Supplemental Digital Content is available in the text
Keywords: bradycardia, cardiopulmonary resuscitation, epinephrine, hypotension, inferior vena cava
Abstract
Rationale:
Bezold–Jarisch reflex (BJR) occurs when the cardioinhibitory receptors in the walls of ventricles are activated by various stimuli, with typical features of bradycardia, vasorelaxation, and hypotension. This reflex usually happens in parturient intrathecal anesthesia, as a result of decreased venous return by compression of inferior vena cava, but it is only rarely reported during general anesthesia.
Patient concerns:
Severe bradycardia and hypotension, indicating BJR, occurred during the induction of general anesthesia in a 3-month-old female child with giant intra-abdominal teratoma.
Diagnoses:
A giant intra-abdominal teratoma was detected by computed tomography scanning. The decreased left ventricular ejection faction along with increased troponin I and N-terminal pro-B-type natriuretic peptide indicated a preoperative mild cardiac dysfunction. BJR was diagnosed on the basis of the severe bradycardia and hypotension observed during the induction of general anesthesia,
Interventions:
Atropine failed to increase heart rate. Cardiopulmonary resuscitation was initiated immediately and epinephrine was injected intravenously because of sudden circulatory collapse. Soon after the return of spontaneous circulation, a central venous line was placed and invasive blood pressure was monitored. Vital signs and homeostasis were kept stable during teratoma resection.
Outcomes:
The child was extubated after emergence from anesthesia in the operating room. Eleven days later, she had recovered without complications and was discharged.
Lessons:
General anesthesia should be induced with great care in patients with giant intra-abdominal masses, and the patient should be kept in the left-lateral table tilt position before induction.
1. Introduction
The Bezold–Jarisch reflex (BJR) is a cardioinhibitory reflex, which triggers vasovagal syncope simultaneously.[1] It is attributable to activation of the cardioinhibitory receptors in the walls of ventricles, followed by the inhibition of sympathetic outflow.[2,3] The cardioinhibitory receptors become active in a number of clinical situations, including sudden decrease in venous return.[3,4] Bradycardia, vasorelaxation, and hypotension are its typical clinical features.[1,5] However, BJR is only rarely reported during general anesthesia.
After receiving written consent from her parents, we reported a 3-month-old female patient, undergoing elective giant intra-abdominal teratoma resection, who experienced a transient BJR during anesthesia induction.
2. Case report
A 3-month-old female child, weighing 5.5 kg, was scheduled for exploratory laparotomy due to a giant mass occupying the intra-abdominal space. Her parents complained that the child's abdomen has become distended during the previous 2 months, with anorexia in the previous 2 days, but they said she showed no vomiting, diarrhea, astriction, sweating, or dyspnea. No unusual complaints were found in the past, personal, or family histories. Vital signs were stable, with blood pressure around 105/70 mm Hg. Preoperatively, computed tomography scanning revealed a giant intra-abdominal space-occupying mass with a maximal transdiameter of 143 mm × 83 mm, and teratoma was suspected (Fig. 1A). Findings on chest x-ray were normal except for cardiac enlargement. Echocardiography indicated an enlarged left ventricle with decreased systolic function (erection fraction 48%) and moderate mitral regurgitation. Electrocardiogram was normal. Serum troponin I (0.211 ng/L) and N-terminal pro-B-type natriuretic peptide (NT-proBNP >35,000 pg/ml) were significantly high. Alpha-fetoprotein increased to 443.7 ng/L. A mild microcytic hypochromic anemia was diagnosed with hemoglobin concentration 90 g/L. For this reason, 50 mL packed red blood cells (PRBCs) were transfused on the day before surgery. The other laboratory results, including arterial blood gas, electrolytes, liver function, and renal function tests, were almost within the normal range.
Figure 1.
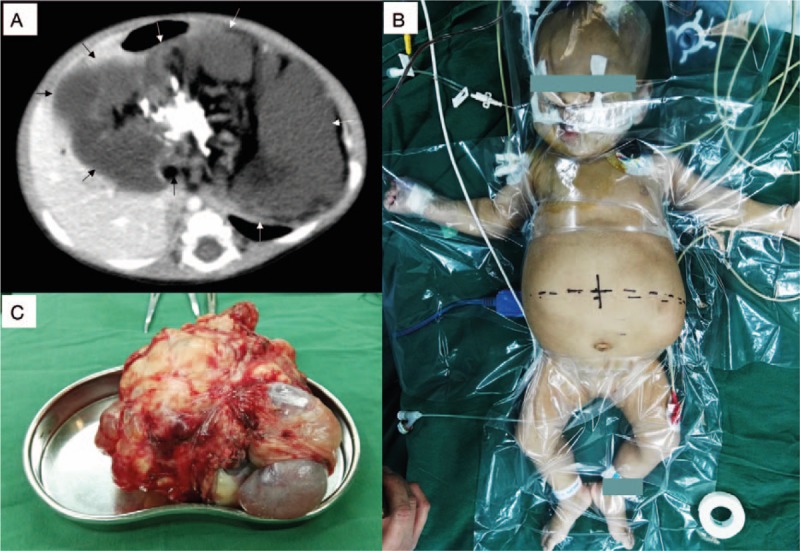
(A) Giant intra-abdominal teratoma. Computed tomography evidence of the teratoma (arrows). (B) Abdomen before operation. (C) Gross morphology of the teratoma.
The child was monitored routinely before induction of anesthesia. The vital signs were stable, with blood pressure 100/50 mm Hg, heart rate 129 beats per minute, and respiratory rate 35 breaths per minute. The abdomen was protuberant and distended (Fig. 1B). A nasogastric tube had been already inserted, and the contents of the stomach were drained out by suction. Then, the child was oxygenated and general anesthesia was induced with cisatracurium 0.2 mg/kg, propofol 2.5 mg/kg, and fentanyl 3.5 μg/kg. The heart rate rapidly decreased below 60 beats per minute in a minute, the blood pressure dropped sharply and became undetectable. The BJR was diagnosed primarily. Atropine (0.02 mg/kg) was injected intravenously, but the heart rate did not increase. Pulsation of common carotid artery was undetectable by palpation. Thus, cardiopulmonary resuscitation (CPR) procedure was initiated with chest compression. Intubation was completed shortly, and 10 μg/kg epinephrine i.v. was administered immediately. After a short while, the spontaneous circulation returned, the heart rate increased above 110 beats per minute, the blood pressure increased to 110/60 mm Hg, and the both vital signs remained stable. The total duration of remarkable bradycardia was about 5 minutes. Then, 2% sevoflurane was inhaled for maintenance of anesthesia.
After that, a double-lumen catheter was placed through right internal jugular vein and central venous pressure was measured. Invasive blood pressure was monitored by right radial artery cannulation. Arterial blood gas and electrolytes were examined, and 20 mL 2.5% sodium bicarbonate was used to normalize metabolic acidosis. Central venous pressure was maintained within 5 to 10 mm Hg during the operation. Dopamine was infused intravenously at a speed of 5 to 7.5 μg/kg/min to improve cardiac function and maintain blood pressure. One hour later, arterial blood gas, hemoglobin concentration, and electrolytes were re-examined. This indicated that metabolic acidosis had been normalized, but hemoglobin dropped to 20% at this time because of blood loss. We immediately transfused 100 mL PRBCs, followed by another 100 mL PRBCs because of ongoing blood loss. Arterial blood gas, together with hemoglobin concentration and electrolytes, were re-examined 80 minutes after the previous examination, which indicated that hemoglobin had already recovered, but metabolic acidosis reoccurred. Then, treatment with 12 mL 0.25% sodium bicarbonate was repeated. Electrolytes were kept within the normal range without any intervention. The operation lasted 160 minutes, and total blood loss was about 170 mL. The tumor was removed intact and confirmed as a cystic and solid mature teratoma by postoperative pathologic diagnosis. Its size was about 180 mm × 150 mm × 130 mm (Fig. 1C) and it was much bigger than that had been estimated. The child was extubated after emergence from anesthesia in the operating room and transferred to the pediatric intensive care unit. Eleven days later, she had recovered without any complications and was discharged. “Timeline of interventions and outcomes” is available in Supplemental figure.
3. Discussion
In this case, severe bradycardia and hypotension, indicating occurrence of BJR, might be attributable to either of 2 causes—inferior vena cava compression and direct cardio-depression.
The afferent limb of the cardioinhibitory receptors in the walls of ventricles is comprised of nonmyelinated, type C vagal fibers.[2] These fibers are responsive to both chemical and mechanical stimuli.[2,6] Mechanical stimuli, such as decreases in cardiac pressure, inotropism, or volume may evoke BJR.[5] Relevant cases have been reported as a result of decreased venous return. These include changes in posture[7,8] and supine hypotensive syndrome in parturient.[9,10]
It is likely that the giant intra-abdominal teratoma caused aortocaval compression in this case. This, in turn, significantly impaired venous return, and the BJR took place subsequently. This situation is very similar to supine hypotensive syndrome in parturient, which is attributable to inferior vena cava compression by a gravid uterus.[1,11] However, induction of anesthesia can decrease central venous pressure in patients with large intra-abdominal tumors.[12] Left-lateral table tilt position or a pelvic wedge has been believed to relieve compression and restore venous return.[13,14] Accordingly, this child should have been placed in left-lateral table tilt position before induction, which may have prevented the BJR.
Preoperative mild cardiac dysfunction was diagnosed as a slight decrease in the left ventricular ejection faction, with significant increase in troponin I and NT-proBNP. However, the cardiac function might be overestimated by the ejection faction value alone, due to the existence of moderate mitral regurgitation. At worst, intravenous propofol, which causes vasodilation and cardio-depression,[15] further aggravates cardiac dysfunction. As mentioned above, impaired cardiac inotropism is another incentive of BJR.[5] These additive effects resulted in profound cardioinhibition. BJR was also reported by Lim et al[16] in a patient with supraventricular tachycardia after administration of midazolam and fentanyl. For this reason, an alternative sedative drug, ketamine, might have been safer for this child, because ketamine was able to stimulate the cardiovascular system, and thus increase blood pressure and heart rate.[15] Etomidate, which can maintain the stability of hemodynamics in pediatric patients,[17] may be another candidate sedative drug for induction.
As stipulated in the 2015 American Heart Association (AHA) updated guidelines for CPR and emergency cardiovascular care,[18] chest compression was performed immediately because the heart rate decreased below 60 beats per minute with unpalpable great artery. In this case, atropine failed to increase the heart rate, and epinephrine was used without hesitation. It happens that there is a similar case with ineffective atropine treatment.[7] At low doses, atropine may cause paradoxical bradycardia. However, 0.02 mg/kg atropine we used was at a high dose for pediatric bradycardia treatment.[19] According to AHA guidelines for CPR, atropine may be repeated 3 to 5 minutes later, but we were not able to wait that long because of the patient's circulatory collapse. Epinephrine is recommended as the first choice for CPR. For another reason, it has potent cardiotonic and vasoconstrictive effects that may improve the cardiac function and restore volume. Ephedrine, another vasoactive drug, has also been recommended for BJR.[1] Atropine, together with ephedrine, may also be a suitable treatment, but we did not attempt it in this particular case.
There were some other insufficiencies in the management of anesthesia in this case. First, the circulatory status was overestimated. The child's blood pressure was around the upper limit of the normal range.[20] The patient's enlarged heart might be a result of gradually increased afterload, followed by an impaired myocardium. However, the patient's relative high blood pressure and lack of symptoms of cardiac dysfunction indicated good circulatory compensation, although she did indeed have cardiac dysfunction. For this reason, we induced general anesthesia in the routine manner. Second, invasive monitoring should have been performed before induction. Invasive monitoring for this child was essential. It is very important to monitor circulatory status and goal-directed fluid therapy during the operation. However, central venous cannulation and arterial cannulation are both very difficult in infants, especially when the infant is awake. Considering this infant had a stable hemodynamics, we planned to perform cannulation after induction of anesthesia. Fortunately, we completed it very quickly. It may be possible to perform cannulation under mild sedation plus local anesthesia before anesthesia induction. Third, appropriate amounts of fluid may be preloaded to optimize volume status. The only venous access was a 24-gauge indwelling needle cannula in the scalp vein, but this could not ensure rapid fluid infusion. Central venous access should be established as discussed above. Preoperatively, this child was fed with milk and vitamins through a nasogastric tube, and fluid therapy was also maintained in the ward. Although the child was fasted before anesthesia, a mixture of 10% glucose 20 mL with 80 mL normal saline was continued from the ward until induction. For this reason, we did not preload additional fluid before induction. The volume status may be assessed by monitoring central venous pressure if a central venous line is inserted, or by measuring the diameter of inferior vena cava with ultrasound. But these evaluations were not performed before anesthesia induction in this case.
4. Conclusions
In conclusion, to avoid BJR, general anesthesia should be induced with great care in patients with giant intra-abdominal masses, and the patient should be kept in the left-lateral table tilt position before induction.
Acknowledgment
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Supplementary Material
Footnotes
Abbreviations: AHA = American Heart Association, BJR = Bezold–Jarisch reflex, CPR = cardiopulmonary resuscitation, NT-proBNP = N-terminal pro-B-type natriuretic peptide, PRBCs = packed red blood cells.
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Supplemental Digital Content is available for this article.
References
- [1].Kinsella SM, Tuckey JP. Perioperative bradycardia and asystole: relationship to vasovagal syncope and the Bezold-Jarisch reflex. Br J Anaesth 2001;86:859–68. [DOI] [PubMed] [Google Scholar]
- [2].Donald DE, Shepherd JT. Reflexes from the heart and lungs: physiological curiosities or important regulatory mechanisms. Cardiovasc Res 1978;12:446–69. [PubMed] [Google Scholar]
- [3].Oberg B, Thoren P. Increased activity in left ventricular receptors during hemorrhage or occlusion of caval veins in the cat. A possible cause of the vaso-vagal reaction. Acta Physiol Scand 1972;85:164–73. [DOI] [PubMed] [Google Scholar]
- [4].Secher NH, Jacobsen J, Friedman DB, et al. Bradycardia during reversible hypovolaemic shock: associated neural reflex mechanisms and clinical implications. Clin Exp Pharmacol Physiol 1992;19:733–43. [DOI] [PubMed] [Google Scholar]
- [5].Campagna JA, Carter C. Clinical relevance of the Bezold-Jarisch reflex. Anesthesiology 2003;98:1250–60. [DOI] [PubMed] [Google Scholar]
- [6].Aviado DM, Guevara Aviado D. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann N Y Acad Sci 2001;940:48–58. [PubMed] [Google Scholar]
- [7].So J, Shin WJ, Shim JH. A cardiovascular collapse occurred in the beach chair position for shoulder arthroscopy under general anesthesia: a case report. Korean J Anesthesiol 2013;64:265–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim YH, Kim DJ, Kim WY. Bezold-Jarisch reflex caused by postural change. J Anesth 2015;29:158. [DOI] [PubMed] [Google Scholar]
- [9].Ou CH, Tsou MY, Ting CK, et al. Occurrence of the Bezold-Jarisch reflex during Cesarean section under spinal anesthesia: a case report. Acta Anaesthesiol Taiwan 2004;42:175–8. [PubMed] [Google Scholar]
- [10].Jang YE, Do SH, Song IA. Vasovagal cardiac arrest during spinal anesthesia for Cesarean section: a case report. Korean J Anesthesiol 2013;64:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kinsella SM, Lohmann G. Supine hypotensive syndrome. Obstet Gynecol 1994;83(5 Pt 1):774–88. [PubMed] [Google Scholar]
- [12].Pilat J, Dabrowski W, Biernacka J, et al. Changes in intra-abdominal, iliac venous and central venous pressures in patients undergoing abdominal surgery due to large tumors of the colon: a pilot study. Acta Clin Croat 2010;49:381–8. [PubMed] [Google Scholar]
- [13].Lee AJ, Landau R, Mattingly JL, et al. Left lateral table tilt for elective cesarean delivery under spinal anesthesia has no effect on neonatal acid-base status: a randomized controlled trial. Anesthesiology 2017;127:241–9. [DOI] [PubMed] [Google Scholar]
- [14].Kinsella SM, Harvey NL. A comparison of the pelvic angle applied using lateral table tilt or a pelvic wedge at elective caesarean section. Anaesthesia 2012;67:1327–31. [DOI] [PubMed] [Google Scholar]
- [15].Vuyk J, Sitsen E, Reekers M. Miller RD, Cohen NH, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL. Intravenous anesthetics. Miller's Anesthesia 8th ed.Philadelphia: Elsevier Saunders; 2015. 821–63. [Google Scholar]
- [16].Lim R, Kilgar J, Cayo S, et al. Complication after treatment for resistant supraventricular tachycardia: the Bezold-Jarisch reflex. Am J Emerg Med 2013;31:1425.e1423-1424. [DOI] [PubMed] [Google Scholar]
- [17].Sarkar M, Laussen PC, Zurakowski D, et al. Hemodynamic responses to etomidate on induction of anesthesia in pediatric patients. Anesth Analg 2005;101:645–50. [table of contents]. [DOI] [PubMed] [Google Scholar]
- [18].Atkins DL, Berger S, Duff JP, et al. Part 11: pediatric basic life support and cardiopulmonary resuscitation quality: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132(18 suppl 2):S519–525. [DOI] [PubMed] [Google Scholar]
- [19].Sivak EL, Hall-Burton DM. Davis PJ, Cladis FP, Motoyama EK. Anesthetic adjuncts. Smith's Anesthesia for Infants and Children 8th ed.Philadelphia, PA: Mosby; 2011. 258–64. [Google Scholar]
- [20].Baum VC, Yuki K, Souza GDd. Davis PJ, Cladis FP, Motoyama EK. Cardiovascular physiology. Smith's Anesthesia for Infants and Children 8th ed.Philadelphia, PA: Mosby; 2011. 73–107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


