Abstract
Early diagnosis is pivotal for prognosis of lung cancer patients. Positron emission tomography/computed tomography (PET-CT) is a useful method for human cancer diagnosis. In this study, we aimed to explore the false positive diagnosis of PET-CT in lung cancer
In total, 754 patients diagnosed with lung cancer via PET-CT were retrospectively collected in this study. Histopathological detection served as gold standard. The diagnostic accuracy of PET-CT was defined as the proportion of lung cancer cases confirmed by pathological diagnosis in the study subjects, and the percentages of misdiagnosed cases represented the false positive diagnosis of PET-CT. Chi-square test and logistic regression analysis were used to analyze the association of pathologically confirmed result with clinical characteristics.
Among all the patients, 705 cases were pathologically confirmed with lung cancer. The diagnostic accuracy of PET-CT was 93.5%, and the false positive rate was 6.50%. Among the false positive patients, inflammatory pseudotumor (42.86%) and tuberculoma (36.74%) were the most pathological types. In the positive detection group, adenocarcinoma (57.16%) and squamous carcinoma (33.19%) were the main pathological types, and 68.09% of the lung cancer patients were at the advanced stages. The false positive rate were related with age, diabetes, interleukin-6 (IL-6) level, and T-spot test (all P < .05).
PET-CT could be a good diagnostic method for lung cancer, but the false positive cases could appear. Detection of inflammatory indicators such as IL-6 and T-spot TB test may help improve the diagnostic accuracy of PET-CT.
Keywords: diagnosis, false positive, lung cancer, PET-CT
1. Introduction
Lung cancer is a common cancer and has become a malignancy with the highest incidence and mortality.[1] Also, it is the leading cause of cancer-related death among males and females around the world.[2,3] In China, the death rate of lung cancer increases more than 4 times in the past 30 years.[4] Among lung cancer, about 80% to 85% are nonsmall cell lung cancer (NSCLC), including adenocarcinoma, squamous carcinoma, large cell carcinoma, and unclassified cell carcinoma.[2] With the development of technology, the progress of the diagnosis and treatment for lung cancer has been improved.[5,6] However, there was a study which found that most lung cancer patients suffered from metastasis or developed at the advanced stages when initially diagnosed.[7] Thus, the early diagnosis is very important for lung cancer patients.
Positron emission tomography/computed tomography (PET-CT) is a kind of reliable diagnostic method to detect primary and metastatic malignant tumors.[8] In 2001, the first PET-CT scanner appeared, and at present, there are more than 2000 PET-CT scanners are operational around the world.[9] PET can react the metabolism of lesions, and CT can display the morphological structure of lesions. Thus, PET-CT is a good method to detect the staging and metastatic survey of cancer. 18F-deoxyglucose (18F-FDG) PET-CT is the most widely approach in the clinical. The absorbed quantity of FDG in cells is associated with the metabolic rate of glucose. In cancer cells, FDG can absorb more energy than that in normal cells; thus, cancers can be marked by 18F-FDG PET-CT.[10] Although PET-CT is a relatively preferable diagnostic tool for cancers, it can also show false positives and negatives. For examples, the differences in metabolic rates of glucose between young and old people may cause errors to the diagnosis. In addition, reactive hyperplasia or active inflammation may also cause a false positive result in application of PET-CT.[11] However, the reports of false positive diagnosis of PET-CT in lung cancer patients are few.
In this study, we aimed to retrospectively review the false positive diagnosis of PET-CT scan in lung cancer patients, and to analyze the clinical characteristics and indicators for false positive diagnosis of PET-CT in these patients.
2. Materials and methods
2.1. Study population
In our study, a total of 754 patients with lung diseases were recruited from Daping hospital. They either got surgeries or had clear pathological results with percutaneous lung biopsy in respiratory disease department and chest surgery, and all accepted the PET-CT before surgery. Our study was approved by the Ethics Committee of Daping Hospital, and the written consents were also obtained from all patients and their families.
2.2. Clinical data of lung cancer patients and false positive patients diagnosed by PET-CT
In this study, we collected the clinical data of lung cancer patients and the false positive patients diagnosed with PET-CT, and the data were listed in Tables 1 and 2. All the basic clinical features included age, gender, diseased region, pathological type, stage, and diabetes history. Other clinical indicators were detected and listed in Table 3, including the inflammatory markers PCT and IL-6, leucocyte, tumor markers CEA, NSE and keratin, t-spot A and t-spot B.
Table 1.
The pathological types of lung cancer and false positive patients and the stages of lung cancer patients.
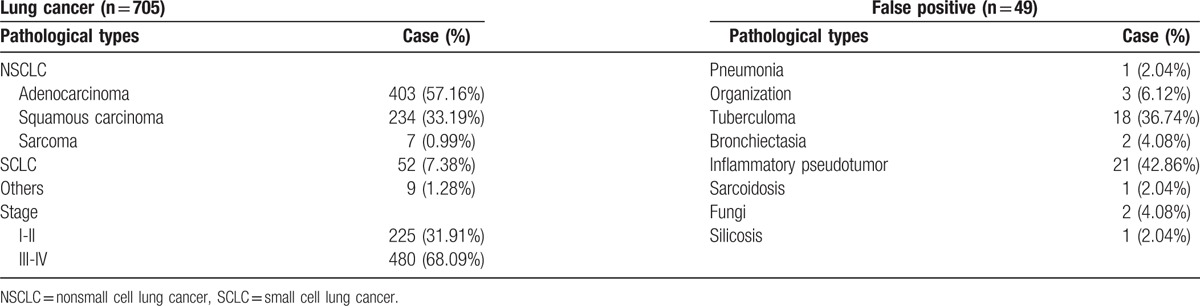
Table 2.
The clinical characteristics of the lung cancer patients and false positive patients with PET-CT.
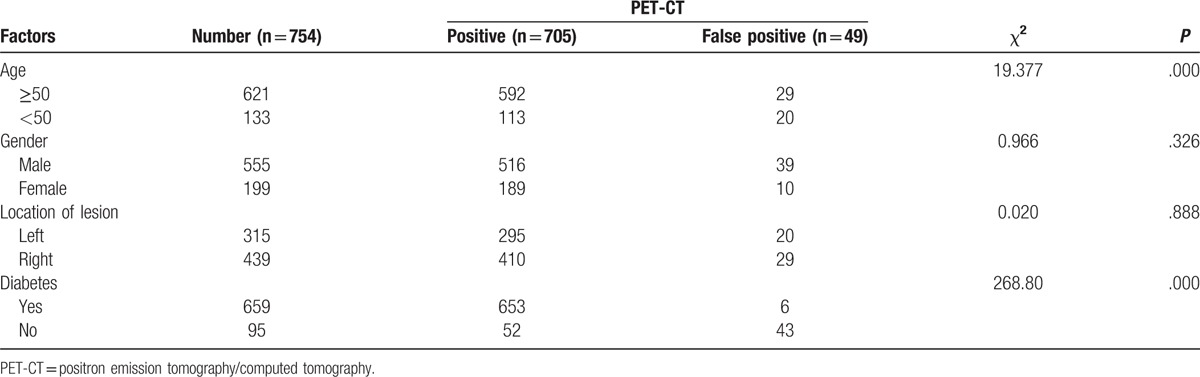
Table 3.
The comparison of clinical indicators between the lung cancer patients and false positive patients.

2.3. Statistical analysis
All statistical analyses were performed using the software of SPSS 19.0 (SPSS Inc., Chicago, IL). The data were summarized and presented as means ± SD. The clinicopathological characteristics and clinical indicators among the lung cancer patients and false positive patients were assessed using Chi-square tests and logistic regression analysis. In this study, all P values less than .05 were considered statistically significant.
3. Results
3.1. The false positive rate of PET-CT
A total of 754 patients (mean age, 59.38 ± 9.28 years; age range 31–88 years) were recruited in our study. Among the lung cancer patients diagnosed by PET-CT, pathological diagnosis demonstrated that 705 cases were confirmed with lung cancer, and 49 cases were diagnosed with benignant disease. The false positive rate of PET-CT was 6.50%.
3.2. The pathological types and stages of the patients diagnosed by PET-CT
Table 1 listed the data of pathological types and stages. We could see that more than a half patients were adenocarcinoma (57.16%) in the positive detection group, followed by squamous carcinoma (33.19%). Also, 68.09% of the lung cancer patients were at the advanced stages. In the false positive group, inflammatory pseudotumor (42.86%) and tuberculoma (36.74%) were the most common misdiagnosed diseases.
3.3. The clinical data of the patients
Tables 2 and 3 were the comparison of the clinical data between the lung cancer patients and the false positive patients. In Table 2, the results indicated that both age and diabetes were related with the false positive outcome (both P = .000). Most of the lung cancer patients were more than 50 years old, and more than 90% lung cancer patients suffered from diabetes. However, in the false positive patients, almost 90% patients were without diabetes. The results of logistic regression analysis showed that age (OR = 2.940, 95%CI = 1.315–6.575, P = .009) and diabetes (OR = 85.277, 95%CI = 34.414–211.314, P = .000) were independent risk factors for the false positive of PET-CT in lung cancer patients (Table 4). The patients who were under 50 years old, or those without diabetes were more likely to undergo false positive detection of PET-CT.
Table 4.
The adjusted effects of age and diabetes on diagnostic accuracy of PET-CT in lung cancer.
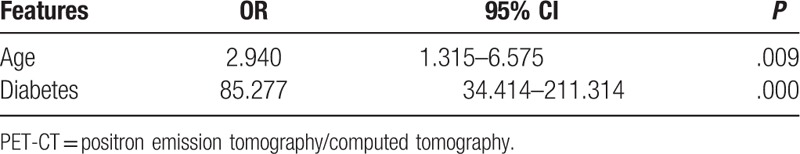
In addition, among the clinical indicators, IL-6 and T-spot-B were both significantly different between the lung cancer patients and the false positive patients, with P value .004 and .002, respectively (Table 3).
4. Discussion
Lung cancer is one of the most common cancers in the world, with increasing morbidity in recent years. At present, around the world, lung cancer has become the malignant tumor with the highest morbidity and mortality, posing great threat to human health.[12–14] Although the treatments of lung cancer have developed constantly, the prognosis of lung cancer patients is still very poor. According to the reports, the prognosis is associated with the stages of clinical diagnosis, the 5-year survival rate of I stage is 38% to 67%, but that of IV stage is only 1%.[13] If the lung cancer patients accept surgery at early stages, the 10-year survival rate can be 88%.[15] Thus, early diagnosis and timely treatment are very important for lung cancer patients.
The diagnosis of lung cancer includes imaging diagnosis and pathology diagnosis. Before the appearance of CT, the diagnosis of lung cancer mainly depends on the x-ray examination; however, its resolution discovery rate is very low.[16] And then with the development of the image technology, CT is widely used in the diagnosis of lung cancer. PET-CT can obtain the PET images and CT images at the same time; thus, the organization function of metabolism and the anatomical and pathological morphology of lesions can be all got. Therefore, PET-CT can achieve the purpose of early diagnosis.[17] Many researchers have used the PET-CT to diagnose lung cancer, but false positive can appear.
In our study, we explored the false positive diagnosis with PET-CT in lung cancer patients in our hospital. In the 754 patients diagnosed with PET-CT, 705 cases were confirmed by pathological detection, and the false positive rate was 6.5%. Among all the lung cancer patients, more than a half patients were adenocarcinoma, and the patients with advanced stages accounted for the large proportion. And among the false positive patients, most of the patients suffered from pseudotumor and tuberculoma, this result was consistent with previous researches. Atypical tuberculosis and inflammation were the main diseases to be misdiagnosed as lung cancer by PET-CT.[18] Some scholars even thought that PET-CT could not discriminate between lung cancer and tuberculosis.[19] From the PET images, the mediastinal lymph node false positives are common,[20] especially that it is difficult to distinguish the lymph node tuberculosis without calcified and metastatic lymph nodes, because of inactive TB lymph nodes without viable TB bacilli.[21] Atypical tuberculosis can be expressed in a variety of forms, often manifested as nodules or lumps, which would be easily confused with lung cancer.[22,23] Kang et al[24] suggested that 18F-FDG showed low diagnosis sensitivity in differentiating between nonsmall cell lung cancer (NSCLC) and lung tuberculosis. The study reported by Shaw et al. showed that in a high tuberculosis-endemic area, PET-CT was still a valuable method for excluding mediastinal lymph node involvement in NSCLC. However, the PET-CT positive results required pathological verification, to exclude the potential possibility of tuberculosis.[25] Therefore, tuberculosis is known cause for to false-positive PET-CT findings. To enhance the control of tuberculosis in tuberculosis-endemic countries, such as India, Indonesia, China, and so on, may help improve the diagnostic accuracy of PET-CT in lung cancer. In addition, to improve the cut-off value of PET-CT in lung cancer diagnosis may also promote its diagnostic value. Shaw et al reported that compared with a cut-off of 2.5, a SUVmax cut-off of 4.5 yielded could promote the diagnostic accuracy from 64.0% to 84.7%.[25] Thus, to progress in tuberculosis control and optimize the standard of PET-CT technique may be promising approaches to reduce the false positive rate of PET-CT in lung cancer.
Additionally, in order to investigate the influence factors for diagnostic accuracy of PET-CT in lung cancer, we also compared the clinical characteristics and tumor biomarkers between the positive group and false positive group. We found that IL-6 and T-spot showed significant differences between the positive detection group and the false positive group. The data revealed that inflammation and potential tuberculosis might influence the diagnostic accuracy of PET-CT. In addition, we also found that age and diabetes might contribute to false positive diagnosis. The pulmonary diseases patients with the age over 50 years and without diabetes were more likely to be misdiagnosed by PET-CT. More attentions should be payed to these patients. However, the underlying cause for the effects of diabetes on diagnostic accuracy of PET-CT was not investigated in the current study, which required further researches.
In conclusion, the results in our study showed that although PET-CT could be a good diagnostic method for lung cancer patients, there should be an awareness of the possibility of false positive. In order to minimize the false positive diagnosis of PET-CT for lung cancer patients, it should combine with other clinical indicators, such as inflammation marker IL-6 detection and T-spot-B test.
Footnotes
Abbreviations: 18F-FDG = 18F-deoxyglucose, CEA = carcinoembryonic antigen, IL-6 = interleukin- 6, NSCLC = nonsmall cell lung cancer, NSE = neuron-specific enolase, PCT = procalcitonin, PET-CT = positron emission tomography/computed tomography.
The authors have no funding and conflicts of interest to disclose.
References
- [1].McErlean A, Ginsberg MS. Epidemiology of lung cancer. Semin Roentgenol 2011;46:173–7. [DOI] [PubMed] [Google Scholar]
- [2].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- [3].Ramalingam SS, Owonikoko TK, Khuri FR. Lung cancer: new biological insights and recent therapeutic advances. CA Cancer J Clin 2011;61:91–112. [DOI] [PubMed] [Google Scholar]
- [4].She J, Yang P, Hong Q, et al. Lung cancer in China: challenges and interventions. Chest 2013;143:1117–26. [DOI] [PubMed] [Google Scholar]
- [5].Rao JS, Gondi C, Chetty C, et al. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther 2005;4:1399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stinchcombe TE, Socinski MA. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc 2009;6:233–41. [DOI] [PubMed] [Google Scholar]
- [7].Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92–8. [DOI] [PubMed] [Google Scholar]
- [8].Kamiyama H, Sakamoto K, Niwa K, et al. Unusual false-positive mesenteric lymph nodes detected by PET/CT in a metastatic survey of lung cancer. Case Rep Gastroenterol 2016;10:275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Townsend DW. Combined positron emission tomography-computed tomography: the historical perspective. Semin Ultrasound CT MR 2008;29:232–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Divgi CR. Molecular imaging of pulmonary cancer and inflammation. Proc Am Thorac Soc 2009;6:464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li S, Zheng Q, Ma Y, et al. Implications of false negative and false positive diagnosis in lymph node staging of NSCLC by means of 18F-FDG PET/CT. PloS One 2013;8:e78552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ginsberg MS. Epidemiology of lung cancer. Semin Roentgenol 2005;40:83–9. [DOI] [PubMed] [Google Scholar]
- [13].Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- [14].Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clinic Proc 2008;83:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mazzone P, Obuchowski N, Mekhail T, et al. Lung cancer screening: is it time for a change in policy? Cleveland Clin J Med 2007;74:441–8. [DOI] [PubMed] [Google Scholar]
- [16].Roberts PF, Straznicka M, Lara PN, et al. Resection of multifocal non-small cell lung cancer when the bronchioloalveolar subtype is involved. J Thorac Cardiovasc Surg 2003;126:1597–602. [DOI] [PubMed] [Google Scholar]
- [17].Orlacchio A, Schillaci O, Antonelli L, et al. Solitary pulmonary nodules: morphological and metabolic characterisation by FDG-PET-MDCT. La Radiol Med 2007;112:157–73. [DOI] [PubMed] [Google Scholar]
- [18].Zheng Z, Pan Y, Guo F, et al. Multimodality FDG PET/CT appearance of pulmonary tuberculoma mimicking lung cancer and pathologic correlation in a tuberculosis-endemic country. Southern Med J 2011;104:440–5. [DOI] [PubMed] [Google Scholar]
- [19].Sathekge MM, Maes A, Pottel H, et al. Dual time-point FDG PET-CT for differentiating benign from malignant solitary pulmonary nodules in a TB endemic area. S Afr Med J 2010;100:598–601. [DOI] [PubMed] [Google Scholar]
- [20].Toba H, Kondo K, Otsuka H, et al. Diagnosis of the presence of lymph node metastasis and decision of operative indication using fluorodeoxyglucose-positron emission tomography and computed tomography in patients with primary lung cancer. J Med Invest 2010;57:305–13. [DOI] [PubMed] [Google Scholar]
- [21].Lee SH, Min JW, Lee CH, et al. Impact of parenchymal tuberculosis sequelae on mediastinal lymph node staging in patients with lung cancer. J Korean Med Sci 2011;26:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fu G, Wu E, Yin W, et al. CT diagnosis of atypical pulmonary tuberculosis easily misdiagnosed as lung cancer. J Pract Radiol 2011;27:358–61. [Google Scholar]
- [23].Lv Y, Xie R, Zhou X, et al. CT imaging of coexisting pulmonary tuberculosis and lung cancer. Chin J Radiol 2013;47:8–12. [Google Scholar]
- [24].Kang F, Wang S, Tian F, et al. Comparing the diagnostic potential of 68Ga-alfatide II and 18F-FDG in differentiating between non-small cell lung cancer and tuberculosis. J Nucl Med 2016;57:672–7. [DOI] [PubMed] [Google Scholar]
- [25].Shaw JA, Irusen EM, von Groote-Bidlingmaier F, et al. Integrated positron emission tomography/computed tomography for evaluation of mediastinal lymph node staging of non-small-cell lung cancer in a tuberculosis-endemic area: a 5-year prospective observational study. S Afr Med J 2015;105:145–50. [DOI] [PubMed] [Google Scholar]


