Abstract
Background:
The aim of this study was to explore the effects of action observation therapy on motor function of upper extremity, activities of daily living, and motion evoked potential in cerebral infarction patients.
Method:
Cerebral infarction survivors were randomly assigned to an experimental group (28 patients) or a control group (25 patients). The conventional rehabilitation treatments were applied in both groups, but the experimental group received an additional action observation therapy for 8 weeks (6 times per week, 20 minutes per time). Fugl-Meyer assessment (FMA), Wolf Motor Function Test (WMFT), Modified Barthel Index (MBI), and motor evoked potential (MEP) were used to evaluate the upper limb movement function and daily life activity.
Results:
There were no significant differences between experiment and control group in the indexes, including FMA, WMFT, and MBI scores, before the intervention. However, after 8 weeks treatments, these indexes were improved significantly. MEP latency and center-motion conduction time (CMCT) decreased from 23.82 ± 2.16 and 11.15 ± 1.68 to 22.69 ± 2.11 and 10.12 ± 1.46 ms. MEP amplitude increased from 0.61 ± 0.22 to 1.25 ± 0.38 mV. A remarkable relationship between the evaluations indexes of MEP and FMA was found.
Conclusions:
Combination of motion observation and traditional upper limb rehabilitation treatment technology can significantly elevate the movement function of cerebral infarction patients in subacute seizure phase with upper limb dysfunction, which expanded the application range of motion observation therapy and provided an effective therapy strategy for upper extremities hemiplegia in stroke patients.
Keywords: action observation therapy, cerebral infarction, mirror neuron system, motor evoked potential, rehabilitation, upper extremity motor function
1. Introduction
Cerebral ischemic stroke, which is also known as cerebral infarction, accounts for about 60% to 80% of stroke and is an important cause of upper extremities hemiplegia, causing the severe disability in hand functions, especially loss of grip, because of complex modes of upper limbs dyskinesia. Furthermore, pain, joint contracture, and discomfort exerting by the upper limbs dyskinesia results in limb disuse and hinders the long-term functional recovery.[1,2] As much as 80% stroke patients have upper extremities hypokinesia in the acute phase and only 5% to 20% patients can recover upper extremities function even if accepting rehabilitation therapy.[3,4] Hence, it is urgent to find effective ways to improve recovery efficacy in cerebral infarction patients, especially in the acute phase.
Mirror neuron system (MNS) is a new recovery strategy for upper extremities in cerebral infarction patients. When patients are observing or imitating other's movements, the same neuron system, as their own movements, can be activated in some degrees. Since the discovery of MNS, it has been used in motion and understanding of the behavior intention, speech, empathy, and social interaction.[5,6] The physical therapy intervention has been implemented based on the neural system in cerebral infarction patients because the physical functions such as cognitive, perceptive, visual, and emotional has been damaged in cerebral infarction patients. Relative study[7] has demonstrated mirror neurons were activated in monkeys’ cerebral cortex, which promoted the current treatments of many diseases with the mirror therapy. Burns[8] applied motor acts observation in rehabilitation recovery of stroke patients and found that it could accelerate the return to functional activities. However, the mechanism of how MSN improves the movement function of cerebral infarction patients has not been well revealed.
Previous studies have pointed out a relationship between motor evoked potential (MEP) and functional rehabilitation.[9,10] Based on the aforesaid facts, we deduced that MSN may improve recovery efficacy in cerebral infarction patients through mediating MEP. Hence, based on the theory of mirror neurons, this study applied action observation therapy to stimulate the rebuilding of the movement function of upper extremities and activities of daily living in cerebral infarction patients and determined the change of brain excitability using motion evoked potential.
2. Materials and methods
2.1. Patients
Stroke patients who were hospitalized in Zhejiang Jiaxing Second Hospital Rehabilitation Center between June 2014 and September 2016 were recruited in this study. Eligible patients had to meet the following inclusion criteria: conform to cerebral infarction diagnostic criteria formulated by Chinese Society of Neurology, Chinese Medical Association; unilateral hemiplegia; first-episode of cerebral infarction determined by CT and MRI; stable vital signs; disease course of 2 to 6 months; age of 40 to 75 years; mini-mental state examination (MME) score ≥27 and treatment instructions can be performed; Fugl-Meyer assessment (FMA) score ≥20 for upper extremity motor function; binocular vision or corrected visual acuity ≥1.0; everyday treatment can be tolerant; and providing informed consent and willingness to participate in the study. Some patients were excluded if they had cerebral hemorrhage, subarachnoid hemorrhage, venous sinus thrombosis, transient ischemic attack, and progressive or reversible ischemic cerebral apoplexy; lesions located in bilateral cerebral hemisphere, cerebellum, or brain stem; joint and other diseases affecting patients sitting or activity; heart, lung, liver, kidney, and other serious diseases; metal implantation in the body; a history of epilepsy; and skull defect. The experiment was approved by Ethics Committee in Zhejiang Jiaxing Second Hospital. All participants gave written informed consent.
2.2. Treatment methods
Eligible participants were randomly divided to receive conventional therapy (control group,) or additional action observation (experimental group) based on a random number table (Fig. 1).
Figure 1.
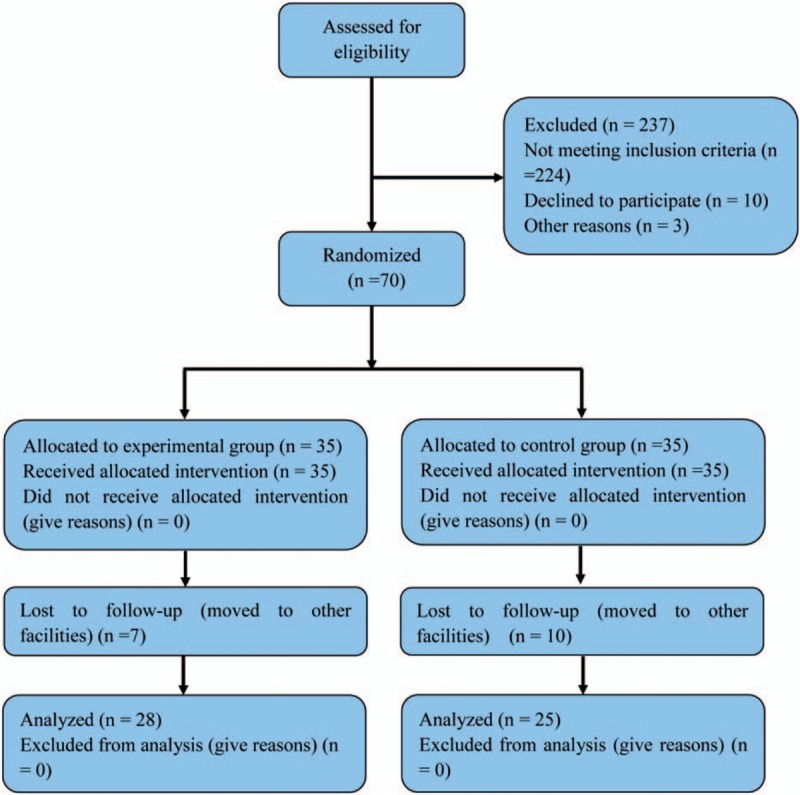
Flow chart of the cases included in this study.
Patients in both groups were treated with drugs to control blood pressure, blood glucose, antiplatelet aggregation and lipid plaque stabilization, and so on. In addition, traditional rehabilitation treatment such as Bobath, Brunnstrom, proprioceptive neuromuscular facilitation, and daily activity ability training were also used in 2 groups. Patients in experimental group received additional action observation treatment which was carried on 6 d/w, 1 time/d and 20 min/time, including watching action video for 10 minutes and imitating 10 minutes. Action observation therapy was performed as follows (Fig. 2): Participants sat before a 42-in color TV with a distance of 2 m and lay their hemiplegia side arm on the table. Participants were requested to watch upper limbs motion video and informed to imitate the action in the video. There were 30 actions in the video, including shoulder joint, elbow joint, wrist joints, forearm, and hand movements in all directions. All the action was recorded by the same model and every action was filmed from 2 different angles for 50 seconds. The action was numbered according to their complexity. No. 1 is the easiest and No. 30 is the most difficult. The action with the similar complexity was set as a group. Action No. 1 to No. 6 was named as group 1. The rest can be done in the same manner. Participants watched the video from group 1 and then imitated the action. If they cannot complete, they would continue to watch the same video and imitate. If not, they would watch the video and imitate in the next group. This treatment lasted 8 weeks. Patients in control group watched different geometric patterns and digit symbol, and did the action picked from the following 30 actions in the video 6 d/w, 1 time/d, and 20 min/time.
Figure 2.

Action observation therapies for participants.
2.3. Assessment methods
FMA, Wolf Motor Function Test (WMFT), and Modified Barthel Index (MBI) were used to assess the motor function and the ability of daily living activities before and after 8 weeks of treatment. FMA items include shoulder, elbow, wrist flexion and extension cooperative movement, wrist joint stability, coordination ability, and speed of small joint movement (such as hand grip and finger side pinched). The assessment was divided into 10 categories and 33 events. The highest score in each event is 2 and the total score is 66.[11,12] WMFT was used to evaluate upper extremity performance while providing insight into joint-specific and total limb movements. It includes 15 tasks, 6 timed joint-segment movements and 9 timed integrative functional movements. The completion time was recorded and the quality of action was graded. The highest score is 5 and the total score is 75.[13,14] MBI includes 10 items to assess the activities of daily living eating, including dressing, shower, and so on. The total score is 100.[15,16]
Motion evoked potential (MEP) was determined using RAPID2 transcranial magnetic stimulator (Magstim, England) and RAPID2 MEP kit (Magstim, England). The determination progress was as follows: cerebral cortex M1 area and seventh cervical spinous process was selected as stimulation point. A stimulation intensity of 120% threshold was given to the stimulation point. Five waves with good repeatability and big amplitude were selected to calculate the MEP latency, amplitude, and center-motion conduction time (CMCT). MEP latency is the delay between the onset of stimulation on affected side cortexes M1 phase and the initiation of the MEP in contralateral thumb short abductor muscle. MEP amplitude means potential difference between the highest and lowest motion evoked potential. CMCT indicate the difference between cortexes latency and spinal latency. All these assessments were made by 2 trained professional therapists and the whole process was recorded. Subsequently, the average score from another 2 trained professional therapists was used to be analyzed.
2.4. Statistical analysis
Statistical analysis was performed by using SPSS 17.0 (SPSS Inc, Chicago, IL). One-simple Kolmogorov-Smirnov test showed all measurement data conform to normal distribution. All data were expressed as mean ± SD. Paired t test was used to perform intragroup comparison and nonpaired t test was used for comparison among groups.
Pearson method was performed to judge the correlation between FMA scores and MEP latency, MEP amplitude, and CMCT. P value <.05 was considered to be statistically significant.
3. Results
3.1. Patients
There were 53 participants included in this study. They were randomly divided into a control group (25 patients) and an experimental group (28 patients). A comparison of general material such as gender, age, course of the disease, and hemiplegia were conducted in 2 groups and no significant differences were found (Table 1).
Table 1.
General material of participants in 2 groups.

3.2. Treatment effect
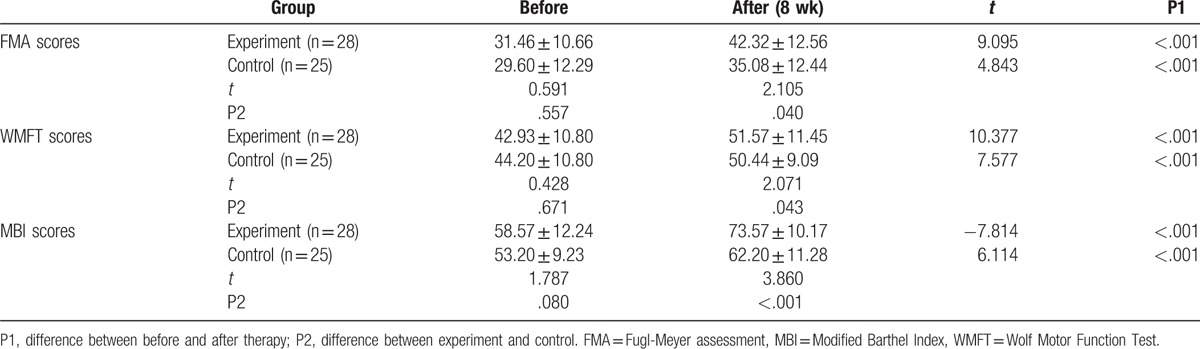
To determine the treatment effect of MSN, the FMA, WMFT, and MBI scores were determined to assess the motor function and the ability of daily living activities, respectively. As shown in Table 2, no significant differences were shown in FMA, WMFT, and MBI scores between experiment group and control group before therapy (P > .05). After therapy for 8 weeks, FMA, WMFT, and MBI scores increased significantly compared with that before therapy both in experiment group and control group. Moreover, these indexes in experimental group were higher than the control group, and a remarkable difference could be found (P < .05). These results showed that combined MSN and tradition rehabilitation therapy could improve motor function and the ability of daily living activities in cerebral infarction patients effectively.
Table 2.
FMA, WMFT, and BI scores comparison between 2 groups before and after therapy.
3.3. Movement transmission mechanism
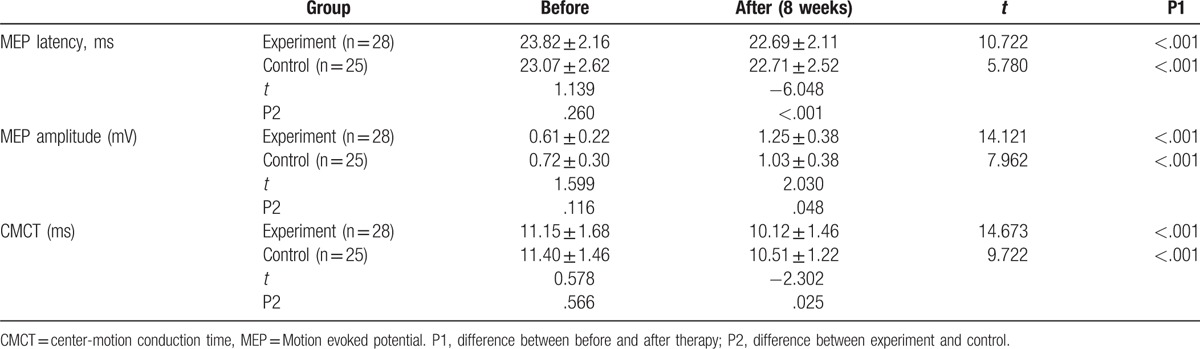
To reveal the mechanism of how MSN improves motor function and the ability of daily living activities, MEP latency, MEP amplitude, and CMCT were measured. The results are listed in Table 3. Significant statistical differences could be found in MEP latency, MEP amplitude, and CMCT between before and after treatment both in experiment and control group (P < .05). In experiment group, MEP latency and CMCT were remarkably shorter from 23.82 ± 2.16 and 11.15 ± 1.68 to 22.69 ± 2.11 and 10.12 ± 1.46 ms, respectively (P < .05). And MEP amplitude was increased from 0.61 ± 0.22 to 1.25 ± 0.38 mV, significantly (P < .05).
Table 3.
MEP comparison between 2 groups patients before and after therapy.
In addition, a significant negative correlation could be found in FMA scores and MEP latency, and FMA scores and CMCT, and a positive correlation in FMA scores and MEP amplitude (Fig. 3). These results indicated that MSN improved motor function and the ability of daily living activities by mediating MEP and CMCT.
Figure 3.
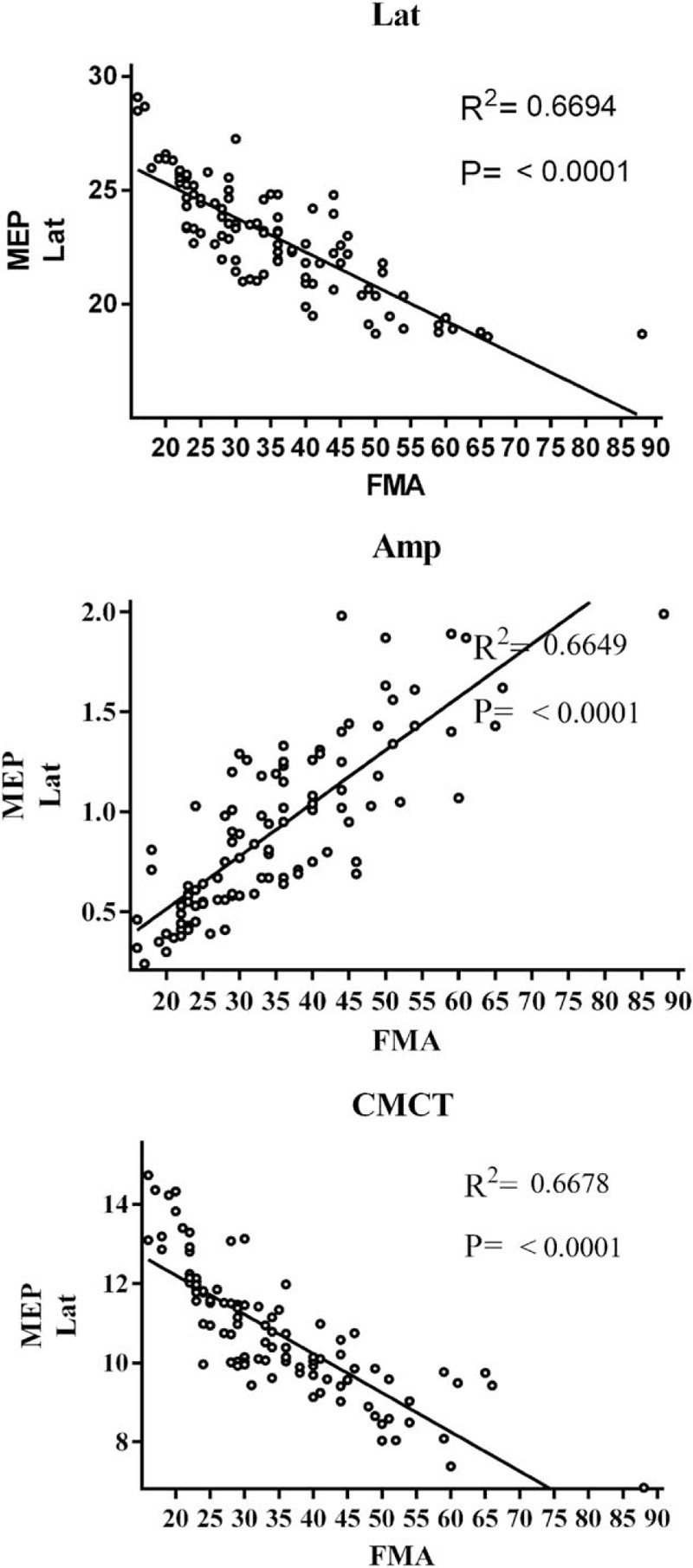
The correlation between FMA scores and MEP latency, and FMA scores and CMCT. CMCT = center-motion conduction time, FMA = Fugl-Meyer assessment, MEP = motion-evoked potential.
4. Discussion
The incidence of disability in stroke patients is still high although its mortality has decreased significantly with the improvement of medical level.[17] Especially, the upper limb movement function in stroke patients has the characteristics of high incidence and poor treatment effect and has always been a big problem to be solved urgently in the international field of neurological rehabilitation. In this study, we found that a combination of MSN and traditional rehabilitation therapy could significantly elevate extremity motor function in subacute stroke patients and improve the MEP.
Action observation therapy has been demonstrated to elevate the upper extremity motor function in chronic stroke patients in previous studies.[18–20] For example, Ertelt[21] applied action observation therapy to cure 8 chronic stroke patients for 4 weeks. The results showed upper extremity motor function was improved remarkably. Through functional magnetic resonance imaging, they found bilateral ventral premotor cortex, bilateral superior temporal gyrus, the supplementary motor area (SMA), and the contralateral supramarginal gyrus were elevated in experimental group than that in control group. Furthermore, action observation therapy also has a positive effect on walking and language ability.[22,23] Kim et al[24] studied the relative alpha power and relative beta power change after action observation and the motor imagery training. They found action observation induces stronger cognitive activity. Mattia[25] and Liepert[26] determined the effects of action observation on damaged motor circuits induced by transcranial magnetic stimulation (TMS) and the results indicate action observation might have a positive influence on the recovery of motor functions after acute and chronic stroke.
With the rapid development of magnetic stimulation technology, the determination of MEP induced by TMS has been a popular method to judge the change of central nervous system plasticity of rehabilitation therapy in stroke patients. MEP abnormal mainly depends on the corticospinal tract conduction function of movement.[27] It is closely related to the degree of patients’ paralysis. MEP latency reflects the transmission from cortex to muscle and the integrity of the conductive pathway.[28] CMCT reflects the function of upper motor neuron and motor neuron. In this study, MEP latency and CMCT reflects had no significant difference between 3 groups without intervention. However, MEP latency was improved and CMCT was shortening significantly compared with pretreatment after intervention. Meantime, the experimental groups were superior to the control group. These results indicated action observation therapy could improve motor nerve excitability. In addition, we found a negative relationship between FMA scores and MEP latency and CMCT, and a positive relationship between FMA scores and MEP amplitude. These results also demonstrate MEP could be used to assess upper limb movement. In this study, we applied FMA, WMFT, MBI, and MEP to evaluate the upper limb movement function and daily life activity in patients with cerebral infarction. This method is widely used in assessment of movement function in stroke patients, and its test-retest reliability and inter-rater reliability has been investigated. We found motor observation treatment based on mirror neurons theory improves the upper limb movement function and daily life activities in patients with cerebral infarction.
Mirror neurons theory has been seen as the theoretical basis of motor observation therapy.[29] Mirror neurons refer to the neurons that encode an action is activated in the observer's cortical motor system when an individual observes another individual performing an action.[30] There is a correlation between different mirror neurons. These mirror neurons which have correlation were named as mirror neuron system (MNS). Iacoboni et al[31] found mirror neurons excitability was elevated when participant imitates a finger movement after observing. Buccino[32] also acquired the similar conclusion. Musically naive participants’ mirror neurons became active during the observation and imitation of the guitar chords. The excitability of premotor cortex and secondary somatosensory cortex contralateral were affected after receiving motor rehabilitation therapy.[33]
The findings suggest that mirror neurons theory could be used in rehabilitation therapy on stroke patients. Through watching and imitating action, the MNS could be activated which could promote the patients to acquire new motor skills. For stroke patients with hemiplegia, by observing the upcoming training movement, mirror neurons which control the same action could be activated and its excitability could be increased. The ability of mirror neurons to complete the training was therefore improved. So learning by imitating can improve the motor function. This study found combination of motor observation and traditional upper limb rehabilitation strategy could effectively improve the upper limb movement function in subacute stroke patients. Meantime, it also indicates that motor observation is an effective way to elevate human motor function. We deduced that MNS is the main mechanism for improving limb movement function in subacute stroke patients.
This study was aimed at cerebral infarction patients in subacute seizure phase with upper limb dysfunction and made a combination of motion observation and traditional upper limb rehabilitation treatment technology. The results showed that this strategy can significantly elevate the movement function of cerebral infarction patients in subacute seizure phase with upper limb dysfunction, which expanded the application range of motion observation therapy and provided an effective therapy strategy for upper extremities hemiplegia in stroke patients. However, this experiment was restricted by the amount of samples and a further supplement was needed.
Footnotes
Abbreviations: CMCT = center-motion conduction time, FMA = Fugl-Meyer assessment, MBI = Modified Barthel Index, MEP = motor evoked potential, MNS = mirror neuron system, TMS = transcranial magnetic stimulation, WMFT = Wolf Motor Function Test.
The study was supported by Science and Technology Planning Project of Jiaxing City, Zhejiang Province, Project No. 2014AY21031-9; National Natural Science Foundation of China, Project No. 81201504; and General plan for medical and health research in Zhejiang Province, Project No. 2014KYA212.
The authors have no conflicts of interest to disclose.
References
- [1].Cirstea MC, Levin MF. Improvement of arm movement patterns and endpoint control depends on type of feedback during practice in stroke survivors. Neurorehabil Neural Repair 2007;21:398–411. [DOI] [PubMed] [Google Scholar]
- [2].Cacchio A, De BE, De BV, et al. Mirror therapy in complex regional pain syndrome type 1 of the upper limb in stroke patients. Neurorehabil Neural Repair 2009;23:792. [DOI] [PubMed] [Google Scholar]
- [3].Kwakkel G, Kollen BJ, Van der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34:2181–6. [DOI] [PubMed] [Google Scholar]
- [4].Spieler JF, Lanoë JL, Amarenco P. Costs of stroke care according to handicap levels and stroke subtypes. Cerebrovasc Dis 2004;17:134–42. [DOI] [PubMed] [Google Scholar]
- [5].Minichino A, Cadenhead K. Mirror neurons in psychiatric disorders: from neuroception to bio-behavioral system dysregulation. Neuropsychopharmacology 2017;42:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yarmand H, Ashayeri H, Golfam A, et al. The effect of mirror neurons stimulation on syntax development of female Persian autistic children 2016;6:67. [Google Scholar]
- [7].Werf YDVD, Helm EVD, Schoonheim MM, et al. Learning by observation requires an early sleep window. Proc Natl Acad Sci USA 2009;106:18926–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Burns MS. Application of neuroscience to technology in stroke rehabilitation. Top Stroke Rehabil 2008;15:570–9. [DOI] [PubMed] [Google Scholar]
- [9].Kim GW, Yu HW, Park SH, et al. Can motor evoked potentials be an objective parameter to assess extremity function at the acute or subacute stroke stage? Ann Rehabil Med 2015;39:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee SY, Lim JY, Kang EK, et al. Prediction of good functional recovery after stroke based on combined motor and somatosensory evoked potential findings. J Rehabil Med 2010;42:16–20. [DOI] [PubMed] [Google Scholar]
- [11].Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 2002;16:232. [DOI] [PubMed] [Google Scholar]
- [12].Fu TS, Wu CY, Lin KC, et al. Psychometric comparison of the shortened Fugl-Meyer Assessment and the streamlined Wolf Motor Function Test in stroke rehabilitation. Clin Rehabil 2012;26:1043–7. [DOI] [PubMed] [Google Scholar]
- [13].Wolf SL, Catlin PA, Ellis M, et al. Assessing Wolf motor function test as outcome measure for research in patients after stroke 2001;32:1635–9. [DOI] [PubMed] [Google Scholar]
- [14].Chen HF, Wu CY, Lin KC, et al. Measurement properties of streamlined wolf motor function test in patients at subacute to chronic stages after stroke. Neurorehabil Neural Repair 2014;28:839. [DOI] [PubMed] [Google Scholar]
- [15].Duffy L, Gajree S, Langhorne P, et al. Reliability (inter-rater agreement) of the Barthel Index for Assessment of stroke survivors systematic review and meta-analysis. Stroke 2013;44:462–8. [DOI] [PubMed] [Google Scholar]
- [16].van Exel NJ, Scholte op Reimer WJ, Koopmanschap MA. Assessment of post-stroke quality of life in cost-effectiveness studies: the usefulness of the Barthel Index and the EuroQoL-5D. Qual Life Res 2004;13:427–33. [DOI] [PubMed] [Google Scholar]
- [17].Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol 2010;9:1228–32. [DOI] [PubMed] [Google Scholar]
- [18].Franceschini M, Agosti M, Cantagallo A, et al. Mirror neurons: action observation treatment as a tool in stroke rehabilitation. Eur J Phys Rehabil Med 2010;46:517–23. [PubMed] [Google Scholar]
- [19].Sale P, Franceschini M. Action observation and mirror neuron network: a tool for motor stroke rehabilitation. Eur J Phys Rehabil Med 2012;48:313–8. [PubMed] [Google Scholar]
- [20].Eunjoo K, Kyeongmi K. Effect of purposeful action observation on upper extremity function in stroke patients. J Phys Ther Sci 2015;27:2867–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ertelt D, Small S, Solodkin A, et al. Action observation has a positive impact on rehabilitation of motor deficits after stroke. NeuroImage 2007;36:T164–73. [DOI] [PubMed] [Google Scholar]
- [22].Park HJ, Oh DW, Choi JD, et al. Action observation training of community ambulation for improving walking ability of patients with post-stroke hemiparesis: a randomized controlled pilot trial. Clin Rehabil 2016;31:1078–86. [DOI] [PubMed] [Google Scholar]
- [23].Chen W, Ye Q, Ji X, et al. Mirror neuron system based therapy for aphasia rehabilitation. Front Psychol 2015;6:1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim J, Lee B, Lee HS, et al. Differences in brain waves of normal persons and stroke patients during action observation and motor imagery. J Phys Ther Sci 2014;26:215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Marangon M, Priftis K, Fedeli M, et al. Lateralization of motor cortex excitability in stroke patients during action observation: a TMS Study. Biomed Res Int 2014;2014:251041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liepert J, Greiner J, Dettmers C. Motor excitability changes during action observation in stroke patients. J Rehabil Med 2014;46:400. [DOI] [PubMed] [Google Scholar]
- [27].Steppan J, Meaders T, Muto M, et al. A metaanalysis of the effectiveness and safety of ozone treatments for herniated lumbar discs. J Vasc Int Radiol 2010;21:534. [DOI] [PubMed] [Google Scholar]
- [28].Fujiki M, Kobayashi H, Abe T, et al. Repetitive transcranial magnetic stimulation for protection against delayed neuronal death induced by transient ischemia. J Neurosurg 2003;99:1063. [DOI] [PubMed] [Google Scholar]
- [29].Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci 2010;11:264–74. [DOI] [PubMed] [Google Scholar]
- [30].Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci 2004;27:169–92. [DOI] [PubMed] [Google Scholar]
- [31].Iacoboni M, Woods RP, Brass M, et al. Cortical mechanisms of human imitation. Science 1999;286:2526. [DOI] [PubMed] [Google Scholar]
- [32].Buccino G, Vogt S, Ritzl A, et al. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron 2004;42:323–34. [DOI] [PubMed] [Google Scholar]
- [33].Johansenberg H, Dawes H, Guy C, et al. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 2002;125:2731–42. [DOI] [PubMed] [Google Scholar]


