91–1.44, P = .234). No obvious between-study heterogeneity was observed for OS and DFS (I2 = 42.5%, P for heterogeneity = .052; I2 = 36.4%, P for heterogeneity = .164, respectively). Sensitivity analysis demonstrated that the results were stable and did not change upon omitting each study. Subgroup analyses based on different follow-up and cutoffs to determine PCNA positivity were performed, and the results were stable and did not change. This meta-analysis demonstrated that osteosarcoma patients with cutoff >10, 25, 50, 75 of PCNA expression were significantly associated with low OS rate. No significant association of DFS and osteosarcoma patients with cutoffs >5 and 40 of PCNA expression was found which is consistent with the previous conclusion. These findings from our meta-analysis facilitated precise assessment of the value of PCNA expression in patients with osteosarcoma.
Several limitations exist in this meta-analysis. First, few studies were included and their sample sizes were also small. Some subgroup analyses with only 2 studies in the meta-analysis may make conclusions less reliable. Second, many studies were excluded because they did not provide enough data to calculate the RR and 95% CI, which might introduce biases in the conclusion. Third, even though we tried to retrieve the data not provided in the original articles from the investigators, some data such as gender, inclusion period and Enneking stage were not accessible. Finally, there was variability in the antibodies used against PCNA and their sources; different studies used antibodies from different companies. Moreover, the stages of osteosarcoma and their follow-up also varied in these investigations. Therefore, heterogeneity was unavoidable.
5. Conclusion
In summary, the findings from this meta-analysis suggested that PCNA expression is an effective biomarker for poor prognosis in patients with osteosarcoma for OS. However, more large-scale studies are needed to further support this conclusion.
Footnotes
Abbreviations: CBM = Chinese Biological Medical, CI = confidence intervals, CNKI = China National Knowledge Internet, DFS = disease-free survival, NOS = Newcastle-Ottawa Scale, OS = overall survival, PCNA = proliferating cell nuclear antigen, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RR = relative ratios.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Bertucci F, Araujo J, Giovannini M. Pancreatic metastasis from osteosarcoma and Ewing sarcoma: literature review. Scand J Gastroenterol 2013;48:4–8. [DOI] [PubMed] [Google Scholar]
- [2].Rosen G, Murphy ML, Huvos AG, et al. Chemotherapy, en bloc resection, and prosthetic bone replacement in the treatment of osteogenic sarcoma. Cancer 1976;37:1–1. [DOI] [PubMed] [Google Scholar]
- [3].Scotlandi K, Serra M, Nicoletti G, et al. Multidrug resistance and malignancy in human osteosarcoma. Cancer Res 1996;56:2434–9. [PubMed] [Google Scholar]
- [4].Gorlick R. Current concepts on the molecular biology of osteosarcoma. Cancer Treat Res 2009;152:467–78. [DOI] [PubMed] [Google Scholar]
- [5].Hagleitner MM, de Bont ES, te Loo DMWM. Survival trends and long-term toxicity in pediatric patients with osteosarcoma. Sarcoma 2012;2012:636405.doi: 10.1155/2012/636405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fuchs B, Zhang K, Schabel A, et al. Identification of twenty-two candidate markers for human osteogenic sarcoma. Gene 2001;278:245–52. [DOI] [PubMed] [Google Scholar]
- [7].Durnali A, Alkis N, Cangur S, et al. Prognostic factors for teenage and adult patients with high-grade osteosarcoma: an analysis of 240 patients. Med Oncol 2013;30:624. [DOI] [PubMed] [Google Scholar]
- [8].Tang Y, Yang C, Guo Z, et al. P16 protein expression as a useful predictive biomarker for neoadjuvant chemotherapy response in patients with high-grade osteosarcoma: A systematic meta-analysis under guideline of PRISMA. Medicine 2017;96:e6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang Q, Liu F, Wang B, et al. HER-2 expression in biopsy and surgical specimen on prognosis of osteosarcoma: a systematic review and meta-analysis of 16 studies. Medicine 2016;95:e3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. J Immunol 1978;121:2228–34. [PubMed] [Google Scholar]
- [11].Bolton WE, Mikulka WR, Healy CG, et al. Expression of proliferation associated antigens in the cell cycle of synchronized mammalian cells. Cytometry 1992;13:117–26. [DOI] [PubMed] [Google Scholar]
- [12].Mathews MB, Bernstein RM, Franza BR, et al. Identity of the proliferating cells nuclear antigen and cycling. Nature 1984;309:374–6. [DOI] [PubMed] [Google Scholar]
- [13].Antonescu CR, Leung DH, Dudas M, et al. Alterations of cell cycle regulators in localized synovial sarcoma: a multifactorial study with prognostic implications. Am J Pathol 2000;156:977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in metaanalyses. Ann Intern Med 2001;135:982. [DOI] [PubMed] [Google Scholar]
- [15].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- [17].Shiraishi H, Kotake T. Usefulness of PCNA labeling index and p53 expression in determining prognosis in osteosarcoma. J Orthop Sci 1997;2:57–63. [Google Scholar]
- [18].Junior AT, de Abreu AF, Pinto CA, et al. Clinicopathological and immunohistochemical analysis of twenty-five head and neck osteosarcomas. Oral Oncol 2003;39:521–30. [DOI] [PubMed] [Google Scholar]
- [19].Unal VS, Ayhan A, Tokgozoglu MA. Proliferating cell nuclear antigen index and nm23 expression in osteosarcoma in relation to disease- free survival and tumor grade. Saudi Med J 2005;26:1475–7. [PubMed] [Google Scholar]
- [20].Oliveira CR, Mendonca BB, Camargo OP, et al. Classical osteoblastoma, atypical osteoblastoma, and osteosarcoma: a comparative study based on clinical, histological, and biological parameters. Clinics (Sao Paulo) 2007;62:167–74. [DOI] [PubMed] [Google Scholar]
- [21].Huang J, Zhu B, Lu L, et al. The expression of novel gene URG4 in osteosarcoma: correlation with patients’ prognosis. Pathology 2009;41:149–54. [DOI] [PubMed] [Google Scholar]
- [22].Tao H, Yang D, Fan S. Clinical significance of measurement of proliferative cell nuclear antigen (PCNA) in osteosarcoma. Chin J Orthop 1996;16:321–3. [Google Scholar]
- [23].Zhang C, Liao W, Li F, et al. Expressions of P21 WAF1 protein, PCNA and Ki-67 and their significance in osteosarcoma. China Oncol 2001;11:109–12. [Google Scholar]
- [24].Zhang GQ, Zheng J, Zhao JC, et al. VEGF and PCNA gene expression and their correlation with microvessel density in human osteosarcoma tissue. J Zhengzhou Univ Med Sci 2005;40:339–41. [Google Scholar]
- [25].Lu WB, Ding H. Study of the expression of PCNA and AgNOR in osteosarcoma and their relationship to the prognosis of this disease. Mod Med Health 2005;21:1328–9. [Google Scholar]
- [26].Luo XZ, Ni JD. Expression and clinical significance of MMP-9 and PCNA in osteosarcoma. J Clin Res 2006;23:682–4. [Google Scholar]
- [27].Guo CX, Wang LM, Guo XW. Study on relation of proliferating cell nuclear antigen and p27 protein to pathological character and prognosis of osteosarcoma. J Med Forum 2006;27:25–7. [Google Scholar]
- [28].Lei NW, Pan XJ, Qi YL, et al. Expressions of P16 and proliferating cell nuclear antigen in osteosarcoma and clinical significances. J Jilin Univ Med Ed 2009;35:519–21. [Google Scholar]
- [29].Zeng XY. Expressions of PCNA and bcl-2 in osteosarcoma and clinical significances. Asia Pac Tradit Med 2009;5:12–3. [Google Scholar]
- [30].Wang GQ. Expressions of caspase-3 and PCNA in osteosarcoma and clinical significances. Bone Setting Tradit Chin Med 2010;22:31–3. [Google Scholar]
- [31].Xu M, Wang W, Yu XC. Cox multivariate regression analysis of survival rate for osteosarcoma. Orthop J China 2011;19:36–9. [Google Scholar]
- [32].Zhen WY, Yu X. Expressions of P27 and PCNA in osteosarcoma and clinical significances. Qiqihar Med J 2011;32:1037–8. [Google Scholar]
- [33].Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol 2010;8:705–18. [PubMed] [Google Scholar]
- [34].Siclari VA, Qin L. Targeting the osteosarcoma cancer stem cell. J Orthop Surg Res 2010;5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Waseem NH, Lane DP. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci 1990;96(part 1):121–9. [DOI] [PubMed] [Google Scholar]
- [36].Gerdes J, Schwab U, Lemke H, et al. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 1983;31:13–20. [DOI] [PubMed] [Google Scholar]
- [37].van Dierendonck JH, Wijsman JH, Keijzer R, et al. Cell-cycle-related staining patterns of anti-proliferating cell nuclear antigen monoclonal antibodies. Comparison with BrdUrd labeling and Ki-67 staining. Am J Pathol 1991;138:1165–72. [PMC free article] [PubMed] [Google Scholar]
- [38].Welkoborsky HJ, Hinni M, Dienes HP, et al. Predicting recurrence and survival in patients with laryngeal cancer by means of DNA cytometry, tumor front grading, and proliferation markers. Ann Otol Rhinol Laryngol 1995;104:503–10. [DOI] [PubMed] [Google Scholar]


