Abstract
Background:
To assess the efficacy and safety of immunomodulatory drugs in patients with noninfectious anterior uveitis (AU).
Methods:
Systematic review of studies were retrieved from Medline (1961 to March 2016), Embase (1961 to March 2016), and Cochrane Library (up to March 2016), and a complementary hand search was also performed. The selection criteria were as follows: (population) noninfectious AU patients, adults; (intervention) immunomodulatory drugs (any dose, regimen, route of administration, duration of treatment); (outcome) control of inflammation, steroid-sparing effect, AU flares, adverse events, and so on; (study design) systematic literature reviews, randomized controlled trials, and observational studies. The study quality was assessed using the Jadad scale and according to The Oxford Centre for Evidence-based Medicine (update 2009).
Results:
We included 13 studies of moderate-poor quality, with a mean duration from 5 months to 20 years, and number of AU patients ranging from 9 to 274. Patient's demographic and clinical characteristics were very heterogeneous. In most cases, uveitis anatomic classification criteria and outcomes definitions were unclear. Some of the studies only included AU patients with a systemic disease associated, mostly spondyloarthritis, others, mixed populations (idiopathic and systemic disease associated patients), and in some articles this data is not described. We found that methotrexate, cyclosporine A, azathioprine, adalimumab, and golimumab might prevent AU flares, improve ocular inflammation and visual acuity, and decrease systemic steroids doses.
Conclusions:
Although there is a lack of robust evidence, methotrexate, cyclosporine A, azathioprine, adalimumab, and golimumab might be effective in AU patients.
Keywords: anterior uveitis, immunomodulatory drugs, systematic review
1. Introduction
Anterior uveitis (AU) is the most common pattern of uveitis, accounting for 50% to 92% of uveitis cases in western countries.[1–3] A significant proportion of patients have no evidence of an underlying disorder and are labeled as idiopathic, but there is also an important percentage of patients with an associated systemic disorder such as spondyloarthritis (SpA).[4]
AU usually responds well to topical corticosteroids.[5] However, there are cases, especially those associated with systemic disorders that may require additional drugs. For example, HLA-B27 AU, is typically more severe, recurrent, and associated with a higher incidence of ocular complications,[6] including wide anterior and posterior synechiae, secondary glaucoma, and cystoid macular edema.[7,8] For these patients, periocular corticosteroid injection is an option as well as systemic corticosteroid therapy.[9] Corticosteroids alone might help decrease ocular inflammation during exacerbations. However, they are not sufficient for many cases of chronic uveitis and do not prevent further relapses. Besides, long-term corticosteroid therapy also incurs significant risk of unacceptable adverse events (AE) like cushingoid changes, iatrogenic diabetes, osteoporosis, and hypercholesterolemia.[10]
On the other hand, immunomodulatory drugs have been widely used in patients with uveitis for decades. Classical immunomodulators such as salazopyrin (SSZ) or methotrexate (MTX) have been shown effective in controlling ocular inflammation, preventing AU flares and potential visual loss, and in decreasing the corticosteroids need.[11,12] Nevertheless, patients could be refractory or intolerant to these classical drugs. In recent years, the use of off-label biologic agents, particularly tumor necrosis factor-alpha (TNF-α) inhibitors, has spread worldwide for treatment of patients with noninfectious uveitis resistant to traditional immunosuppressors showing encouraging results.[13] This provides new options for the treatment of AU, which, in turn, calls for the need of updating the evidence in order to establish a framework for supporting treatment recommendations.
Finally, taking also into account that therapeutic decision-making in infectious and malignant AU is much less controversial, the aim of this paper was to perform a systematic and critical review of the literature on the use of immunomodulatory drugs in adult patients with noninfectious and nonmalignant AU.
2. Methods
In context of a clinical practice guideline for the management of uveitis, a systematic literature review (SLR) was performed to address the experts’ question on the efficacy and safety of current available immunomodulatory drugs in patients with noninfectious nonmalignant AU. In accordance with the experts, a review protocol was established for this purpose and we followed the indications of the PRISMA statement. As this is an SLR, not an interventional study, an ethical approval was not necessary. The same way patients were not included and therefore informed consent was not given.
2.1. Search strategy
The studies were identified by sensitive search strategies in the main medical databases. We have listed the search strategies in the supplementary data. For this purpose, an expert librarian collaborated and checked the search strategies. The following bibliographic databases were screened: Medline (PubMed) and Embase (Embase.com) from 1961 to March 2016, and The Cochrane Library (including Cochrane Central Register of Controlled Trials, i.e., CENTRAL and the Database of Reviews of Effectiveness, i.e., DARE) up to March 2016. We used specific MeSH headings and additional keywords to identify studies on AU and different types of immunomodulatory drugs. The strategy combines disease and treatment terms as listed previously and a controlled vocabulary for describing any of them. All the retrieved references were managed in Endnote X5 (Thomson Reuters).
Finally, a hand search was completed by reviewing the references of the included studies, and all the publications or other information provided by the experts related to SLR were also examined.
2.2. Selection criteria
The studies retrieved by the search strategies were included if they met the following pre-established criteria: Patients had to be diagnosed with active noninfectious nonmalignant AU, 18 years or older, taking an immunomodulatory drug, including SSZ, MTX, cyclosporine A (CsA), azathioprine (AZA), leflunomide, chlorambucil, cyclophosphamide, mycophenolate, and tacrolimus, or biologic therapies (anti-TNFα drugs and others). There was no restriction regarding the type of drug, dose, route of administration, concomitant use of other drugs, or treatment duration. Different outcomes were considered such as control of inflammation, steroid-sparing effect, visual acuity, reduction of the number of uveitis flares, or AE. Only SLR, randomized controlled trials (RCT), or observational studies (study sample size ≥10 patients) were included as well as studies in English, French, or Spanish language. Studies analyzing patients with uveitis from different or various anatomic sites other than anterior segment were excluded unless they performed subanalysis with those with AU.
2.3. Screening of studies, data collection, and data analysis
Screening of studies, data collection, and analysis was performed by 2 reviewers (AG and EL). First, both reviewers screened the titles and abstracts of the retrieved articles for selection criteria independently. This process was done in 20 minutes sessions. If, while doing this, the reviewers found any discrepancy between them, then, a consensus was reached by asking a third reviewer (LC). The same process was afterward undertaken. The articles from the previous selection process were read in detail, and at the end of this phase a list of included studies was established.
The collection of data from the included studies was carried out by two reviewers independently for every article. As in previous processes, in case of discrepancies, a consensus was reached by looking at the original article or by asking the third reviewer (LC). Articles that did not fulfil all the inclusion criteria or that had insufficient data were excluded.
To grade the quality and risk of bias, we used the Jadad score[14] for RCT and a modification of The Oxford Centre for Evidence-based Medicine Levels of Evidence in its May 2011 update,[15] in which articles are classified as follows: systematic reviews of RCT with homogeneity; individual RCT with narrow confidence intervals; trials in which all patients get harm or none does; systematic reviews of cohort studies with homogeneity; individual cohort study, or low quality RCTs; “Outcomes” Research and Ecological studies; systematic reviews of case-control studies with homogeneity; individual case-control study; case-series and poor quality cohort and case-control studies; and expert opinion without explicit critical appraisal, or based on physiology, bench research, or “first principles.”
Evidence tables were produced. Descriptive analyses were performed. To describe the included article samples, we used the distribution of frequencies, the mean and standard deviation, or the median and interquartile range, depending on the distribution. Comparisons were performed using the Student t test or the chi-square test. Meta-analysis was only planned in case enough homogeneity was present among the included studies.
3. Results
The search strategies retrieved 2166 references (Fig. 1), of which 425 were duplicates. After the selection by title and abstract, 98 references were selected for review in detail. After this process, 85 were excluded mainly due to lack of data regarding AU patients or to the absence of a clear anatomic classification of the uveitis (Table 1).[12,13,16–98] As a result, 13 articles (Tables 2 and 3) were finally included.[11,99–110] The articles found in the hand search were also excluded.
Figure 1.
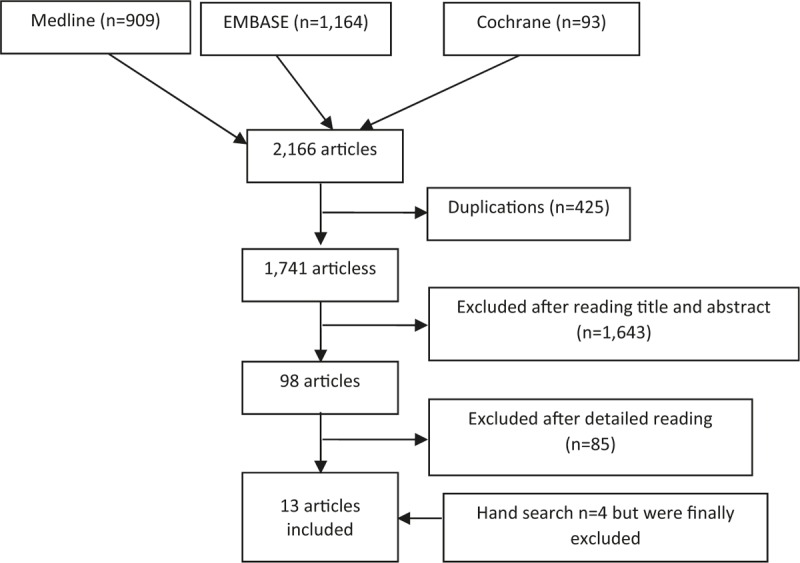
Studies flow chart.
Table 1.
Excluded articles and reason for exclusion.

Table 2.
Main characteristics of the included studies.

Table 3.
Main results of the included studies.

The quality of the included articles was in general poor or moderate. We found 2 RCTs,[11,105] the rest were observational studies. Their mean study duration varied from 5 months[108] to 20 years,[102] and the number of AU patients from 9[12] to 274,[108] in whom clinical characteristics were also very heterogeneous (see Table 1). In most cases, criteria to define the anatomic classification of uveitis and efficacy definitions were not clear. Besides, some of the studies only included AU patients with a systemic disease associated, basically SpA,[11,100,102,108,110] others mixed populations[101,104,106] and in some articles this data was not described (probably idiopathic AU patients).[99,103,105,107,109]
AU was treated with different immunomodulatory drugs, including MTX (mean doses from 7.5 to 25 mg/wk),[99,103,109] SSZ (doses from 500 mg to 4 g/d),[11,102,106] AZA 100 mg/d),[105,107] CsA (data regarding doses were not provided)[104] and anti-TNFα drugs, ADA, and golimumab (GLM)[100,101,108,110] following similar doses to those recommended for rheumatologic conditions.
The number of AU flares before and after treatment was the most evaluated outcome along with AU activity and corticosteroids use. However, we found a great variability between studies in the type of outcomes and definitions.
3.1. Methotrexate
In patients with idiopathic AU or associated systemic disease, most of them MTX and biologics naïve, MTX significantly decreased the number of AU flares and activity, and increased the time interval between flares (Tables 2 and 3). MTX doses in these patients ranged from 7.5 to 25 mg/wk and this effect was described in the short and long term. In the subgroup of patients taking systemic corticosteroids at baseline, the dose of these drugs was progressively tapered until discontinuation in many of them.[99,103] One study also depicted the same results regardless of HLA-B27 status (positive or negative).[99] Reported AEs were the same as those previously described for MTX.
3.2. Salazopyrin
SSZ (from 500 mg to 2 g/d for 3 years) was evaluated in a low-quality RCT[11] that revealed a significant reduction in the number of AU flares and an improvement in visual acuity of those patients diagnosed with ankylosing spondylitis (AS)-associated AU. No relevant AEs were recorded. In other observational studies, a decrease of UA flares was also observed, without relevant AEs.[102,106] SSZ has been primarily used in idiopathic and AS/SpA-associated AU.
3.3. Azathioprine
A 3-months RCT published in 1969 compared AZA (100 mg/d) with placebo in 16 patients with AU. The authors did not find differences in visual acuity, number of anterior chamber cells, AU flares, or intraocular pressure after 3 months of treatment.[105] Another prospective study analyzed the effect of AZA in AU patients of whom 24% were refractory to other immunomodulators.[107] AZA significantly improved ocular inflammation and decreased systemic corticosteroids doses. At 6 months and 1 year, 24% and 35% of patients, respectively, showed no ocular activity. AEs were the same as those usually registered for this drug.
3.4. Cyclosporine A
Regarding CsA, in a moderate quality observational study,[104] that included AU patients (almost 75% with a systemic disease-associated AU), 33% by 6 months and 51% by 1 year gained sustained and complete control of inflammation over at least 2 visits spanning at least 28 days. Besides, a steroid-sparing success was achieved by 22.1% by 6 months and 36.1% within 1 year. The most frequent AE in this study was renal toxicity.
3.5. Anti-TNFα agents
We included 3 articles reporting the outcomes of adalimumab (ADA) in AU. All were observational studies in which the majority of participants were SpA-associated AU patients (up to 40% refractory to other anti-TNFα agents). In this population, ADA improved different outcomes, including the number of AU flares, ocular inflammation, and dose of corticosteroids. This effect remained in the long term.[101,108,109] One of these studies also showed that the rate of AU flares was reduced by 51% in all study patients, by 58% in 274 patients with a history of AU, by 68% in 106 patients with a recent history of AU, and by 50% in 28 patients with symptomatic AU at baseline. AU flares during ADA treatment in this work were predominantly mild.[108] Expected AE were registered in all studies.
Two more reports analyzing GLM in patients with AU, refractory to immunomodulators including biologic therapies in many patients were included.[100,110] Both studies analyzed a total of 27 patients with SpA-associated AU. The first one depicted a significant improvement in visual acuity, number of UA flares, and need of systemic steroids during a mean follow-up of almost 1 year.[110] On the other hand, 1 patient developed a malignant hypertension and stopped GLM. In the second one, most patients had rapid and progressive improvement in visual acuity and inflammatory parameters as well as in the steroid need. The number of AU flares also decreased but this difference was nonsignificant. In this study, 87% of patients also reached clinical remission after a median follow-up of 23 months.[100]
4. Discussion
We have performed an SLR to analyze the efficacy and safety of immunomodulators when used for treatment of adult patients with noninfectious and nonmalignant AU. To our knowledge, this is the first one specifically designed to analyze patients with AU.
Currently, there is a lack of robust evidence in clinical practice regarding the use of immunomodulators in these patients. Even with this limitation, there is some evidence supporting the use of MTX, SSZ, AZA, CsA, ADA, and GLM.
More specifically, as first line immunomodulators, but also in patients resistant to other immunosuppressive agents, MTX, SSZ, and CsA have shown effectiveness to prevent AU flares, improve visual acuity, and to decrease systemic steroids dose in the short and the long term (up to 3 years). These results have been described in patients with idiopathic AU and patients with an associated systemic disease. In the case of AZA, this drug could also be effective in improving ocular inflammation and in reducing systemic corticosteroids need, in patients who are naïve or refractory to other immunomodulators. This effect has been depicted in the short and long term as well. On the other hand, the evidence also supports the use of ADA and GLM, in different clinical aspects of AU (including refractory patients to other immunomodulators), as they have improved outcomes of interest including AU flares, degree of ocular inflammation, and the need for corticosteroids treatment. In addition, we have evidence of immunomodulators’ benefit in the short and the long term. Besides, the AEs reported did not differ from those reported when used these drugs for treatment of other immune-mediated conditions.[111]
As commented before, regarding the study populations, the included studies analyzed patients with idiopathic AU and patients with an associated systemic disease in whom immunomodulators achieved a good response in many of them. In the case of patients with an associated systemic disease, most of them were SpA patients, especially AS, but the studies also included patients with other types of SpA like psoriatic arthritis. Moreover, 1 study found that MTX improved outcomes in both, HLA-B27 positive and negative patients.[99] In this article, although the rate of flares decreased, all the observed flares occurred in the HLA-B27 positive patients.
The selection criteria of the immunomodulators were not described in detail. Classical immunomodulators were used as first-line agents in patients with inadequate response to topical treatments and/or systemic corticosteroids, but also in refractory patients to other immunomodulators, as depicted for anti-TNFα therapies. Doses and routes of administration were those recommended in the summary of products characteristics, and almost 100% of treatments with immunomodulatory drugs were used in monotherapy. Unfortunately there were no comparative studies between immunomodulators.
The main limitation of this SLR is the quality of the included studies that was quite poor in general, limiting the generalization of conclusions. This lack of robust evidence probably, at least in part, might have been solved in daily practice using the evidence and experience from other chronic immune-mediated diseases. Another of the main limitations of the SLR is the lack of proper standardization of the uveitis anatomic classification and definition of outcomes. Therefore, we excluded many articles that actually analyzed patients with AU but did not perform subanalysis of patients with AU. The same way comparisons between studies results were very complicated and a meta-analysis was not possible.
Interestingly, we did not include any article with other biologics like infliximab or tocilizumab. We found some reports during the selection process but eventually excluded them because they did not meet the inclusion criteria, mainly due to lack of subanalysis or due to the sample size of the studies. However, in the literature there are some case series suggesting that these drugs could be effective as those reported with ADA or GLM.[112–114] In the case of etanercept, observational reports have indicated lower effectiveness and some paradoxical occurrence of uveitis following treatment with this agent.[115]
In summary, even with all the limitations exposed previously, immunomodulators could be effective in patients with noninfectious and nonmalignant AU in order to prevent flares and improve other ocular outcomes. However, more research is needed in order to properly define the role of each immunomodulator in this population.
Footnotes
Abbreviations: ADA = adalimumab, AE = adverse events, AS = ankylosing spondylitis, AU = anterior uveitis, AZA = azathioprine, CsA = cyclosporine A, GLM = golimumab, g = gram, mg = milligram, MTX = methotrexate, RCT = randomized controlled trials, SLR = systematic literature review, SpA = spondyloarthritis, SSZ = salazopyrin, TNF-α = tumor necrosis factor-alpha.
The project was funded by an unrestricted grant of the Spanish Society of Ocular Inflammation (SEIO). GE has received honoraria from GSK y Actelion, MCC from Abbvie, Merck Sharp & Dohme y Allergan, JMH from Allergan and Abbvie.
The rest of authors have no conflicts of interest to disclose.
References
- [1].Chang JH, Wakefield D. Uveitis: a global perspective. Ocul Immunol Inflamm 2002;10:263–79. [DOI] [PubMed] [Google Scholar]
- [2].Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol 1987;103:234–5. [DOI] [PubMed] [Google Scholar]
- [3].Chang JH, McCluskey PJ, Wakefield D. Acute anterior uveitis and HLA-B27. Surv Ophthalmol 2005;50:364–88. [DOI] [PubMed] [Google Scholar]
- [4].Munoz-Fernandez S, Martin-Mola E. Uveitis. Best practice and research. Clin Rheumatol 2006;20:487–505. [DOI] [PubMed] [Google Scholar]
- [5].Careless DJ, Inman RD. Acute anterior uveitis: clinical and experimental aspects. Semin Arthritis Rheum 1995;24:432–41. [DOI] [PubMed] [Google Scholar]
- [6].Loh AR, Acharya NR. Incidence rates and risk factors for ocular complications and vision loss in HLA-B27-associated uveitis. Am J Ophthalmol 2010;150:534–42.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Labalette P. Refractory anterior uveitis. J Fr Ophtalmol 2011;34:122–6. [DOI] [PubMed] [Google Scholar]
- [8].Power WJ, Rodriguez A, Pedroza-Seres M, et al. Outcomes in anterior uveitis associated with the HLA-B27 haplotype. Ophthalmology 1998;105:1646–51. [DOI] [PubMed] [Google Scholar]
- [9].Byun YS, Park YH. Complications and safety profile of posterior subtenon injection of triamcinolone acetonide. J Ocul Pharmacol Ther 2009;25:159–62. [DOI] [PubMed] [Google Scholar]
- [10].Foster CS, Kothari S, Anesi SD, et al. The Ocular Immunology and Uveitis Foundation preferred practice patterns of uveitis management. Surv Ophthalmol 2016;61:1–7. [DOI] [PubMed] [Google Scholar]
- [11].Benitez-Del-Castillo JM, Garcia-Sanchez J, Iradier T, et al. Sulfasalazine in the prevention of anterior uveitis associated with ankylosing spondylitis. Eye 2000;14:340–3. [DOI] [PubMed] [Google Scholar]
- [12].Munoz-Fernandez S, Garcia-Aparicio AM, Hidalgo MV, et al. Methotrexate: an option for preventing the recurrence of acute anterior uveitis. Eye 2009;23:1130–3. [DOI] [PubMed] [Google Scholar]
- [13].Guignard S, Gossec L, Salliot C, et al. Efficacy of tumour necrosis factor blockers in reducing uveitis flares in patients with spondylarthropathy: a retrospective study. Ann Rheum Dis 2006;65:1631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [15].CEBM. CEBM Levels of Evidence 2011. University of Oxford; 2011. Available at: http://wwwcebmnet/indexaspx?o=1025. Accessed March 10, 2016. [Google Scholar]
- [16].Abu El-Asrar AM, Al Tamimi M, Hemachandran S, et al. Prognostic factors for clinical outcomes in patients with Vogt-Koyanagi-Harada disease treated with high-dose corticosteroids. Acta Ophthalmol 2013;91:e486–93. [DOI] [PubMed] [Google Scholar]
- [17].Akman-Demir G, Ayranci O, Kurtuncu M, et al. Cyclosporine for Behcet's uveitis: is it associated with an increased risk of neurological involvement? Clin Exp Rheumatol 2008;26(suppl 50):S84–90. [PubMed] [Google Scholar]
- [18].Al Rashidi S, Al Fawaz A, Kangave D, et al. Long-term clinical outcomes in patients with refractory uveitis associated with Behcet disease treated with infliximab. Ocul Immunol Inflamm 2013;21:468–74. [DOI] [PubMed] [Google Scholar]
- [19].Alpsoy E, Durusoy C, Yilmaz E, et al. Interferon alfa-2a in the treatment of Behcet disease: a randomized placebo-controlled and double-blind study. Arch Dermatol 2002;138:467–71. [DOI] [PubMed] [Google Scholar]
- [20].Androudi S, Brazitikos P, Iaccheri B, et al. Outcomes of early and late immunomodulatory treatment in patients with HLA-B27-associated chronic uveitis. Graefes Arch Clin Exp Ophthalmol [Albrecht von Graefes Archiv für klinische und experimentelle Ophthalmologie] 2003;241:1000–5. [DOI] [PubMed] [Google Scholar]
- [21].Arcinue CA, Durrani K, Artornsombudh P, et al. The efficacy and safety of adalimumab in ocular inflammatory disease. Orphan Drugs Res Rev 2015;5:69–74. [Google Scholar]
- [22].Arevalo JF, Lasave AF, Al Jindan MY, et al. Uveitis in Behcet disease in a tertiary center over 25 years: the KKESH Uveitis Survey Study Group. Am J Ophthalmol 2015;159:177–82. [DOI] [PubMed] [Google Scholar]
- [23].Aydinoglu-Candan O, Araz-Ersan B, Gul A, et al. Anti-interferon alpha antibodies and autoantibodies in patients with Behcet's disease uveitis treated with recombinant human interferon alpha-2a. Graefes Arch Clin Exp Ophthalmol [Albrecht von Graefes Archiv für klinische und experimentelle Ophthalmologie] 2015;253:457–65. [DOI] [PubMed] [Google Scholar]
- [24].Barreiro-de-Acosta M, Lorenzo A, Dominguez-Munoz JE. Efficacy of adalimumab for the treatment of extraintestinal manifestations of Crohn's disease. Revista espanola de enfermedades digestivas: organo oficial de la Sociedad Espanola de Patologia Digestiva 2012;104:468–72. [DOI] [PubMed] [Google Scholar]
- [25].Baughman RP, Lower EE, Bradley DA, et al. Etanercept for refractory ocular sarcoidosis: results of a double-blind randomized trial. Chest 2005;128:1062–147. [DOI] [PubMed] [Google Scholar]
- [26].Bernauer W, Pleisch B, Brunner M. Five-year outcome in immune-mediated scleritis. Graefes Arch Clin Exp Ophthalmol [Albrecht von Graefes Archiv für klinische und experimentelle Ophthalmologie] 2014;252:1477–81. [DOI] [PubMed] [Google Scholar]
- [27].Biasi D, Carletto A, Caramaschi P, et al. Efficacy of methotrexate in the treatment of ankylosing spondylitis: a three-year open study. Clin Rheumatol 2000;19:114–7. [DOI] [PubMed] [Google Scholar]
- [28].Braun J, Baraliakos X, Listing J, et al. Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum 2005;52:2447–51. [DOI] [PubMed] [Google Scholar]
- [29].Buggage RR, Levy-Clarke G, Sen HN, et al. A double-masked, randomized study to investigate the safety and efficacy of daclizumab to treat the ocular complications related to Behcet's disease. Ocul Immunol Inflamm 2007;15:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Calvo-Rio V, Blanco R, Beltran E, et al. Anti-TNF-alpha therapy in patients with refractory uveitis due to Behcet's disease: a 1-year follow-up study of 124 patients. Rheumatology 2014;53:2223–31. [DOI] [PubMed] [Google Scholar]
- [31].Cervantes-Castaneda RA, Bhat P, Fortuna E, et al. Induction of durable remission in ocular inflammatory diseases. Eur J Ophthalmol 2009;19:118–23. [DOI] [PubMed] [Google Scholar]
- [32].Chipont E, Espana E, Sanchez S, et al. Intraocular penetration of cyclosporin A in uveitis. Archivos de la Sociedad Espanola de Oftalmologia 1993;64:487–94. [Google Scholar]
- [33].Cordero-Coma M, Yilmaz T, Onal S. Systematic review of anti-tumor necrosis factor-alpha therapy for treatment of immune-mediated uveitis. Ocul Immunol Inflamm 2013;21:12–20. [DOI] [PubMed] [Google Scholar]
- [34].Cordero-Coma M, Calvo-Rio V, Adan A, et al. Golimumab as rescue therapy for refractory immune-mediated uveitis: a three-center experience. Mediators Inflamm 2014;2014:717598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cuchacovich M, Gatica H, Verdaguer JI, et al. Treatment of non infectious ocular inflammatory disease with low doses of cyclosporin A. Tratamiento con dosis bajas de ciclosporina A en pacientes con enfermedad ocular inflamatoria de etiologia no infecciosa 1999;127:277–85. [PubMed] [Google Scholar]
- [36].Davatchi F, Shahram F, Chams H, et al. High dose methotrexate for ocular lesions of Behcet's disease. Preliminary short-term results. Adv Exp Med Biol 2003;528:579–84. [DOI] [PubMed] [Google Scholar]
- [37].de Fidelix TSA, Vieira LA, de Freitas D, et al. Biologic therapy for refractory scleritis: a new treatment perspective. Int Ophthalmol 2015;35:903–12. [DOI] [PubMed] [Google Scholar]
- [38].Demiroglu H, Ozcebe OI, Barista I, et al. Interferon alfa-2b, colchicine, and benzathine penicillin versus colchicine and benzathine penicillin in Behcet's disease: a randomised trial. Lancet 2000;355:605–9. [DOI] [PubMed] [Google Scholar]
- [39].Deuter CME, Zierhut M, Mohle A, et al. Long-term remission after cessation of interferon-alpha treatment in patients with severe uveitis due to Behcet's disease. Arthritis Rheum 2010;62:2796–805. [DOI] [PubMed] [Google Scholar]
- [40].Diaz-Llopis M, Garcia-Delpech S, Salom D, et al. Adalimumab therapy for refractory uveitis: a pilot study. J Ocul Pharmacol Ther 2008;24:351–61. [DOI] [PubMed] [Google Scholar]
- [41].Diaz-Llopis M, Salom D, Garcia-de-Vicuna C, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology 2012;119:1575–81. [DOI] [PubMed] [Google Scholar]
- [42].Dick AD, Tugal-Tutkun I, Foster S, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology 2013;4:777–87. [DOI] [PubMed] [Google Scholar]
- [43].Durrani K, Kempen JH, Ying GS, et al. Adalimumab for ocular inflammation. Ocular Immunol Inflamm 2016;25:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Flores M, Gudino Perez R, Rios Prado R, et al. Comparative study of the treatment of autoimmune uveitis with prednisone and with cyclophosphamide and azathioprine. Estudio comparativo entre el tratamiento de la uveitis autoinmune con prednisona y con ciclofosfamida y azatioprina 2001;48:75–9. [PubMed] [Google Scholar]
- [45].Foster CS, Tufail F, Waheed NK, et al. Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate. Arch Ophthalmol 2003;4:437–40. [DOI] [PubMed] [Google Scholar]
- [46].Fujino Y, Joko S, Masuda K, et al. Ciclosporin microemulsion preconcentrate treatment of patients with Behcet's disease. Jpn J Ophthalmol 1999;43:318–26. [DOI] [PubMed] [Google Scholar]
- [47].Galor A, Jabs DA, Leder HA, et al. Comparison of antimetabolite drugs as corticosteroid-sparing therapy for noninfectious ocular inflammation. Ophthalmology 2008;115:1826–32. [DOI] [PubMed] [Google Scholar]
- [48].Galor A, Perez VL, Hammel JP, et al. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology 2006;113:2317–23. [DOI] [PubMed] [Google Scholar]
- [49].Giardina A, Ferrante A, Ciccia F, et al. One year study of efficacy and safety of infliximab in the treatment of patients with ocular and neurological Behcet's disease refractory to standard immunosuppressive drugs. Rheumatol Int 2011;31:33–7. [DOI] [PubMed] [Google Scholar]
- [50].Gueudry J, Wechsler B, Terrada C, et al. Long-term efficacy and safety of low-dose interferon alpha2a therapy in severe uveitis associated with Behcet disease. Am J Ophthalmol 2008;146:837–44.e1. [DOI] [PubMed] [Google Scholar]
- [51].Hasanreisoglu M, Cubuk MO, Ozdek S, et al. Interferon alpha-2a therapy in patients with refractory Behçet uveitis. Ocular Immunol Inflamm 2016;25:71–5. [DOI] [PubMed] [Google Scholar]
- [52].Hogan AC, McAvoy CE, Dick AD, et al. Long-term efficacy and tolerance of tacrolimus for the treatment of uveitis. Ophthalmology 2007;114:1000–6. [DOI] [PubMed] [Google Scholar]
- [53].Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2010;52:ra72. [DOI] [PubMed] [Google Scholar]
- [54].Interlandi E, Leccese P, Olivieri I, et al. Adalimumab for treatment of severe Behcet's uveitis: a retrospective long-term follow-up study. Clin Exp Rheumatol 2014;32(suppl 84):S58–62. [PubMed] [Google Scholar]
- [55].Isnard Bagnis C, Tezenas du Montcel S, Beaufils H, et al. Long-term renal effects of low-dose cyclosporine in uveitis-treated patients: follow-up study. J Am Soc Nephrol 2002;13:2962–8. [DOI] [PubMed] [Google Scholar]
- [56].Joshi L, Talat L, Yaganti S, et al. Outcomes of changing immunosuppressive therapy after treatment failure in patients with noninfectious uveitis. Ophthalmology 2014;121:1119–24. [DOI] [PubMed] [Google Scholar]
- [57].Jouve L, Benrabah R, Héron E, et al. Multiple sclerosis-related uveitis: Does MS treatment affect uveitis course? Ocular Immunol Inflamm 2016;25:302–7. [DOI] [PubMed] [Google Scholar]
- [58].Kaplan-Messas A, Barkana Y, Avni I, et al. Methotrexate as a first-line corticosteroid-sparing therapy in a cohort of uveitis and scleritis. Ocular Immunol Inflamm 2003;11:131–9. [DOI] [PubMed] [Google Scholar]
- [59].Kavandi H, Khabbazi A, Kolahi S, et al. Long-term efficacy and safety of interferon α-2a therapy in severe refractory ophthalmic Behcet's disease. Clin Rheumatol 2003;35:2765–9. [DOI] [PubMed] [Google Scholar]
- [60].Krause L, Altenburg A, Pleyer U, et al. Longterm visual prognosis of patients with ocular Adamantiades-Behcet's disease treated with interferon-alpha-2a. J Rheumatol 2008;35:896–903. [PubMed] [Google Scholar]
- [61].Kruh JN, Yang P, Suelves AM, et al. Infliximab for the treatment of refractory noninfectious Uveitis: a study of 88 patients with long-term follow-up. Ophthalmology 2014;121:358–64. [DOI] [PubMed] [Google Scholar]
- [62].Larkin G, Lightman S. Mycophenolate mofetil. A useful immunosuppressive in inflammatory eye disease. Ophthalmology 1999;106:370–4. [DOI] [PubMed] [Google Scholar]
- [63].Lau CH, Comer M, Lightman S. Long-term efficacy of mycophenolate mofetil in the control of severe intraocular inflammation. Clin Exp Ophthalmol 2003;31:487–91. [DOI] [PubMed] [Google Scholar]
- [64].Lee SH, Chung H, Yu HG. Clinical outcomes of cyclosporine treatment for noninfectious uveitis. Korean J Ophthalmol 2012;26:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lian F, Zhou J, Wei C, et al. Anti-TNFα agents and methotrexate in spondyloarthritis related uveitis in a Chinese population. Clin Rheumatol 2015;34:1913–20. [DOI] [PubMed] [Google Scholar]
- [66].Martel JN, Esterberg E, Nagpal A, et al. Infliximab and adalimumab for uveitis. Ocul Immunol Inflamm 2012;20:18–26. [DOI] [PubMed] [Google Scholar]
- [67].Ozyazgan Y, Yurdakul S, Yazici H, et al. Low dose cyclosporin A versus pulsed cyclophosphamide in Behçet's syndrome: a single masked trial [Internet]. Br J Ophthalmol. 1992;4:241–3. Available at: http://onlinelibrarywileycom/o/cochrane/clcentral/articles/283/CN-00087283/framehtml. Accessed March 10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Papaliodis GN, Chu D, Foster CS. Treatment of ocular inflammatory disorders with daclizumab. Ophthalmology 2003;110:786–9. [DOI] [PubMed] [Google Scholar]
- [69].Prete M, Guerriero S, Dammacco R, et al. Autoimmune uveitis: a retrospective analysis of 104 patients from a tertiary reference center. J Ophthalmic Inflamm Infect 2014;4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Riancho-Zarrabeitia L, Calvo-Rio V, Blanco R, et al. Anti-TNF-alpha therapy in refractory uveitis associated with sarcoidosis: multicenter study of 17 patients. Semin Arthritis Rheum 2015;45:361–8. [DOI] [PubMed] [Google Scholar]
- [71].Rudwaleit M, Rosenbaum JT, Landewé R, et al. Observed incidence of uveitis following certolizumab pegol treatment in patients with axial spondyloarthritis. Arthritis Care Res 2016;68:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Saenz A, Ausejo M, Shea B, et al. Pharmacotherapy for Behcet's syndrome. Cochrane Database of Syst Rev 2000;2:CD001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sainz de la Maza M, Molina N, Gonzalez-Gonzalez LA, et al. Scleritis therapy. Ophthalmology 2012;119:51–8. [DOI] [PubMed] [Google Scholar]
- [74].Sainz de la Maza M, Molina N, Gonzalez-Gonzalez LA, et al. Scleritis associated with relapsing polychondritis. Br J Ophthalmol 2016;100:1290–4. [DOI] [PubMed] [Google Scholar]
- [75].Sakai T, Watanabe H, Kuroyanagi K, et al. Health- and vision-related quality of life in patients receiving infliximab therapy for Behcet uveitis. Br J Ophthalmol 2013;97:338–42. [DOI] [PubMed] [Google Scholar]
- [76].Shakoor A, Esterberg E, Acharya NR. Recurrence of uveitis after discontinuation of infliximab. Ocul Immunol Inflamm 2014;22:96–101. [DOI] [PubMed] [Google Scholar]
- [77].Sieper J, Koenig A, Baumgartner S, et al. Analysis of uveitis rates across all etanercept ankylosing spondylitis clinical trials. Ann Rheum Dis 2010;69:226–9. [DOI] [PubMed] [Google Scholar]
- [78].Simonini G, Cimaz R, Jones GT, et al. Non-anti-TNF biologic modifier drugs in non-infectious refractory chronic uveitis: The current evidence from a systematic review. Semin Arthritis Rheum 2015;45:238–50. [DOI] [PubMed] [Google Scholar]
- [79].Smith JR, Levinson RD, Holland GN, et al. Differential efficacy of tumor necrosis factor inhibition in the management of inflammatory eye disease and associated rheumatic disease. Arthritis Rheum 2001;45:252–7. [DOI] [PubMed] [Google Scholar]
- [80].Sobaci G, Erdem U, Durukan AH, et al. Safety and effectiveness of interferon alpha-2a in treatment of patients with Behcet's uveitis refractory to conventional treatments. Ophthalmology 2010;117:1430–5. [DOI] [PubMed] [Google Scholar]
- [81].Sobrin L, Kim EC, Christen W, et al. Infliximab therapy for the treatment of refractory ocular inflammatory disease. Arch Ophthalmol 2007;125:895–900. [DOI] [PubMed] [Google Scholar]
- [82].Sobrin L, Christen W, Foster CS. Mycophenolate mofetil after methotrexate failure or intolerance in the treatment of scleritis and uveitis. Ophthalmology 2008;115:1416–21.e1. [DOI] [PubMed] [Google Scholar]
- [83].Suhler EB, Smith JR, Wertheim MS, et al. A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol 2005;123:903–12. [DOI] [PubMed] [Google Scholar]
- [84].Suhler EB, Lowder CY, Goldstein DA, et al. Adalimumab therapy for refractory uveitis: results of a multicentre, open-label, prospective trial. Br J Ophthalmol 2013;97:481–6. [DOI] [PubMed] [Google Scholar]
- [85].Suhler EB, Lim LL, Beardsley RM, et al. Rituximab therapy for refractory scleritis: results of a phase I/II dose-ranging, randomized, clinical trial. Ophthalmology 2014;121:1885–91. [DOI] [PubMed] [Google Scholar]
- [86].Sullu Y, Oge I, Erkan D, et al. Cyclosporin: a therapy in severe uveitis of Behcet's disease. Acta Ophthalmol Scand 1998;76:96–9. [DOI] [PubMed] [Google Scholar]
- [87].Takeuchi M, Asukata Y, Kawagoe T, et al. Infliximab monotherapy versus infliximab and colchicine combination therapy in patients with Behcet's disease. Ocul Immunol Inflamm 2012;20:193–7. [DOI] [PubMed] [Google Scholar]
- [88].Takeuchi M. A systematic review of biologics for the treatment of noninfectious uveitis. Immunotherapy 2013;5:91–102. [DOI] [PubMed] [Google Scholar]
- [89].Takeuchi M, Kezuka T, Sugita S, et al. Evaluation of the long-term efficacy and safety of infliximab treatment for uveitis in Behcet's disease: a multicenter study. Ophthalmology 2014;121:1877–84. [DOI] [PubMed] [Google Scholar]
- [90].Tugal-Tutkun I, Guney-Tefekli E, Urgancioglu M. Results of interferon-alfa therapy in patients with Behcet uveitis. Graefes Arch Clin Exp Ophthalmol [Albrecht von Graefes Archiv für klinische und experimentelle Ophthalmologie] 2006;244:1692–5. [DOI] [PubMed] [Google Scholar]
- [91].Tugal-Tutkun I, Kadayifcilar S, Khairallah M, et al. Safety and efficacy of gevokizumab in patients with Behçet's disease uveitis: results of an exploratory phase 2 study. Ocul Immunol Inflamm 2016;25:62–70. [DOI] [PubMed] [Google Scholar]
- [92].Vallet H, Riviere S, Sanna A, et al. Efficacy of anti-TNF alpha in severe and/or refractory Behcet's disease: multicenter study of 124 patients. J Autoimmun 2015;62:67–74. [DOI] [PubMed] [Google Scholar]
- [93].Vallet H, Seve P, Biard L, et al. Infliximab versus adalimumab in the treatment of refractory inflammatory uveitis: a multicenter study from the French Uveitis Network. Arthritis Rheumatol 2016;68:1522–30. [DOI] [PubMed] [Google Scholar]
- [94].Vitale AT, Rodriguez A, Foster CS. Low-dose cyclosporin A therapy in treating chronic, noninfectious uveitis. Ophthalmology 1996;103:365–74. [DOI] [PubMed] [Google Scholar]
- [95].Wieringa WG, Wieringa JE, ten Dam-van Loon NH, et al. Visual outcome, treatment results, and prognostic factors in patients with scleritis. Ophthalmology 2013;120:379–86. [DOI] [PubMed] [Google Scholar]
- [96].Wu D, Guo Y-Y, Xu N-N, et al. Efficacy of anti-tumor necrosis factor therapy for extra-articular manifestations in patients with ankylosing spondylitis: a meta-analysis. BMC Musculoskelet Disord 2015;16:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Yazici H, Pazarli H, Barnes CG, et al. A controlled trial of azathioprine in Behçet's syndrome [Internet]. N Engl J Med. 1990;5:281–5. Available at: http://onlinelibrarywileycom/o/cochrane/clcentral/articles/848/CN-00064848/framehtml. Accessed March 10, 2016. [DOI] [PubMed] [Google Scholar]
- [98].Zaghetto JM, Yamamoto MM, Souza MB, et al. Chlorambucil and cyclosporine A in Brazilian patients with Behcet's disease uveitis: a retrospective study. Arq Bras Oftalmol 2010;73:40–6. [DOI] [PubMed] [Google Scholar]
- [99].Bachta A, Kisiel B, Tłustochowicz M, et al. High efficacy of methotrexate in patients with recurrent idiopathic acute anterior uveitis: a prospective study. Arch Immunol Ther Exp 2016;65:93–7. [DOI] [PubMed] [Google Scholar]
- [100].Calvo-Río V, Blanco R, Santos-Gómez M, et al. Golimumab in refractory uveitis related to spondyloarthritis. Multicenter study of 15 patients. Semin Arthritis Rheum 2016;46:95–101. [DOI] [PubMed] [Google Scholar]
- [101].Dobner BC, Max R, Becker MD, et al. A three-centre experience with adalimumab for the treatment of non-infectious uveitis [Internet]. Br J Ophthalmol. 2013;2:134–8. Available at: http://onlinelibrarywileycom/o/cochrane/clcentral/articles/415/CN-00859415/framehtml. Accessed March 10, 2016. [DOI] [PubMed] [Google Scholar]
- [102].Dougados M, Berenbaum F, Maetzel A, et al. Prevention of acute anterior uveitis associated with spondylarthropathy induced by salazosulfapyridine. Rev Rhum Ed Fr 1993;60:81–3. [PubMed] [Google Scholar]
- [103].Gangaputra S, Newcomb CW, Liesegang TL, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology 2009;116:2188–98.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kacmaz RO, Kempen JH, Newcomb C, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology 2010;117:576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Mathews JD, Crawford BA, Bignell JL, et al. Azathioprine in active chronic iridocyclitis. A double-blind controlled trial [Internet]. Br J Ophthalmol. 1969;5:327–30. Available at: http://onlinelibrarywileycom/o/cochrane/clcentral/articles/222/CN-00003222/framehtml. Accessed March 11, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Munoz-Fernandez S, Hidalgo V, Fernandez-Melon J, et al. Sulfasalazine reduces the number of flares of acute anterior uveitis over a one-year period. J Rheumatol 2003;30:1277–9. [PubMed] [Google Scholar]
- [107].Pasadhika S, Kempen JH, Newcomb CW, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol 2009;148:500–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Rudwaleit M, Rodevand E, Holck P, et al. Adalimumab effectively reduces the rate of anterior uveitis flares in patients with active ankylosing spondylitis: results of a prospective open-label study. Ann Rheum Dis 2009;68:696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Samson CM, Waheed N, Baltatzis S, et al. Methotrexate therapy for chronic noninfectious uveitis: analysis of a case series of 160 patients. Ophthalmology 2001;108:1134–9. [DOI] [PubMed] [Google Scholar]
- [110].Yazgan S, Celik U, Işık M, et al. Efficacy of golimumab on recurrent uveitis in HLA-B27-positive ankylosing spondylitis. Int Ophthalmol 2016;37:139–45. [DOI] [PubMed] [Google Scholar]
- [111].de La Forest Divonne M, Gottenberg JE, Salliot C. Safety of biologic DMARDs in RA patients in real life: a systematic literature review and meta-analyses of biologic registers. Joint Bone Spine 2016;84:133–40. [DOI] [PubMed] [Google Scholar]
- [112].Matsuda J, Kaburaki T, Kobayashi S, et al. Treatment of recurrent anterior uveitis with infliximab in patient with ankylosing spondylitis. Jpn J Ophthalmol 2013;57:104–7. [DOI] [PubMed] [Google Scholar]
- [113].El-Shabrawi Y, Hermann J. Anti-tumor necrosis factor-alpha therapy with infliximab as an alternative to corticosteroids in the treatment of human leukocyte antigen B27-associated acute anterior uveitis. Ophthalmology 2002;109:2342–6. [DOI] [PubMed] [Google Scholar]
- [114].Calvo-Rio V, Santos-Gomez M, Calvo I, et al. Anti-IL6-R Tocilizumab for Severe Juvenile Idiopathic Arthritis-Associated Uveitis Refractory to anti-TNF therapy. A multicenter study of 25 patients. Arthritis Rheumatol 2016;69:668–75. [DOI] [PubMed] [Google Scholar]
- [115].Fabiani C, Vitale A, Lopalco G, et al. Different roles of TNF inhibitors in acute anterior uveitis associated with ankylosing spondylitis: state of the art. Clin Rheumatol 2016;35:2631–8. [DOI] [PubMed] [Google Scholar]


