Abstract
The aim of the study is to evaluate the use of the tumor border in peripheral non-small cell lung cancer (NSCLC) as an indicator of pleural invasion.
This retrospective study was performed at a single tertiary center. The analysis of 136 patients with peripheral NSCLC included 101 (74.3%) patients with pathologically proven pleural invasion and 35 (25.7%) patients without pleural invasion. The tumor borders on conventional computed tomography (CT) were classified into 5 types on lung window setting: type 1, S or reverse S border with a blunt angle; type 2, sharp angle; type 3, concave border with a blunt angle; type 4, straight border with a perpendicular angle; and type 5, convex border with a perpendicular or blunt angle. In patients with more than 1 tumor border type, the priority was type 5, 4, 3, 2, and 1. Blunt angle, pleural contact >3 cm, and adjacent pleural thickening were also recorded for comparison with pleural invasion of peripheral tumors.
Tumor border types 2 and 5 significantly differed between patients with and without pleural invasion (P = .001 and P < .001, respectively). Patients with and without pleural invasion did not significantly differ in tumor border type 1, tumor border type 3, tumor border type 4, blunt angle, pleural contact >3 cm, or pleural thickening. Tumor border type 5 was a moderate indicator of pleural invasion with positive LR, 5.20; accuracy, 57%; sensitivity, 45%; specificity, 91%; PPV, 94%; and NPV, 36%. Tumor border type 2 was a weak indicator of pleural invasion with positive LR, 0.51; accuracy, 34%; sensitivity, 34%; specificity, 34%; PPV, 60%; and NPV, 15%.
Tumor border type 5 has a high PPV and high specificity for predicting pleural invasion by peripheral NSCLC.
Keywords: computed tomography, lung cancer, pleural invasion
1. Introduction
Lung cancer is among common cancers and is the most common cause of cancer-related death worldwide.[1] Approximately 85% of lung cancers are non-small cell lung cancers (NSCLCs).[2] Surgical resection is the best curative treatment in patients with early stage (stages I and II) lung cancer.[3] Unfortunately, most NSCLC patients present with either locally advanced or metastatic disease, and only 25% to 30% of patients are eventually suitable for surgical resection with a curative intent.[3] For patients with T1-sized (≤3 cm) lung cancer, prognosis depends on whether pleural invasion has occurred, and on the depth of invasion. Five-year survival rates for patient with surgically resected NSCLC are 86% for patients without pleural invasion, 62% to 70% for patients with visceral pleural invasion, and 57% for patients with parietal pleural invasion.[4]
Although computed tomography (CT) is widely used for staging lung cancer, its use for diagnosing pleural invasion by lung cancer is limited because contiguity of the tumor with the pleural surface is not necessarily equivalent to invasion.[5] Bony destruction with or without soft tissue mass extending into the chest wall is the only CT finding with a 100% positive predictive value (PPV) for detecting parietal pleura and chest wall invasion.[6]
Proposed predictors of pleural invasion include the presence of blunt angles at the point of contact between the tumor and pleura, contact of >3 cm between the tumor and pleural surface, pleural thickening adjacent to the tumor, and increased density of extrapleural fat.[7–10] However, these signs can also be misleading because they can result from inflammation and fibrosis rather than from tumor invasion. To the best of our knowledge, there is no previous study showing tumor border as an indicator of lung cancer invasion. We postulated 2 indicators of pleural invasion by a tumor: a concave border of a peripheral tumor contaminated by atelectasis or pneumonia and, contrarily, a convex border composed of a space occupying tumor. The purpose of this study was to evaluate the use of the tumor border in peripheral NSCLC as an indicator of pleural invasion.
2. Materials and methods
2.1. Patients
The protocol of this study received institutional review board approval, and the need for informed consent of patients was waived.
This study retrospectively reviewed all patients who had received surgical intervention for NSCLC between January 2012 and August 2015 at a single tertiary center. The inclusion criteria were an available pathology report describing the condition of the lung and assessing the degree of pleural invasion based on orcein staining; lobectomy and margin-free segmentectomy or wedge resection; preoperative CT images on a radiology picture archiving and communication system (PACS) (EBM, Taipei, Taiwan); and peripheral tumors abutting the pleural surface. Patients were excluded if preoperative images were unavailable on the radiology PACS or if the interval between CT imaging and subsequent surgery exceeded 3 months.
2.2. Pathologic analysis
Pathologic specimens were stained with hematoxylin-eosin and orcein to investigate the presence and extent of pleural tumor invasion. Four pathologists with a median of 9.5 years (6–26 years) of experience in pulmonary pathology independently staged the pleural invasion in each patient according to the seventh edition of the TNM staging system as follows: PL0, no pleural involvement; PL1, tumor invasion of the elastic layer of the visceral pleura but without reaching the visceral pleural surface; PL2, tumor invasion to the visceral pleural surface; PL3, tumor invasion of the parietal pleura or chest wall.[11]
2.3. CT imaging
Pathological records and CT images were available for 334 patients who had received lobectomy, margin-free segmentectomy, or wedge resection. After excluding 198 patients whose tumors did not abut pleural surface on CT images, the final analysis included 136 patients. In 118 (86.8%) patients, CT examinations were performed at our hospital using 1 of the 4 CT systems: Siemens Medical Systems, Forscheim, Germany (46 patients); Toshiba Aquilion one TSX-301, Nasu, Japan (44 patients); Brilliance 64, Philips Medical Systems, Haifa, Israel (16 patients); and Optima CT 660, GE, Japan (12 patients). In the remaining 18 (13.2%) patients, CT examinations were performed at the other 9 hospitals. The CT parameters were as follows: detector collimation, 0.6 to 1.25 mm; beam pitch, 1.1 to 1.4; rotation time, 0.5 to 0.8 seconds; tube voltage, 120 kVp; tube current, 110 to 300 mA; and a reconstruction kernel with a high-frequency algorithm. Reconstruction thickness was contiguous 5 mm in axial, coronal, and sagittal sections. Additionally, the reconstruction thickness and interval for thin slices of axial section were 1.0 and 5.0 mm, respectively, in 65 (47.8%) patients; contiguous 2 mm in 44 (32.4%) patients, and 1.25 and 5.0 mm, respectively, in 12 (8.8%) patients. The other 18 (13.2%) patients did not undergo additional thin slice imaging. Each CT image extended from the lower neck to the adrenal gland level. Nonionic contrast medium (Ultravist 300; Schering, Berlin, Germany) at a dose of 2 mL/kg was used for CT examination in 84 (61.8%) patients. The remaining 52 (38.2%) patients did not receive intravenous contrast medium due to renal function impairment, history of asthma, history of allergy to iodine contrast agent, or other reasons. All images were displayed at window settings for lung (center, 600 HU; width, 1500 HU) and soft tissue (center, 40 HU; width, 400 HU).
2.4. Imaging evaluation on CT
A radiologist with 10 years of experience in thoracic radiology reviewed each medical record, measured the tumor size (maximum tumor diameter) and used the annotation function in the radiology PACS to mark targets for CT. This marking system enabled accurate tracking of targets on preoperative imaging. The radiologist also evaluated the presence of 3 CT signs of pleural invasion: blunt angle, pleural contact >3 cm, and adjacent pleural thickening. The blunt angle and pleural contact >3 cm were assessed by lung window imaging and pleural thickening was assessed by soft tissue window imaging. The conventional criteria were used to define chest wall invasion, that is, at least 2 of the aforementioned CT signs.[8,9] The other 2 radiologists with 21 and 24 years of thoracic imaging experience, respectively, independently interpreted target lesions on CT at each examination. Both radiologists were aware that the patients had NSCLC but were blinded to the details of their pathologic reports. The tumor border was assessed on the single tumor image that provided the maximum pleural attachment on lung window imaging. The tumor borders were classified into the 5 types shown in Figures 1 and 2 (type 1: S or reverse S border with a blunt angle of the tumor on pleura; type 2, sharp angle; type 3, concave border with a blunt angle; type 4, straight border with a perpendicular angle; and type 5, convex border with a perpendicular or blunt angle). In patients with more than 1 tumor border type, the tumor borders were prioritized in descending order of complexity (type 5, type 4, type 3, type 2, and type 1). For example, a patient who had both types 5 and 3 tumor borders was categorized as type 5. The tumor border types were also checked for consistency with the description of pleural invasion in the pathologic report.
Figure 1.
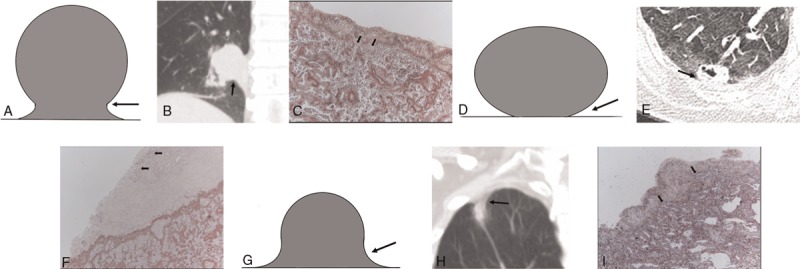
Pictograms, CT images, and photopathologic features of various tumor borders. (A–C) Representative type 1, S or reverse S border with a blunt angle (arrow), in 80-y-old male with adenocarcinoma (arrows) invading of the elastic layer of the visceral pleura but without reaching the visceral pleural surface (PL1) (orcein stain, magnification ×40). (D–F) Type 2, sharp angle (arrow), in 54-y-old female with adenocarcinoma (arrows) invading the visceral pleural surface (PL2) (orcein stain, magnification ×40). (G–I) Type 3, concave border with a blunt angle (arrow), in 58-y-old male with adenocarcinoma (arrows) invading the elastic layer of the visceral pleura (PL1) (orcein stain, magnification ×40).
Figure 2.
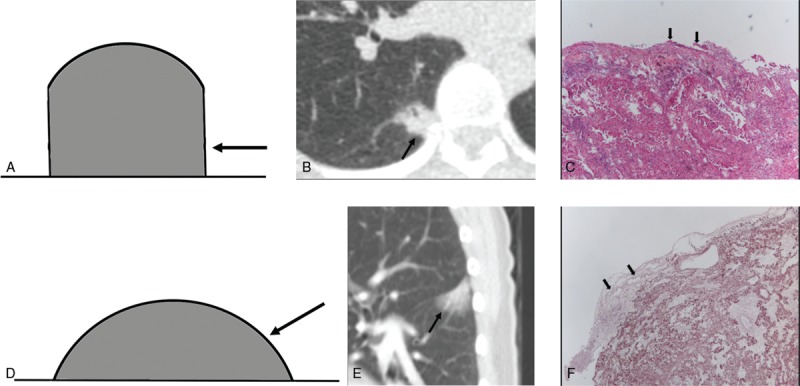
Pictograms, CT images, and photopathologic features of various tumor borders. (A–C) Type 4, straight border with a perpendicular angle (arrow), in 51-y-old female with adenocarcinoma (arrows) invading the visceral pleural surface (PL2) (hematoxylin-eosin stain, magnification ×40). (D–F) Type 5, convex border with a perpendicular or blunt angle (arrow), in 60-y-old woman with adenocarcinoma (arrows) invading the visceral pleural surface (PL2) (orcein stain, magnification ×40).
2.5. Statistical analysis
The Cohen kappa, κ, statistical analysis for categorical data, was performed to determine interobserver agreement. Kappa result was interpreted as follows: values ≤0 as indicating no agreement and 0.01 to 0.20 as none to slight, 0.21 to 0.40 as fair, 0.41 to 0.60 as moderate, 0.61 to 0.80 as substantial, and 0.81 to 1.00 as almost perfect agreement. The Kolmogorov-Smirnov test was used to evaluate whether or not the measurable variables were normally distributed. The χ2 test or Fisher exact test was used to examine the differences between tumor characteristics and pleural status of peripheral NSCLC. Diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and positive likelihood ratio (LR) were calculated separately according to the presence or absence of tumor characteristics. Positive LRs were interpreted as evidence supporting disease diagnosis: >10, strong evidence; 5 to 10, moderate evidence; <5, weak evidence.[12] Factors significantly associated with pleural invasion were also identified by binomial logistic regression analysis. Independent variables, including age, gender, tumor size, tumor histology results, tumor borders, blunt angle, pleural contact >3 cm, associated pleural thickening, and conventional criteria for chest wall invasion were included in the model by forced entry approach. A P value <.05 was considered statistically significant. All data analyses were performed with SPSS statistical software (version 17.0 J; SPSS, Chicago, IL).
3. Results
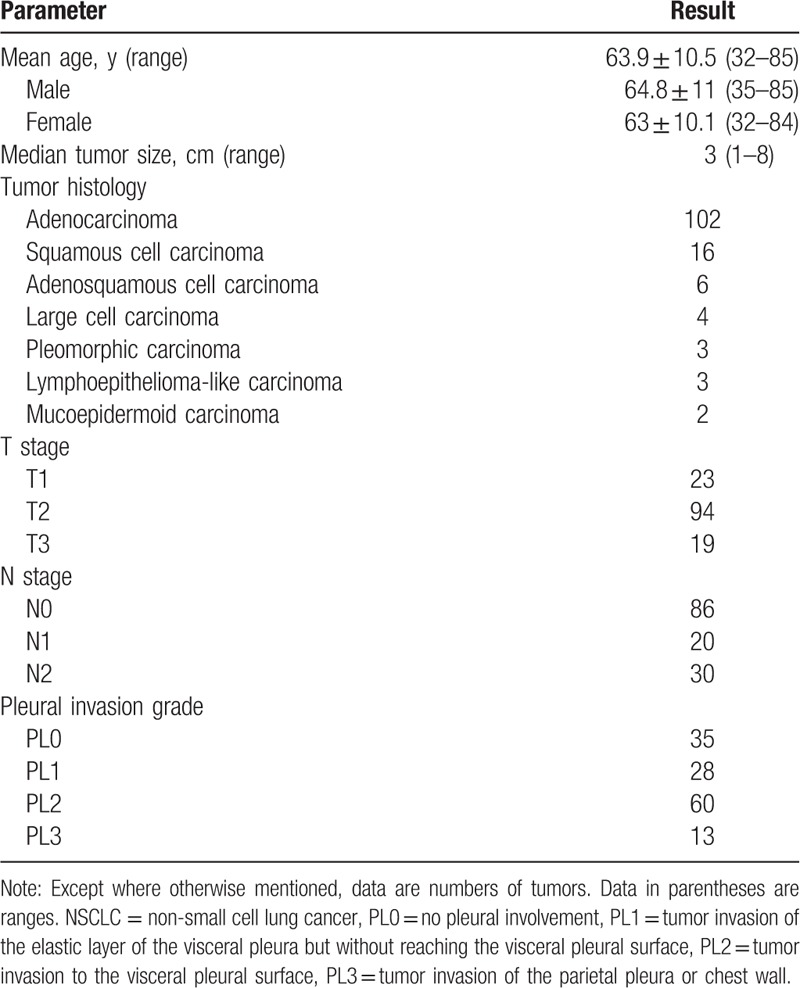
One hundred thirty six patients (66 men and 70 women; mean age, 63.9 years ± 10.5; age range, 32–85 years) were enrolled in this study. Mean age did not significantly differ between men (64.8 years ± 11; range, 35–85) and women (63 years ± 10.1; range, 32–84) (Table 1). The median number of days from diagnostic CT scan to surgery was 24 days (range: 2–86 days). Specimens were obtained by lobectomy in 97 patients, segmentectomy in 11 patients and wedge resection in 28 patients. Histologically, the NSCLCs were diagnosed as adenocarcinoma (n = 102; 75.0%), squamous cell carcinoma (n = 16; 11.8%), adenosquamous cell carcinoma (n = 6; 4.4%), large cell carcinoma (n = 4; 2.9%), pleomorphic carcinoma (n = 3; 2.2%), lymphoepithelioma-like carcinoma (n = 3; 2.2%) or mucoepidermoid carcinoma (n = 2; 1.5%). In 101(74.3%) patients, pleural invasion was confirmed by pathologic analysis (PL1–3), and 25 (25.7%) patients had no pleural invasion (PL0). Pleural invasion was classified as PL1 in 26 patients, PL2 in 60 patients, and PL3 in 13 patients (Table 1). In the 60 patients with PL2 invasion, 3 patients had peripheral adjacent lobe invasion.
Table 1.
Clinicopathologic features of 136 patients with peripheral NSCLC.
Interobserver agreement on tumor border type was substantial agreement (κ = 0.80). Interobserver differences (19 of 136 patients, 14.0%) included 3 patients of type 2, 5 patients of type 3, 4 patients of type 4, and 7 patients of type 5, which were resolved by consensus. The tumor border was type 1 in 6 (4.4%) patients, type 2 in 57 (41.9%) patients, type 3 in 10 (7.4%) patients, type 4 in 15 (11.0%) patients, and type 5 in 48 (35.3%) patients. The use of intravenous contrast medium significantly differed by tumor type (P < .001). Intravenous contrast medium was used in 1 of 6 patients with tumor border type 1, in 44 of 57 patients with tumor border type 2, in 8 of 10 patients with tumor border type 3, in 3 of 15 patients with tumor border type 4, and in 31 of 48 patients with tumor border type 5.
Binomial logistic modeling showed that pleural invasion was significantly associated with tumor borders types (P = .001) and with tumor size (P = .03). Tumor border types 2 and 5 significantly differed between patients with and without pleural invasion (P = .001 and P < .001, respectively) (Table 2). Patients with and without pleural invasion did not significantly differ in tumor border type 1, tumor border type 3, tumor border type 4, blunt angle, pleural contact >3 cm, or pleural thickening.
Table 2.
Comparison of clinicopathologic features in 136 patients with peripheral NSCLC with and without pleural invasion.
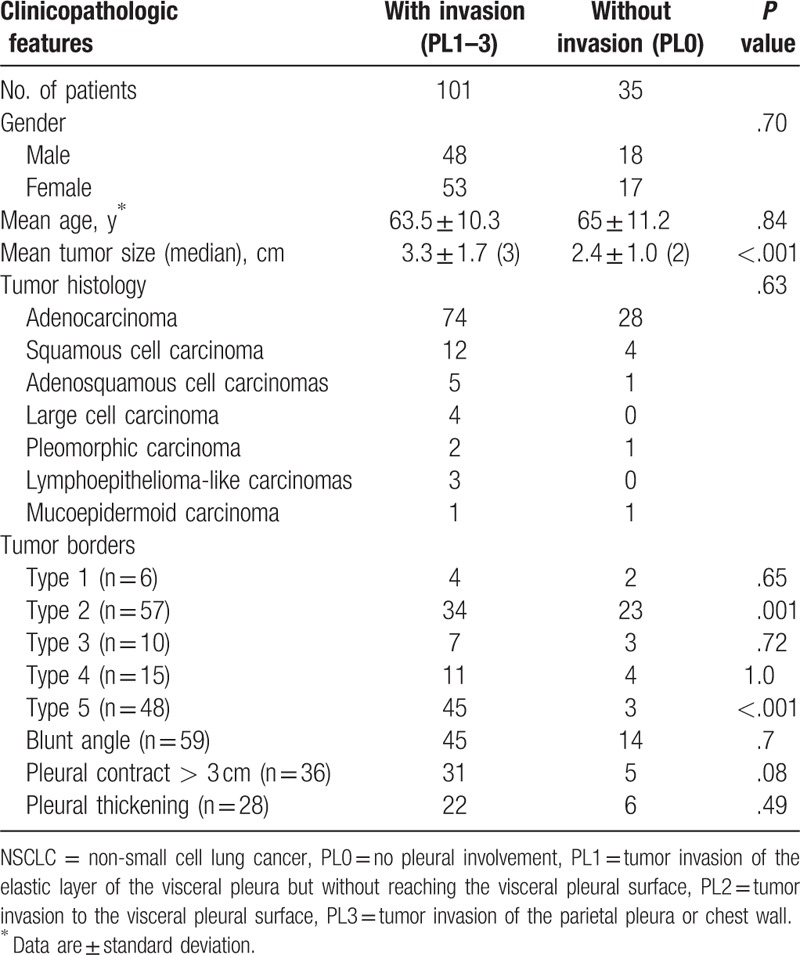
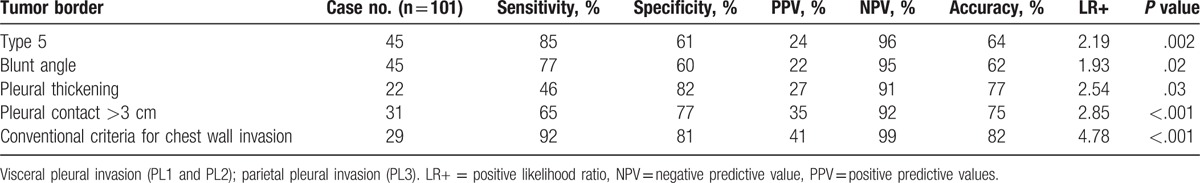
Table 3 shows the sensitivity, specificity, PPV, NPV, accuracy and positive LR of tumor border types, blunt angle, pleural contact >3 cm, and pleural thickening associated with pleural invasion. Tumor border type 5 was considered moderate evidence of pleural invasion with the following results: positive LR, 5.20; accuracy, 57% (77 of 136); sensitivity, 45% (45 of 101); specificity, 91% (32 of 35); PPV, 94% (45 of 48); and NPV, 36% (32/88). Tumor border types 1 to 4, blunt angle, pleural contact >3 cm, and pleural thickening were associated with weak evidence of pleural invasion. Patients with visceral pleural invasion (PL1–2) and patients with parietal pleural invasion (PL3) significantly differed in tumor border type 5 (P = .002), blunt angle (P = .02), pleural contact >3 cm (P < .001), pleural thickening (P = .03), and conventional criteria for chest wall invasion (P < .001) (Table 4). Conventional criteria for chest wall invasion differentiated parietal pleural invasion from visceral pleural invasion with positive LR, 4.78; accuracy, 82% (83/101); sensitivity, 92% (12 of 13); specificity, 80% (71/88); PPV, 41% (12 of 29); and NPV, 99% (71 of 72). Tumor border type 5, blunt angle, pleural contact >3 cm, and pleural thickening were associated with weak evidence to differentiating parietal pleural invasion from visceral pleura invasion. Tumor border types 1 to 5, blunt angle, pleural contact >3 cm, and pleural thickening did not significantly differ between PL1 and PL2. Tumor border type 5 was in none to slight agreement with blunt angle (κ=0.12), pleural contact >3 cm (κ=0.09), and pleural thickening (κ=0.03).
Table 3.
Tumor characteristics associated with pleural invasion.
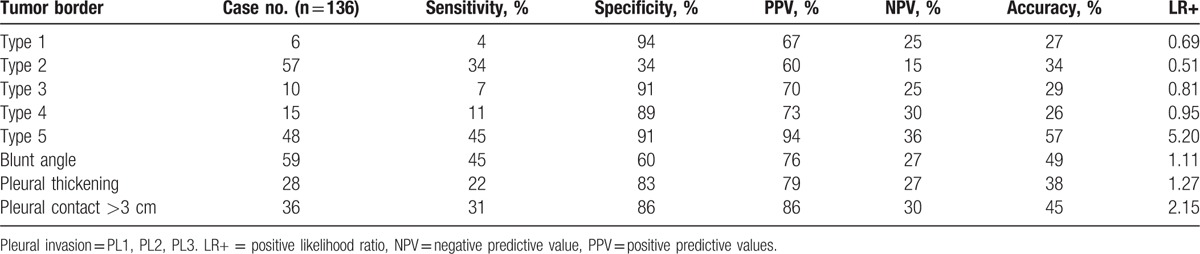
Table 4.
Tumor characteristics associated with visceral and parietal pleural invasion.
Tumor size was non-normally distributed (P < .001). The median tumor size was 3 cm (IQR, 2 cm; range, 1–8 cm). Tumor size was significantly larger in the presence of pleural invasion (median, 3 cm; IQR, 2 cm; range, 1–8 cm) than in the absence of pleural invasion (median, 2; IQR, 1 cm; range 1–4 cm) (P < .001). Age, gender, and tumor histology did not significantly differ between patients with and without pleural invasion by peripheral NSCLC (Table 2).
4. Discussion
This study showed that tumor border type 5 on CT was a moderate diagnostic indicator of pleural invasion by peripheral NSCLC with 94% PPV and 91% specificity. Several studies have investigated the use of CT imaging for diagnosing pleural invasion by lung cancer. Glazer et al[8] stated that blunt angle, pleural contact >3 cm, and pleural thickening were good predictors of pleural invasion on 10-mm thickness CT imaging. In contrast, Ebara et al[7] reported that blunt angle and pleural thickening were not significantly associated with pleural invasion on 1-mm thickness CT imaging. Tanaka et al[13] found that blunt angle was associated with pleural invasion and pleural thickening but was not significantly associated with pleural invasion on 1- to 3-mm thickness CT imaging. Blunt angle and pleural thickening were not significantly associated with pleural invasion in our study, which is consistent with Ebara et al.[7] A possible explanation for these discordant results is differences in patient characteristics and CT parameters. The length of contact with pleura was significantly greater in tumors with pleural invasion than in those without pleural invasion.[7,13] Our study found that pleural contact >3 cm was not significantly associated with pleural invasion. A possible explanation for the discrepant results is that some of our cases might have been contaminated with pneumonia or atelectasis. Tumor size was significantly larger in the presence of pleural invasion than in the absence of pleural invasion, which is consistent with the literature.[7,13] Tumor border type 5 had none to slight agreement with blunt angle, pleural contact >3 cm, and pleural thickening. Therefore, tumor border type 5 could be a distinctive indicator of pleural invasion by peripheral NSCLC. To the best of our knowledge, this study is the first to report this association.
For predicting chest wall invasion in cases where the tumor is adjacent to the chest wall without bone destruction or without a mass involving the chest wall, CT has a limited accuracy (sensitivity, 38–90%; specificity, 40–96%).[7,14] Signs of chest wall invasion (PL3) observed in CT images include blunt angle, pleural contact >3 cm, and adjacent pleural thickening.[7–10] Previous reports show that the conventional criteria used to predict chest wall invasion had accuracy of 68% to 83.3%; sensitivity of 67% to 87%; specificity of 43.9% to 91.3%; PPV of 20.7% to 53.8%; and NPV of 81.8% to 96%.[7–9,15] In the present study, the conventional criteria used to differentiate between parietal pleural invasion (PL3) and visceral pleural invasion (PL1, 2) had positive LR, 4.78; accuracy, 82%; sensitivity, 92%; and specificity, 80%, which is consistent to Imai et al[9] Tumor border type 5, blunt angle, pleural contact >3 cm, and pleural thickening significantly differed between patients with parietal pleural invasion and patients with visceral pleural invasion. However, tumor border type 5, blunt angle, pleural contact >3 cm, and pleural thickening were associated with weak evidence to differentiate parietal pleural invasion from visceral pleura. Imai et al[9] reported that the ratio of tumor-pleura contact to tumor diameter accurately predicted thoracic wall invasion (sensitivity and specificity, 89.7% and 96.0%, respectively). Ebara et al[7] further reported that the ratio of the tumor-pleura interfacial area to tumor size could differentiate between parietal and visceral pleural invasion with 77% accuracy. Tanaka et al[13] used SUVmax 4.3 on 18F-FDG PET/CT as the cutoff value for predicting visceral pleural invasion with 85% accuracy. Other suggested methods for differentiating between parietal and visceral pleural invasion include, respiratory dynamic MR imaging, ultrasonography, and CT combined with artificial pneumothorax.[9,16,17] However, respiratory dynamic MR imaging cannot consistently distinguish between tumor invasion and nonmalignant adhesion. The accuracy of intraoperative ultrasonography is highly dependent on the skill and experience of the operator. Using CT combined with artificial pneumothorax introduces complications.[9]
Tumor border type 5, blunt angle, pleural contact >3 cm, and pleural thickening cannot be used to differentiate PL1 and PL2. Visceral pleural invasion is an important stage-defining feature of NSCLC in the absence of lymph node involvement. In patients with lymph node-negative NSCLC, visceral pleural invasion indicates either stage IB or IIA, depending on tumor size. Ebara et al[7] reported that a skirtlike 3-dimensional pleural pattern predicts pleural invasion of peripheral NSCLC with 77% accuracy. Our previous study[18] further reported that a pleural tag with a soft tissue component at the pleural end on a mediastinal window images predicts visceral pleural invasion of non-abutting NSCLC with 71% accuracy.[18] Whether visceral pleural invasion is an adverse prognostic indicator in stage I NSCLC patients remains controversial, especially in patients with a small tumor size. Huang et al[19] found that visceral pleural invasion was associated with poor overall survival and a high risk of recurrence in stage I patients. However, some studies suggest that visceral pleural invasion does not affect prognosis in tumors <3 cm.[20] When the primary tumor is >3 cm, visceral pleural invasion may indicate the need for adjuvant chemotherapy in patients with stage IIA NSCLC and selected patients with stage IB NSCLC.[21,22] The prognostic value of differentiating between PL1 and PL2 is still controversial. Hung et al[23] reported that PL2 is a significant negative prognostic indicator of recurrence and overall survival in node-negative NSCLC with visceral pleural invasion. Adachi et al[24] reported that survival rates did not significantly differ between patients with PL1 and PL2 disease, regardless of lymph node status.
Some limitations of our study should be noted. First, this retrospective study analyzed a relatively small sample of patients treated at a single tertiary institution. Patient selection bias was inevitable. Second, 4 pathologists independently staged pleural invasion. Interobserver bias was inevitable. Third, different slice thickness may have affected the evaluations of tumor characteristics. Fourth, tumor border types were prioritized in patients with more than 1 lesion type. For example, co-present type 3 and type 5 lesions were simply classified as type 5 lesions. Prioritizing the lesions may have biased our results. Fifth, different proportions of various tumor border types were assessed without intravenous contrast medium in this study. The non-contrast enhanced imaging might have biased the evaluation of tumor characteristics. Sixth, the overall accuracy of 0.57 and a sensitivity of 0.45 might limit the use of tumor border type 5. However, tumor border type 5 had sufficiently high PPV and high specificity for use in clinical diagnosis. Finally, Yang et al[25] reported that survival is similar in patients with peripheral adjacent lobe invasion NSCLC and those with parietal pleural invasion (PL3) disease. However, this study classified peripheral adjacent lobe invasion as PL2, which potentially biased the differentiation between PL2 and PL3 based on tumor border. Further prospective multi-institutional studies are needed to validate the value of tumor border types for diagnosing pleural invasion by peripheral NSCLC.
In conclusion, a tumor border type 5 has sufficiently high PPV and high specificity for use as an indicator of pleural invasion by peripheral NSCLC.
Acknowledgments
The authors thank Chih-Hsiang Hsu (Department of Nursing/Cancer Center) for collecting the patient list and clinical data.
Footnotes
Abbreviations: 18F-FDG PET/CT = positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-d-glucose integrated with computed tomography, CT = computed tomography, IQR = interquartile range, LR+ = positive likelihood ratio, LR = likelihood ratio, MR = magnetic resonance, NPV = negative predictive value, NSCLC = non-small cell lung cancer, PACS = picture archiving and communication system, PL0 = no pleural involvement, PL1 = tumor invasion of the elastic layer of the visceral pleura but without reaching the visceral pleural surface, PL2 = tumor invasion to the visceral pleural surface, PL3 = tumor invasion of the parietal pleura or chest wall, PPV = positive predictive value, SUVmax = maximum standardized uptake value.
The authors report no conflicts of interest.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225–49. [DOI] [PubMed] [Google Scholar]
- [3].Lang-Lazdunski L. Surgery for nonsmall cell lung cancer. Eur Respir Rev 2013;22:382–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Oyama M, Miyagi Maeshima A, Tochigi N, et al. Prognostic impact of pleural invasion in 1488 patients with surgically resected non-small cell lung carcinoma. Jpn J Clin Oncol 2013;43:540–6. [DOI] [PubMed] [Google Scholar]
- [5].UyBico SJ, Wu CC, Suh RD, et al. Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics 2010;30:1163–81. [DOI] [PubMed] [Google Scholar]
- [6].Briediene R. Radiological diagnostics of pleura and mediastinum invasion in lung cancer patients. Acta Med Litu 2005;12:68–72. [Google Scholar]
- [7].Ebara K, Takashima S, Jiang B, et al. Pleural invasion by peripheral lung cancer: prediction with three-dimensional CT. Acad Radiol 2015;22:310–9. [DOI] [PubMed] [Google Scholar]
- [8].Glazer HS, Duncan-Meyer J, Aronberg DJ, et al. Pleural and chest wall invasion in bronchogenic carcinoma: CT evaluation. Radiology 1985;157:191–4. [DOI] [PubMed] [Google Scholar]
- [9].Imai K, Minamiya Y, Ishiyama K, et al. Use of CT to evaluate pleural invasion in non-small cell lung cancer: measurement of the ratio of the interface between tumor and neighboring structures to maximum tumor diameter. Radiology 2013;267:619–26. [DOI] [PubMed] [Google Scholar]
- [10].Verschakelen JA, Bogaert J, De Wever W. Computed tomography in staging for lung cancer. Eur Respir J Suppl 2002;35:40s–8s. [DOI] [PubMed] [Google Scholar]
- [11].Sobin LH, Gospodarowicz M, Wittekind C. UICC TNM Classification of Malignant Tumors. Wiley-Blackwell, 7th ed.New York, NY:2009. [Google Scholar]
- [12].Anvari A, Halpern EF, Samir AE. Statistics 101 for radiologists. Radiographics 2015;35:1789–801. [DOI] [PubMed] [Google Scholar]
- [13].Tanaka T, Shinya T, Sato S, et al. Predicting pleural invasion using HRCT and 18F-FDG PET/CT in lung adenocarcinoma with pleural contact. Ann Nucl Med 2015;29:757–65. [DOI] [PubMed] [Google Scholar]
- [14].Gallardo-Valera G, Trivino-Ramirez A, Congregado M, et al. Usefulness of video-assisted thoracoscopy for correctly staging tumors as T3 because of chest wall invasion. Arch Bronconeumol 2009;45:325–9. [DOI] [PubMed] [Google Scholar]
- [15].Kajiwara N, Akata S, Uchida O, et al. Cine MRI enables better therapeutic planning than CT in cases of possible lung cancer chest wall invasion. Lung Cancer 2010;69:203–8. [DOI] [PubMed] [Google Scholar]
- [16].Bandi V, Lunn W, Ernst A, et al. CT in detecting chest wall invasion by tumor: a prospective study. Chest 2008;133:881–6. [DOI] [PubMed] [Google Scholar]
- [17].Yokoi K, Mori K, Miyazawa N, et al. Tumor invasion of the chest wall and mediastinum in lung cancer: evaluation with pneumothorax CT. Radiology 1991;181:147–52. [DOI] [PubMed] [Google Scholar]
- [18].Hsu JS, Han IT, Tsai TH, et al. Pleural tags on CT scans to predict visceral pleural invasion of non-small cell lung cancer that does not abut the pleura. Radiology 2016;279:590–6. [DOI] [PubMed] [Google Scholar]
- [19].Huang H, Wang T, Hu B, et al. Visceral pleural invasion remains a size-independent prognostic factor in stage I non-small cell lung cancer. Ann Thorac Surg 2015;99:1130–9. [DOI] [PubMed] [Google Scholar]
- [20].Baisi A, Raveglia F, De Simone M, et al. Does visceral pleural invasion affect prognosis in stage I non-small cell lung cancer? Ann Thorac Surg 2015;100:1977. [DOI] [PubMed] [Google Scholar]
- [21].Jiang L, Liang W, Shen J, et al. The impact of visceral pleural invasion in node-negative non-small-cell lung cancer: a systematic review and meta-analysis. Chest 2015;148:903–11. [DOI] [PubMed] [Google Scholar]
- [22].See KC, Lee P. Advances in the diagnosis of pleural disease in lung cancer. Ther Adv Respir Dis 2011;5:409–18. [DOI] [PubMed] [Google Scholar]
- [23].Hung JJ, Jeng WJ, Hsu WH, et al. Prognostic significance of the extent of visceral pleural invasion in completely resected node-negative non-small cell lung cancer. Chest 2012;142:141–50. [DOI] [PubMed] [Google Scholar]
- [24].Adachi H, Tsuboi M, Nishii T, et al. Influence of visceral pleural invasion on survival in completely resected non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;48:691–7. [DOI] [PubMed] [Google Scholar]
- [25].Yang HX, Hou X, Lin P, et al. Peripheral direct adjacent lobe invasion non-small cell lung cancer has a similar survival to that of parietal pleural invasion T3 disease. J Thorac Oncol 2009;4:1342–6. [DOI] [PubMed] [Google Scholar]


