Abstract
This study aimed to analyze the correlation of the plasminogen activator inhibitor (PAI-1) gene polymorphisms (rs6092 and rs7242) with susceptibility of osteonecrosis of the femoral head (ONFH).
This case-control study included 106 ONFH patients and 151 healthy controls. PAI-1 polymorphisms were genotyped by polymerase chain reaction (PCR) with direct sequencing. The genotype distribution of polymorphism in the control group was checked with the status of Hardy–Weinberg equilibrium (HWE). The χ2 test was applied to compare the genotypes of polymorphisms between the case and control groups. The association intensity between PAI-1 polymorphisms and ONFH risk was estimated by odds ratios (ORs) and 95% confidence intervals (95% CI). The linkage disequilibrium of PAI-1 polymorphisms was analyzed by Haploview.
We found that the genotypes and alleles of PAI-1 rs6092 and rs7242 polymorphisms had no obvious association with the risk of ONFH (P >.05). But the strong linkage disequilibrium existed between rs6092 and rs7242 polymorphisms and haplotype G-T was significantly associated with the decreased risk of ONFH occurrence (OR = 0.666, 95%CI = 0.445–0.998).
PAI-1 rs6092 and rs7242 polymorphisms are not associated with ONFH development, but haplotype G-T may be a protective factor of ONFH.
Keywords: haplotype, osteonecrosis of the femoral head, PAI-1, polymorphisms
1. Introduction
Osteonecrosis of the femoral head (ONFH), also called as avascular necrosis of the femoral head,[1,2] is a progressive collapse of the femoral head which is mainly induced by interruption in blood supply and anomalies in the fibrinolytic system.[3,4] The disease seriously affected the life quality of patients, especially the young cases.[5] Until now, hip replacement represents the only effective approach for patients with ONFH at the terminal stage.[6] Understanding the etiology of ONFH is necessary for prevention and treatment. Various risk factors are confirmed to be associated with the onset of ONFH, including trauma, abuse of corticosteroids or alcoholism, Gaucher disease, coagulopathies, Caisson disease, inflammatory or autoimmune diseases, etc.[7] However, the pathogenesis of ONFH cannot be completely explained. ONFH is a complex disease caused by many genetic and environmental factors. Studies have showed that ONFH is associated with the decreased fibrinolytic activity because of the levels of plasminogen activator inhibitor (PAI-1).[8] Increased intravascular coagulation is proposed as the pathogenetic mechanism for interruption of the blood supply. A significantly higher prevalence of coagulation abnormalities is reported in patients with ONFH.[9]
PAI-1 is an important factor for coagulation and fibrinolytic system associated with ONFH development.[4] The alteration of fibrinolytic activity mainly be attributed to the alterations in PAI-1 level.[10] PAI-1 protein is a kind of serine protease inhibitor encoded by the PAI-1 gene. PAI-1 is primarily expressed in the vascular endothelial cells, but it also produced by other tissues like adipose tissue. Growing evidences have demonstrated that the concentration of PAI can be significantly affected by some PAI-1 polymorphisms such as 4G/5G.[11,12] Meanwhile, a meta-analysis suggested that the PAI-1 4G/4G genotype might be a risk factor for ONFH.[13] In addition to 4G/5G, some other polymorphisms of the PAI-1 gene were also reported to be involved in pathological processes in human. For instance, Liu et al[14] suggested that the PAI-1 rs7242 GT/GG genotype might be a risk indicator for the occurrence of grade ≥3 radiation pneumonitis. French et al,[15] reported that the PAI-1 rs6092 polymorphism was significantly associated with susceptibility of osteonecrosis among children with acute lymphoblastic leukemia. However, the potential role of PAI-1 rs6092 and rs7242 polymorphisms in ONFH development was unknown.
The purpose of the study was to investigate the effects of genetic variants on risk of ONFH in a Chinese Han population. In the present study, we selected the common single-nucleotide polymorphisms (SNPs) rs6092 and rs7242 in the PAI-1 gene, and the influence of linkage disequilibrium between the 2 polymorphisms on ONFH development was also analyzed.
2. Materials and methods
2.1. Study objects
The present study was a prospective case-control study. A total of 257 subjects including 106 ONFH patients and 151 healthy controls were collected in the present study from October 2014 to October 2015. The cases were confirmed as ONFH by professional doctor according to clinical features and double hip x-ray examination, CT, or MRI examination in the Department of Orthopaedics of Affiliated Hospital of Taishan Medical University. The diagnosis criteria of ONFH were based on Chinese Orthopaedic Association (2012 edition). The patients would be excluded if their conditions met the following criteria: severe trauma in half a year or major operations; taking drugs affecting blood lipid metabolism; nephrotic syndrome; hypothyroidism; diabetes mellitus; In addition, the patients with insufficient clinical data would also be excluded from the present research. In the meanwhile, 151 healthy individuals (88 men and 63 women, mean age 56.2 ± 13.1) were recruited as the control group from the healthy check-up center of the same hospital. The controls were frequency-matched with the cases in age and gender. All the participators were Chinese Han population without any blood relationship.
This study was approved by the ethic committee of Taishan Medical University (201408) on June 10, 2014. Written informed consents were obtained from all participators before blood collection. Sample collection abides by the Declaration of Helsinki. In the meanwhile, the basic characteristics of subjects were recorded by trained doctor through a questionnaire survey or medical records. The information included age, gender, body mass index (BMI), cigarette consumption, and blood stress (hypertension or not). A smoker was defined that people smoked at least 1 cigarette every day and continued half a year. The determination of hypertension was based on the general criteria.
2.2. Blood collection and DNA extraction
A total of 3 mL fasting peripheral venous blood were collected from each participant in early morning. Then genomic DNA was extracted from the isolated blood samples using Biospin Whole Blood Genomic DNA Extraction Kit (Bioer Technology Co., Ltd, China) according to the introductions. The qualified samples were preserved in −20°C.
2.3. Genotyping method
The PAI-1 gene sequence was obtained from Genebank database (Accession: NC_000007.14). According to the primer design optimization principles, the specific primers of rs6092 and rs7242 SNPs were designed by Primer Premier 5.0 software and were synthesized in Shanghai Sangon Biotech Co., Ltd. The detailed primer sequences were as follows, rs6092: 5‘-TGTCTTCCAGAACGATTCCTTCACC-3′ (forward) and 5′-GTTGTCAGCTGGAGCATGGCC-3′ (reverse); rs7242: 5′-CCTCCCCAGAAACAGTGTGCATGGG-3′ (forward) and 5′-AAGAGCTGGGCACGCATCTGGC-3′ (reverse). PCR system was carried out in a volume of 25.0 μL mixture, containing 20 ng genomic DNA, 12.5 μL 2 × PCR mix, 1.0 μL of forward and reverse primers, and ddH2O. PCR reaction conditions were as the following: initial denaturation at 95°C for 5 minutes followed by 35 loops of denaturation at 95 °C for 1 minute, extension at 72°C for 30 seconds and annealing at 55°C for 30 seconds, followed by a final extension at 72°C for 10 minutes. PCR products were detected for the quality and concentration by 1.0% agarose gel electrophoresis and NanoDrop 2000. Then eligible PCR products were sent to Shanghai Sangon Biotech Co., Ltd for sequencing to determine the genotype of each polymorphism in the case and control groups.
2.4. Statistical analysis
Genotype frequencies of PAI-1 polymorphisms in the study were obtained by direct counting. The status of Hardy–Weinberg equilibrium (HWE) was checked through the analysis of genotype distribution in the control group. Quantitative variables were analyzed by the Student t test. The χ2 test was applied to compare the genotypes and alleles frequencies of polymorphisms between the case and control groups. Relationship strength of PAI-1 polymorphisms and the risk of ONFH were presented by odds ratio (OR) with 95% confidence intervals (95% CI). All the statistical analyses were performed using SPSS 18.0 software. Moreover, linkage disequilibrium between rs6092 and rs7242 polymorphisms was assessed by Haploview software. Significant statistical level was set as P <.05 and all statistical tests were 2-sided.
3. Results
3.1. Baseline characteristics of study objects
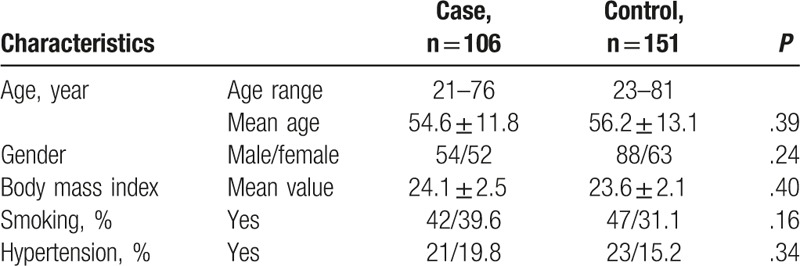
The basic characteristics of subjects in the case and control groups are listed in Table 1. The age of ONFH patients was from 21 to 76 years with the mean age of 54.6 ± 11.8 years and that of healthy controls was from 23 to 81 years with the mean age 56.2 ± 13.1 years. The gender distribution in the 2 groups was 54/52 and 88/63 (male/females). There was no significant difference between the 2 groups in the distribution of age and gender (P >.05 for both). The BMI in cases (24.1 ± 2.5) and controls (23.6 ± 2.1) was not significantly different, either (P = .40). In this study population, smoking and hypertension were not the influence factors of the ONFH occurrence risk (P = .16 and 0.34, respectively).
Table 1.
The baseline characteristics of subjects in the case and control groups.
3.2. Association of PAI-1 gene polymorphisms with the risk of ONFH
Genotype and allele distributions of rs6092 and rs7242 polymorphisms were in accordance with HWE test in control group (P = .071 and .223). The goodness of fit revealed the representative of the subjects.
In ONFH patients, rs6092 GG, GA, and AA genotype frequencies were 62.26%, 27.36%, and 10.38%, respectively, and were 63.58%, 29.14%, and 7.28% in controls. Mutant allele frequencies of the rs6092 polymorphism were 24.06% in cases and 21.85% in controls. The genotype and allele frequencies of the rs6092 polymorphism had no significant differences between cases and controls (Table 2, P >.05 for all). These data showed that the rs6092 polymorphism was not significantly related to the risk of ONFH.
Table 2.
Association of PAI-1 gene polymorphisms with the risk of ONFH.
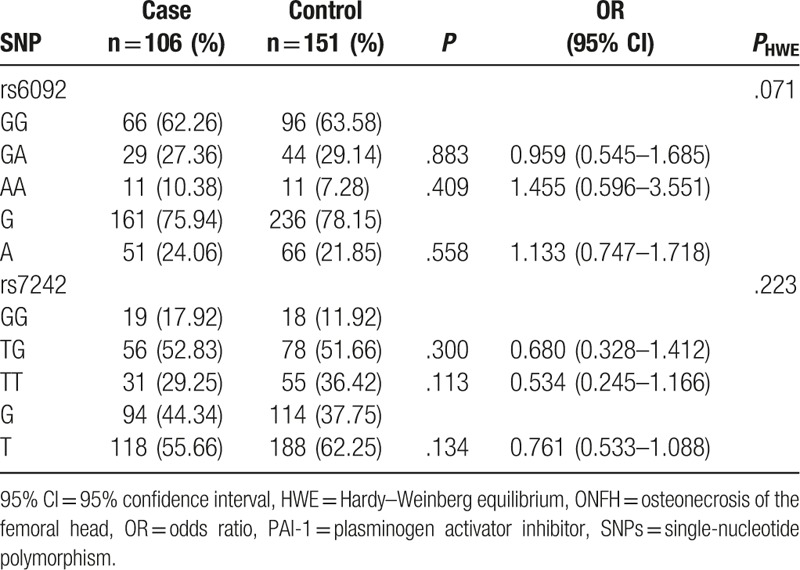
For the rs7242 polymorphism, the TG or TT genotype had no statistically significant differences between case and control groups (Table 2, P >.05 for both). Allele T frequency was higher in controls than that in cases, but the difference was not significant (P = .134). Based on the above results, we indicated that the rs7242 polymorphism had no significant association with the risk of ONFH.
3.3. Haplotype analysis of PAI-1 gene rs6092 and rs7242 polymorphisms in ONFH patients
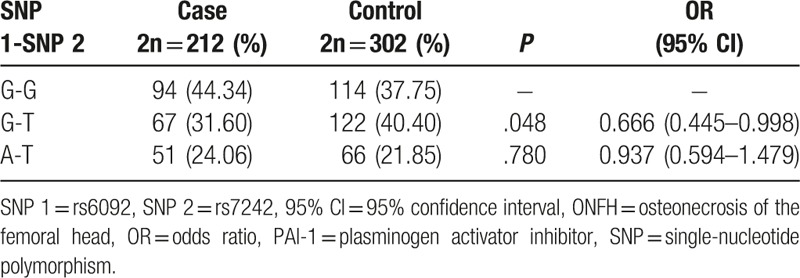
According to Haploview software, the strong linkage disequilibrium was observed between rs6092 and rs7242 polymorphisms (D′ = 1.0, r2 = 0.155). These 2 SNPs constituted 3 haplotypes (G-G, G-T, and A-T). G-T haplotype was obviously of lower frequency in cases than that in controls (Table 3, P = .048), while A-T haplotye had no significant difference between the 2 groups (P >.05), compared with the common haplotype G-G. So the G-T haplotype might be the protective factor for the ONFH occurrence (OR = 0.666, 95% CI = 0.445–0.998).
Table 3.
Haplotype analysis of PAI-1 gene rs6092 and rs7242 polymorphisms in ONFH patients.
4. Discussion
ONFH is a common hip joint disease. Pathological evolution of ONFH may be as the following: abnormal blood supply, which is caused by different risk factors, leads to the degeneration and necrosis of subchondral bone, and then results in the subsite of the femoral head.[16] ONFH can happen in any age, especially in young or middle-age adults. ONFH reduces quality of life, and causes heavy economic toll for the family and society. However, so far, there was no effective therapeutic strategy for patients with ONFH.[17,18] Prevention is a pivotal approach for management of ONFH. Thus, we aimed to identify the high risk population of ONFH through SNPs technology in the present study.
Some research works reported that the abuse of glucocorticoid is the risk factor for the occurrence of ONFH.[19,20] Glucocorticoid could increase the plasma levels of the PAI-1 antigen in ONFH patients.[20] In addition, thrombosis is one of the common causes of abnormal blood supply, and the abnormal blood supply will lead to the occurrence of ONFH. When thrombus occurs, PAI-1 is released by platelet and endothelial cells to inhibit the tissue plasminogen activator-mediated activation of plasma plasminogen, which finally leads to the stability of thrombosis formation, then keep the balance between wound healing and vascular patency.[21–23] All the researches demonstrated that PAI-1 level was significantly associated with the onset and development of ONFH. Relevant studies indicated that PAI-1 polymorphisms could alter the expression of this gene,[10] and even break the balance between thrombosis and fibrinolysis. Thrombosis formation is influenced by PAI-1 gene polymorphisms.[24] Accumulating evidences have suggested that the PAI-1 gene 4G/5G polymorphism might be an etiological factor for ON occurrence in renal transplant patients,[25] post-SARS patients[26] and glucocorticoid-induced ON patients.[27] There also other polymorphisms exist in the PAI-1 gene, such as rs6092 (exon 1) and rs7242 (3′UTR). But the association of these SNPs with the development of ONFH has not been exactly researched.
In present study, we found that all of the genotype and allele frequencies of rs6092 and rs7242 SNPs had no obvious difference between the cases and controls, which indicated that no significant association existed between the 2 SNPs with the occurrence of ONFH. This result was in accordance with a previous study.[28] But French et al[15] suggested that the rs6092 SNP could predict the risk of ON in acute lymphoblastic leukemia children. There was not research about the association of rs7242 and ONFH. But Huang et al[29] suggested that rs7242 did not relate to the occurrence of stroke. Stroke is also caused by abnormal blood supply, so this research confirmed our study from another point of view. A single mutation could not represent the whole gene, so we detected the interaction between these 2 polymorphisms in ONFH patients. Then our data demonstrated that the strong linkage disequilibrium existed between rs6092 and rs7242 SNPs, and the G-T haplotype was correlated to 0.666-fold decreased risk of ONFH. The results showed that when interacted with the rs6092G allele, the rs7242T allele had statistical significance with ONFH. This is the first time to report the influence of PAI-1 rs6092 and rs7242 on the ONFH development in a Chinese population.
There were still several limitations in the present study. First, the small sample size was relatively small in the present study. Second, the information for ethnic was not considered, which might affect the results of our study. Third, the confounding factors like environmental factors were neglected in this study. Therefore, the conclusion in our study should be verified by further studies.
In conclusion, G-T haplotype of rs6092 and rs7242 SNPs may be the protective factor against the onset of ONFH in Chinese Han population.
Footnotes
Abbreviations: 95%CI = 95% confidence interval, ANFH = avascular necrosis of the femoral head, BMI = body mass index, HWE = Hardy–Weinberg equilibrium, MRI = magnetic resonance imaging, ON = osteonecrosis, ONFH = osteonecrosis of the femoral head, OR = odds ratio, PAI-1 = plasminogen activator inhibitor, PCR = polymerase chain reaction, SNPs = single-nucleotide polymorphisms, tPA = tissue plasminogen activator.
The authors have no conflicts of interest to disclose.
References
- [1].Chotivichit A, Korwutthikulrangsri E, Auewarakul C, et al. Core decompression and concentrated autologous bone marrow injection for treatment of osteonecrosis of the femoral head. J Med Assoc Thailand 2012;95(suppl 9):S14–20. [PubMed] [Google Scholar]
- [2].Kaushik AP, Das A, Cui Q. Osteonecrosis of the femoral head: an update in year 2012. World J Orthop 2012;3:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu RW, Wang FS, Ko JY, et al. Comparative serum proteome expression of osteonecrosis of the femoral head in adults. Bone 2008;43:561–6. [DOI] [PubMed] [Google Scholar]
- [4].Lee JS, Koo KH, Ha YC, et al. Role of thrombotic and fibrinolytic disorders in osteonecrosis of the femoral head. Clin Orthop Rel Res 2003;417:270–6. [DOI] [PubMed] [Google Scholar]
- [5].Seki T, Hasegawa Y, Masui T, et al. Quality of life following femoral osteotomy and total hip arthroplasty for nontraumatic osteonecrosis of the femoral head. J Orthop Sci 2008;13:116–21.18392915 [Google Scholar]
- [6].Issa K, Pivec R, Kapadia BH, et al. Osteonecrosis of the femoral head: the total hip replacement solution. Bone Joint J 2013;95-B(11 suppl A):46–50. [DOI] [PubMed] [Google Scholar]
- [7].Zhao DW, Yu XB. Core decompression treatment of early-stage osteonecrosis of femoral head resulted from venous stasis or artery blood supply insufficiency. J Surg Res 2015;194:614–21. [DOI] [PubMed] [Google Scholar]
- [8].Zalavras C, Dailiana Z, Elisaf M, et al. Potential aetiological factors concerning the development of osteonecrosis of the femoral head. Eur J Clin Invest 2000;30:215–21. [DOI] [PubMed] [Google Scholar]
- [9].Pouya F, Kerachian MA. Avascular necrosis of the femoral head: are any genes involved? Arch Bone Joint Surg 2015;3:149–55. [PMC free article] [PubMed] [Google Scholar]
- [10].Liguori R, Quaranta S, Di Fiore R, et al. A novel polymorphism in the PAI-1 gene promoter enhances gene expression. A novel pro-thrombotic risk factor? Thromb Res 2014;134:1229–33. [DOI] [PubMed] [Google Scholar]
- [11].Chi YF, Chai JK, Yu YM, et al. Association between PAI-1 polymorphisms and plasma PAI-1 level with sepsis in severely burned patients. Genet Mol Rese 2015;14:10081–6. [DOI] [PubMed] [Google Scholar]
- [12].Sogutlu Sari E, Yazici A, Eser B, et al. The prevalence of 4G/5G polymorphism of plasminogen activator inhibitor-1 (PAI-1) gene in central serous chorioretinopathy and its association with plasma PAI-1 levels. Cutaneous Ocul Toxicol 2014;33:270–4. [DOI] [PubMed] [Google Scholar]
- [13].Liang XN, Xie L, Cheng JW, et al. Association between PAI-1 4G/5G polymorphisms and osteonecrosis of femoral head: a meta-analysis. Thromb Res 2013;132:158–63. [DOI] [PubMed] [Google Scholar]
- [14].Liu B, Tang Y, Yi M, et al. Genetic variants in the plasminogen activator inhibitor-1 gene are associated with an increased risk of radiation pneumonitis in lung cancer patients. Cancer Med 2017;6:681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].French D, Hamilton LH, Mattano LA, Jr, et al. A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 2008;111:4496–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shah KN, Racine J, Jones LC, et al. Pathophysiology and risk factors for osteonecrosis. Curr Rev Musculoskel Med 2015;8:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang C, Peng J, Lu S. Summary of the various treatments for osteonecrosis of the femoral head by mechanism: a review. Exp Ther Med 2014;8:700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shah SN, Kapoor CS, Jhaveri MR, et al. Analysis of outcome of avascular necrosis of femoral head treated by core decompression and bone grafting. J Clin Orthop Trauma 2015;6:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dong YL, Zhou L, Li YL, et al. Establishment and assessment of rat models of glucocorticoid-induced osteonecrosis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao Acta Academiae Medicinae Sinicae 2015;37:152–6. [DOI] [PubMed] [Google Scholar]
- [20].Kerachian MA, Seguin C, Harvey EJ. Glucocorticoids in osteonecrosis of the femoral head: a new understanding of the mechanisms of action. J Steroid Biochem Mol Biol 2009;114:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brogren H, Karlsson L, Andersson M, et al. Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood 2004;104:3943–8. [DOI] [PubMed] [Google Scholar]
- [22].Al-Horani RA. Serpin regulation of fibrinolytic system: implications for therapeutic applications in cardiovascular diseases. Cardiovasc Hematol Agents Med Chem 2014;12:91–125. [DOI] [PubMed] [Google Scholar]
- [23].Chapman MP, Moore EE, Moore HB, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg 2015;80:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hosseini S, Kalantar E, Hosseini MS, et al. Genetic risk factors in patients with deep venous thrombosis, a retrospective case control study on Iranian population. Thromb J 2015;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ferrari P, Schroeder V, Anderson S, et al. Association of plasminogen activator inhibitor-1 genotype with avascular osteonecrosis in steroid-treated renal allograft recipients. Transplantation 2002;74:1147–52. [DOI] [PubMed] [Google Scholar]
- [26].Sun W, Li Z, Shi Z, et al. Relationship between post-SARS osteonecrosis and PAI-1 4G/5G gene polymorphisms. Eur J Orthop Surg Traumatol 2014;24:525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gong LL, Fang LH, Wang HY, et al. Genetic risk factors for glucocorticoid-induced osteonecrosis: a meta-analysis. Steroids 2013;78:401–8. [DOI] [PubMed] [Google Scholar]
- [28].Bond J, Adams S, Richards S, et al. Polymorphism in the PAI-1 (SERPINE1) gene and the risk of osteonecrosis in children with acute lymphoblastic leukemia. Blood 2011;118:2632–3. [DOI] [PubMed] [Google Scholar]
- [29].Huang X, Li Y, Huang Z, et al. Pai-1 gene variants and COC use are associated with stroke risk: a case-control study in the Han Chinese women. J Mol Neurosci 2014;54:803–10. [DOI] [PubMed] [Google Scholar]


