Abstract
Background:
This meta-analysis aimed to illustrate the efficacy and safety of combined topical and intravenous (IV) tranexamic acid (TXA) for blood loss control in primary total knee arthroplasty (TKA) patients.
Methods:
In April 2017, a systematic computer-based search was conducted in PubMed, EMBASE, Web of Science, Cochrane Database of Systematic Reviews, and Google database. Data on patients prepared for TKA surgery in studies that compared combined topical and IV TXA versus placebo, topical, or IV TXA alone were retrieved. The primary endpoint was the need for transfusion, total blood loss, hemoglobin drop, and the occurrence of deep venous thrombosis (DVT), pulmonary embolism (PE), and the infection. After testing for publication bias and heterogeneity between studies, data were aggregated for random-effects models when necessary.
Results:
Seven clinical studies were ultimately included in the meta-analysis. Compared with IV TXA and control group, combined TXA was associated with less need for transfusion, blood loss, and hemoglobin drop (P < .05). There was no significant difference between the combined TXA and topical TXA in terms of the need for transfusion, total blood loss, and hemoglobin drop (P > .05). There was no significant difference between the complications (DVT, PE, and infection) between the combined TXA, IV TXA, topical TXA, and control group.
Conclusions:
Current meta-analysis suggests that the combined IV and topical TXA was superior than IV TXA or control group. There is still need for more studies to identify whether combined TXA was superior than topical TXA alone.
Keywords: blood loss, combined, meta-analysis, total knee arthroplasty, tranexamic acid
1. Introduction
A total knee arthroplasty (TKA) is a well established surgery that alleviates knee pain and improves function for end-stage knee disease such as osteoarthritis or rheumatoid arthritis.[1,2] However, TKA involves substantial postoperative blood loss and thus subsequently blood transfusion was increased.[3] Allogeneic blood transfusion has potential risks of infection of HIV and other infectious disease.[4,5] Recent studies have explored on topical or intravenous (IV) hemostatic and pharmacological drugs for blood loss control after TKA. Tranexamic acid (TXA), a synthetic lysine analogously drug, inhibits fibrinolysis by competitively bond the lysine-binding sites of plasminogen.[6] Several meta-analyses have shown that TXA can reduce the perioperative blood loss and subsequent blood transfusion in TKA.[7,8] However, the optimal regimen and dose of TXA remained debatable.
IV TXA is most often used, and has been identified to be very safe and effective. Topical TXA during TKA has been described as a safe and cost-effective option to IV TXA. Another method was combined intra-articular and IV TXA for blood loss in TKA. Although several randomized controlled trials (RCTs) have been published and show positive results about combined topical and IV TXA. It should be noted that the sample size of these RCTs was limited (ranging from 30 to 92 patients), which may affect the accuracy of relevant conclusions. Therefore, we undertook a meta-analysis to evaluate whether combined TXA is superior to intra-articular or IV with respect to need for transfusion; total blood loss; hemoglobin drop; and the occurrence of deep venous thrombosis (DVT), pulmonary embolism (PE), and infection.
2. Methods
This meta-analysis was conducted in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions[9] and was written in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) checklist.[10]
2.1. Search strategy
The following electronic databases were systematically searched from their inception through April 2017: PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, and the Google database. The detailed PubMed search strategy was as follows: ((((((“Arthroplasty, Replacement, Knee”[Mesh]) OR TKR) OR TKA) OR total knee replacement) OR total knee arthroplasty)) AND tranexamic acid. This is a meta-analysis and thus no ethical approval was necessary for this meta-analysis.
2.2. Inclusion criteria and exclusion criteria
Inclusion criteria included RCTs; comparison of combined intra-articular TXA and IV TXA versus IV TXA, topical TXA or control group in patients undergoing TKA; the mean age of included patients were older than 18 years; the finally outcomes incorporated need for transfusion, total blood loss, hemoglobin drop, and postoperative complications (DVT, PE, and infection); and the included studies were restricted to English language.
Exclusion criteria included “case report,” “review,” “letters,” “talk,” and “commentary”; and data were duplicated or overlapped.
2.3. Data extraction and outcome measures
For each published study included in the meta-analysis, 2 authors (Zhao Wang and Xiaofei Shen) independently extracted the following data: author, publication year, number of patients, proportion of male patients, mean age of the patients, outcomes, duration of the follow-up period, and surgery type. Any disagreement was resolved by discussion. The outcome measures were total infection rate, and the infection rate among low-risk, moderate-risk, and high-risk category patients. We extracted the mean and standard deviation values using GetData Graph Digitizer software as needed.[9]
2.4. Risk of bias assessment
Two reviewers (Zhao Wang and Xiaofei Shen) independently evaluated the risk of bias of RCTs according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions.[9] A total of 7 items were assessed and a graph was drawn for the summary of the risk of bias.
2.5. Statistical analysis
For dichotomous outcomes (need for transfusion and the occurrence of DVT, PE, and infection), we calculated the risk ratio (RR) and the 95% confidence interval (95% CI). For continuous outcomes (total blood loss and the hemoglobin drop), we calculated weighted mean difference (WMD) and the 95% CI. Heterogeneity was considered to be statistically significant if the I2 value was greater than 50%. A fixed-effects model was applied if the I2 value was less than 50%. All statistical analyses were conducted using Stata 12.0 (Stata Corp., College Station, TX). A P value less than .05 was considered statistically significant. Kappa values were used to measure the degree of agreement between the 2 reviewers and were rated as follows: fair, 0.40 to 0.59; good, 0.60 to 0.74; and excellent, 0.75 or higher.[11]
3. Results
3.1. Search results and general characteristic
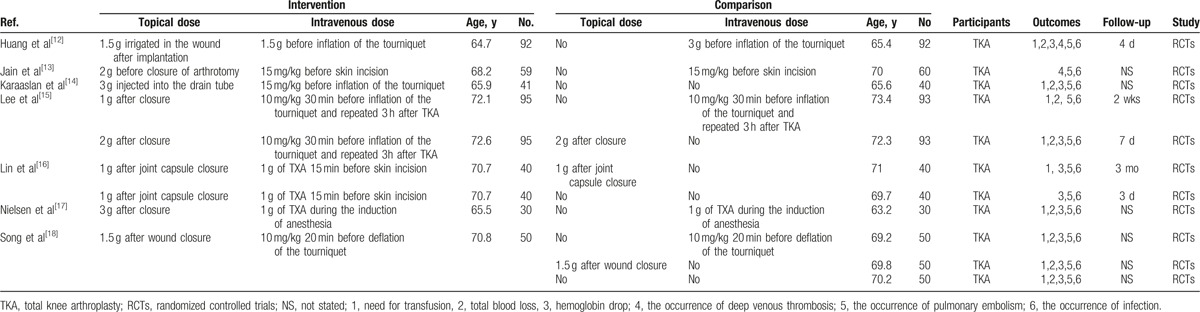
The literature search and selection process are illustrated in Fig. 1. The initial search yielded 288 articles (PubMed = 76, Embase = 98, Web of Science = 75, Cochrane Library = 22, Google database = 17). After excluding duplications, 209 studies were examined. Next, 202 of the studies were excluded on the basis of the inclusion criteria. Finally, we included 7 RCTs in this meta-analysis.[12–18] The general characteristic of the included studies can be obtained in Table 1. The topical TXA dose ranged from 1 to 3 g. The IV dose of TXA ranged from 1 to 3 g. The follow-up ranged from 3 days to 3 months.
Figure 1.
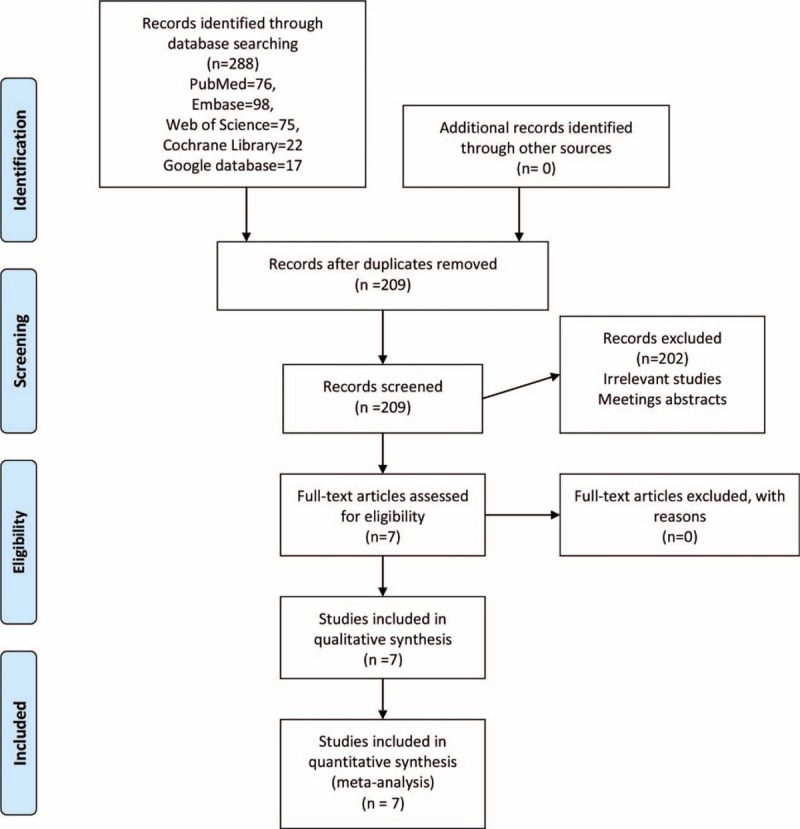
Flowchart of study search and inclusion criteria.
Table 1.
The general characteristics of the included RCTs.
3.2. Risk of bias
The risk of bias graph and risk of bias summary are summarized in Figs. 2 and 3. All trials reported random sequence generation; only 1 study did not report the allocation concealment[17] and identify as an unclear risk of bias. The rest of bias was all with a low risk of bias.
Figure 2.
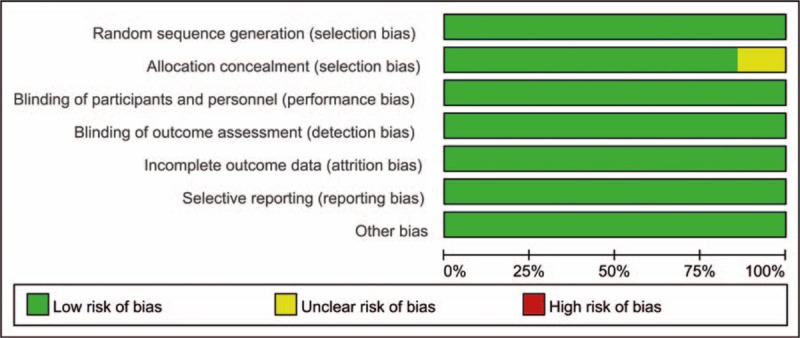
The risk of bias graph.
Figure 3.
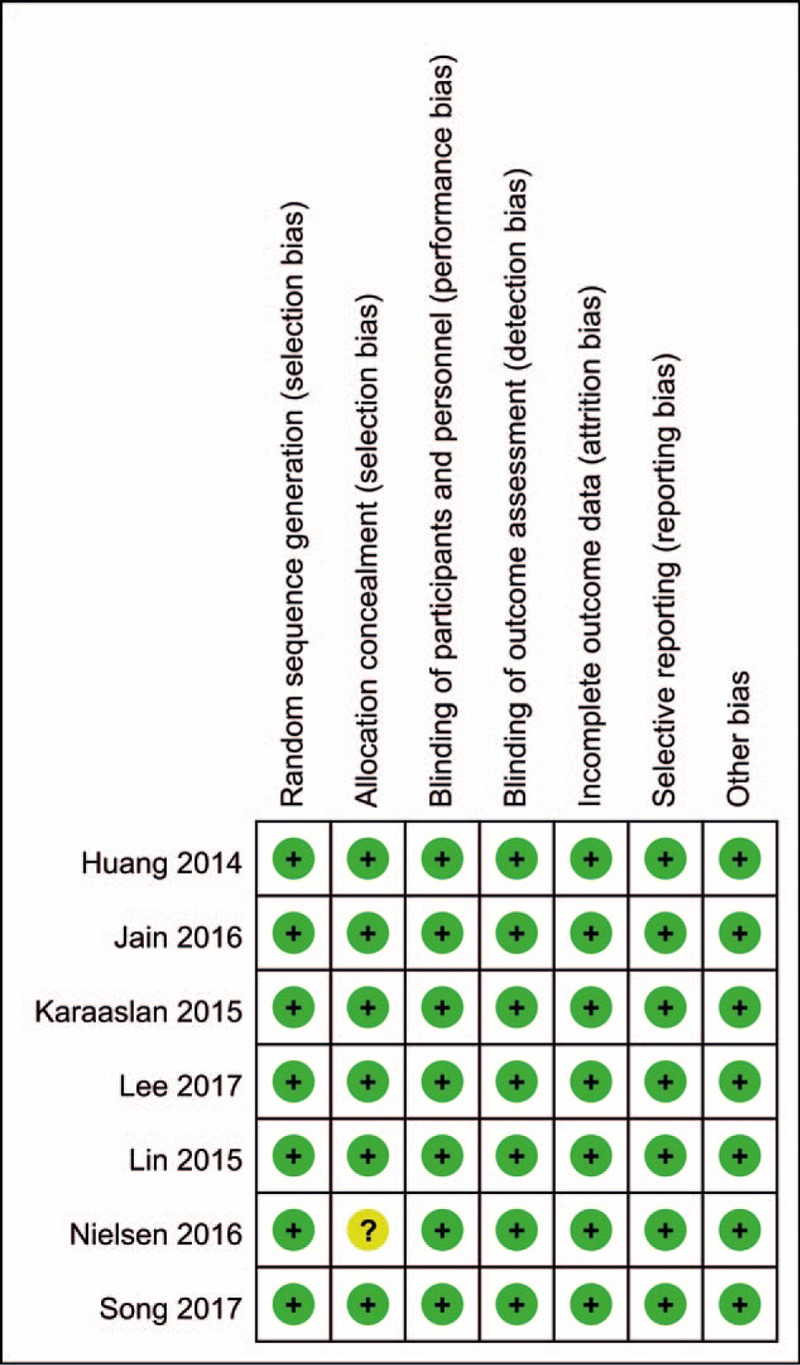
Risk of bias of included randomized controlled trials. +, no bias; −, bias; ?, bias unknown.
3.3. Results of meta-analysis
3.3.1. Need for transfusion
There were a total of 5 studies involving 779 patients reported available data of need for transfusion between the combined group and IV TXA group. There was no heterogeneity between the included studies (I2 = 0.0%, P = .779). Compared with IV TXA group, combined TXA was associated with a less need for transfusion (RR = 0.46, 95% CI = 0.23–0.89, P = .021) (Fig. 4).
Figure 4.
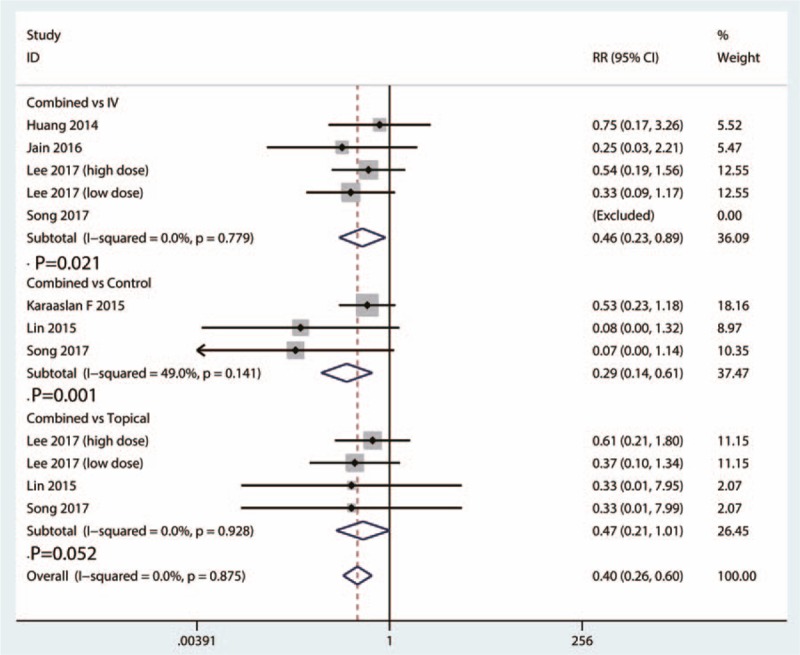
Forest plots of the included studies comparing the need for transfusion.
A total of 3 studies involving 261 patients provided data of need for transfusion between the combined group and control group. There was a moderate statistical evidence of heterogeneity between the included studies (I2 = 49.0%, 95% CI = 32.1–53.7, P = .141). Compared with control group, combined TXA was associated with a less need for transfusion (RR = 0.29, 95% CI = 0.14–0.61, P = .001) (Fig. 4).
A total of 4 studies provided data of need for transfusion between the combined group and topical TXA group. The heterogeneity test indicated no statistical evidence of heterogeneity (I2 = 0.0%, 95% CI = 0.0–0.0, P = .928). There was no statistically significant difference between the need for transfusion between combined group and topical group (RR = 0.47, 95% CI = 0.21–1.01, P = .052) (Fig. 4).
3.4. Total blood loss
Total blood loss between the combined group and IV TXA group was reported in a total of 5 studies. There was a high statistical evidence of heterogeneity between the included studies (I2 = 70.2%, 95% CI = 55.9–81.6, P = .779). Compared with IV TXA group, combined TXA was associated with less total blood loss (WMD = −131.85, 95% CI = −203.65 to −60.05, P = .000) (Fig. 5).
Figure 5.
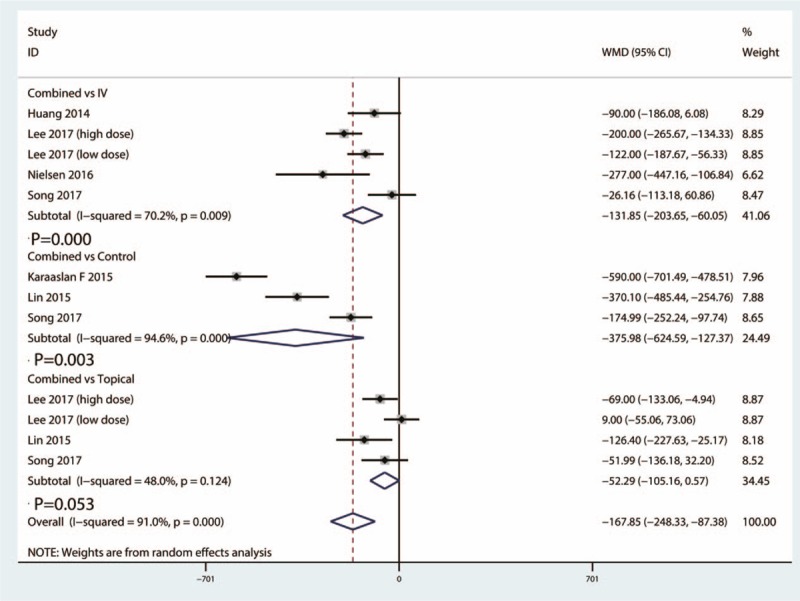
Forest plots of the included studies comparing the total blood loss.
Data from 3 studies reported total blood loss between the combined group and control group. The heterogeneity test indicated high statistical evidence of heterogeneity (I2 = 94.6%, 95% CI = 88.5–97.4, P = .000). Compared with control group, combined TXA was associated with less total blood loss (WMD = −375.98, 95% CI = −624.59 to −127.37, P = .003) (Fig. 5).
Data from 4 studies reported total blood loss between the combined group and topical TXA group. The heterogeneity test indicated moderate statistical evidence of heterogeneity (I2 = 48.0%, 95% CI = 35.1–51.3, P = .124). Final result indicated that there was no significant difference between combined group and topical group in terms of total blood loss (WMD = −52.29, 95% CI = −105.16 to 0.57, P = .053) (Fig. 5).
3.5. Hemoglobin drop
A total of 5 studies provided data of hemoglobin drop between the combined group and IV TXA group. There was a high heterogeneity between the included studies in terms of hemoglobin drop (I2 = 90.1%, 95% CI = 84.3–96.4, P = .000). Compared with IV TXA group, combined TXA was associated with less hemoglobin drop (WMD = −1.27, 95% CI = −1.92 to −0.61, P = .000) (Fig. 6).
Figure 6.
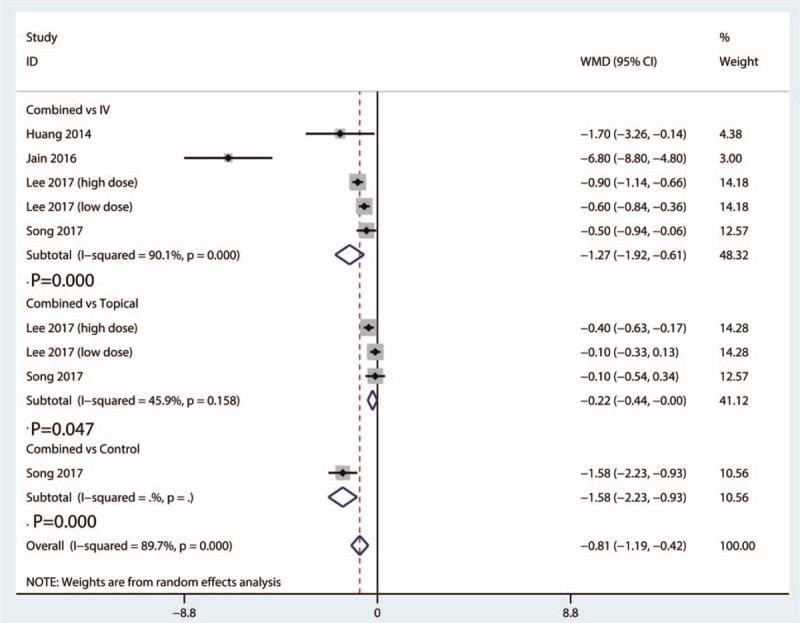
Forest plots of the included studies comparing the hemoglobin drop.
Data from 3 studies provided outcome of hemoglobin drop between the combined group and topical group. There was a moderate heterogeneity between the included studies (I2 = 45.9%, 95% CI = 45.4–50.6, P = .158). Compared with topical group, combined TXA was associated with less hemoglobin drop (WMD = −0.22, 95% CI = −0.44 to −0.00, P = .047) (Fig. 6).
Only 1 study provided hemoglobin drop outcome between the combined group and control TXA group. Compared with control group, combined TXA was associated with less hemoglobin drop (WMD = −1.58, 95% CI = −2.23 to −0.937, P = .000) (Fig. 6).
3.6. The occurrence of DVT
A total of 5 studies provided data of the occurrence of DVT between the combined group and IV TXA group. There was no heterogeneity between the included studies of this outcome (I2 = 0.0%, 95% CI = 0.0–0.0, P = .812). There was no significant difference between the occurrence of DVT between the combined group and IV TXA group [risk difference (RD) = −0.01, 95% CI = −0.02 to 0.01, P = .412] (Fig. 7).
Figure 7.
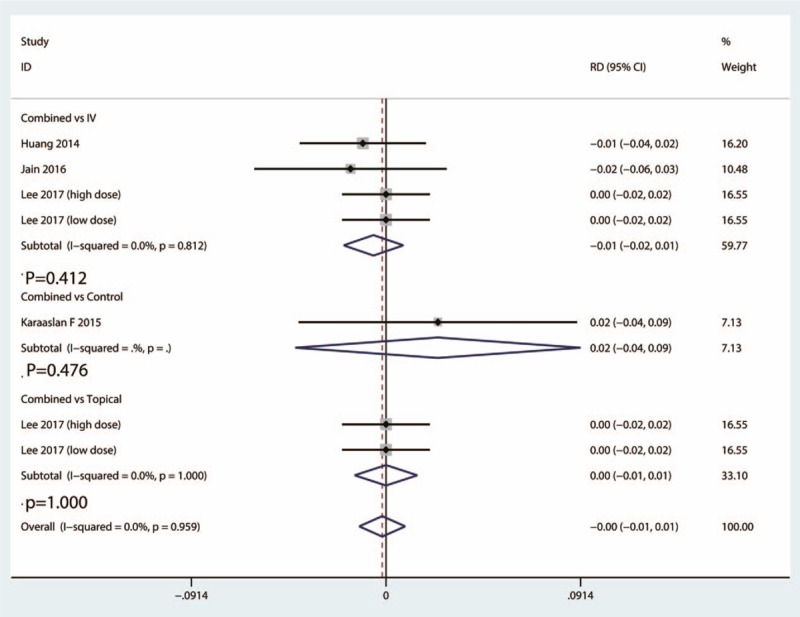
Forest plots of the included studies comparing the occurrence of DVT.
Data from 2 studies provided the occurrence of DVT between the combined group and topical group. There was a moderate heterogeneity between the included studies (I2 = 0.0%, 95% CI = 0.0–0.0, P = 1.000). There was no significant difference between the occurrence of DVT between the combined group and topical TXA group (RD = 0.00, 95% CI = −0.04 to 0.09, P = 1.000) (Fig. 7).
Only 1 study provided the occurrence of DVT between the combined group and control TXA group. There was no significant difference between the occurrence of DVT between the combined group and control group (RD = 0.02, 95% CI = −2.23 to −0.937, P = .476) (Fig. 7).
3.7. The occurrence of PE
Data from 4 studies provided data of the occurrence of PE between the combined group and IV TXA group. There was no statistical evidence of heterogeneity (I2 = 0.0%, 95% CI = 0.0–0.0, P = .885). There was no significant difference between the occurrence of PE between the combined group and IV TXA group (RD = −0.01, 95% CI = −0.02 to 0.01, P = .408) (Fig. 8).
Figure 8.
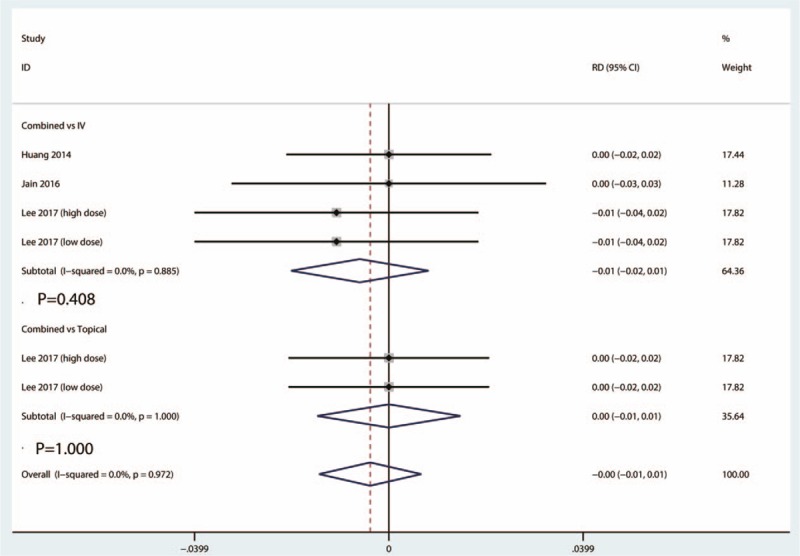
Forest plots of the included studies comparing the occurrence of PE.
Data from 2 studies provided the occurrence of PE between the combined group and topical group. There was no statistical evidence of heterogeneity (I2 = 0.0%, 95% CI = 0.0–0.0, P = 1.000). There was no significant difference between the occurrence of PE between the combined group and topical TXA group (RD = 0.00, 95% CI = −0.01 to 0.01, P = 1.000) (Fig. 8).
3.8. The occurrence of infection
Data from 3 studies provided the occurrence of infection between the combined group and IV TXA group. Heterogeneity test indicated that there was no heterogeneity between the included 3 studies (I2 = 0.0%, 95% CI = 0.0–0.0, P = 1.000). There was no significant difference between the occurrence of infection between the combined group and IV TXA group (RD = 0.00, 95% CI = −0.01 to 0.01, P = 1.000) (Fig. 9).
Figure 9.
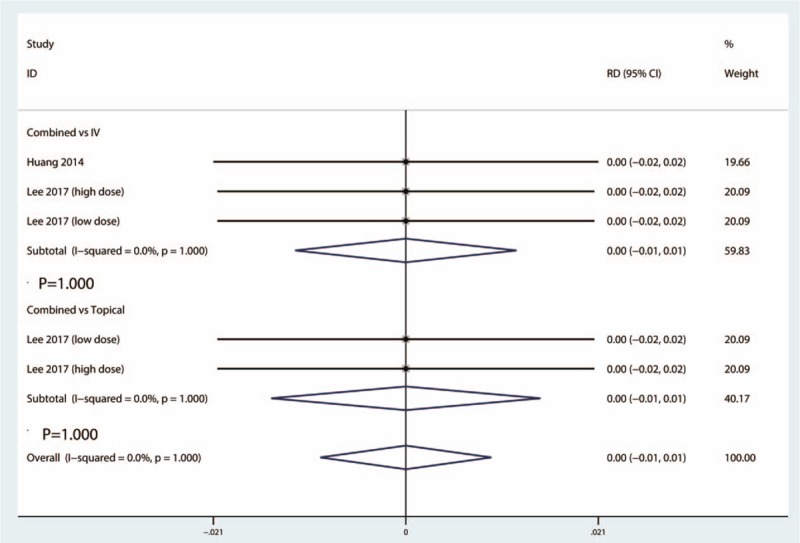
Forest plots of the included studies comparing the occurrence of infection.
A total of 2 studies provided data of the occurrence of infection between the combined group and topical group. The heterogeneity test indicated that there was no statistically heterogeneity between the included 2 studies (I2 = 0.0%, 95% CI = 0.0–0.0, P = 1.000). There was no significant difference between the occurrence of PE between the combined group and topical TXA group (RD = 0.00, 95% CI = −0.01 to 0.01, P = 1.000) (Fig. 9).
4. Discussion
This meta-analysis aimed to illustrate the optimal method to administration TXA in primary TKA patients. The common application methods of TXA include intra-articular, IV, and combined intra-articular and IV TXA. Pooled results indicated that combined intra-articular and IV TXA has similar hemostasis effects when compared with intra-articular TXA. Combined intra-articular and IV TXA was superior than IV TXA and control groups. For the complications (DVT, PE, and infection), there was no significant difference between the 3 application methods. To our knowledge, this is the largest sample meta-analysis comparing the efficacy of combined topical and IV TXA in TKA patients.
The major strength and highlight of this meta-analysis was that we concentrate on the patients of primary TKA patients and included only high-quality RCTs. In order to exclude the heterogeneity, we then conducted a meta-regression to identify whether TXA dose influence the final results. Although we perform a rigorous statistically calculation, the level of evidence was low, as the heterogeneity was large.
The result of our meta-analysis indicated that the combined group was associated with a less need for transfusion than control group and IV TXA group. Song et al[18] revealed that combined topical and IV TXA is not superior to either topical or IV in primary navigated TKA. And Lee et al[15] also revealed that no additional effect was found about topical TXA combined IV TXA in further reducing perioperative blood loss. Current meta-analysis indicated that topical administration TXA has similar hemostasis effects when compared with combined TXA. These outcomes were in contradict with previous meta-analysis.[19–22] Above meta-analysis included TKA and total hip arthroplasty (THA), and obviously, these TKA and THA have different blood loss. Another shortcoming was that non-RCTs were also included, thus the heterogeneity was large.
As for the complications (DVT, PE, and infection), there was no statistically difference between the combined group with control group, topical TXA group, and IV TXA group. TXA, as an antifibrinolytic agent, has potential complications of DVT and PE after TKA. Astedt et al[23] reported that TXA does not inhibit fibrinolytic activity in normal vein wall. Our outcomes are consistent with those of many previous studies, which found that TXA will not increase the incidence of symptomatic DVT or PE with the use of TXA in TKA at any dose, timing, or route of administration.[24–27]
There were a total of 5 limitations to this meta-analysis: only 7 RCTs with small samples were included, which have affected the precision of final outcomes; the follow-up time ranged from 24 to 48 hours after TKA and whether there was a difference at long-term follow up was unknown; the dosage and timing of TXA administration differed between the studies, although a subgroup analysis was conducted to decrease the heterogeneity, which would affect the precision of the results; the tourniquet or bandage administration differed from each other, and the large heterogeneity between the included studies will affect the final results; and we cannot completely exclude the publication bias between the included studies because a total of 7 RCTs were included in this meta-analysis.
5. Conclusion
The use of combined topical and IV TXA was superior than IV TXA alone and control groups in primary TKA patients. The topical administration TXA has similar hemostasis effects when compared with combined topical and IV TXA. There was no significant difference between the combined group, topical group and IV group in terms of the complications. The number of included studies and sample size were limited, and further studies are necessary to identify the optimal dose and protocols of combined TXA for reducing blood loss after TKA.
Footnotes
Abbreviations: CENTRAL = Cochrane Central Register of Controlled Trials, CI = confidence interval, DVT = deep venous thrombosis, PE = pulmonary embolism, RR = risk ratio, THA = total hip arthroplasty, TKA = total knee arthroplasty, TXA = tranexamic acid, WMD = weighted mean difference.
The authors report no conflicts of interest.
References
- [1].Li J, Deng X, Jiang T. Combined femoral and sciatic nerve block versus femoral and local infiltration anesthesia for pain control after total knee arthroplasty: a meta-analysis of randomized controlled trials. J Orthop Surg Res 2016;11:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu SQ, Chen X, Yu CC, et al. Comparison of periarticular anesthesia with liposomal bupivacaine with femoral nerve block for pain control after total knee arthroplasty: a PRISMA-compliant meta-analysis. Medicine (Baltimore) 2017;96:e6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jiang C, Lou J, Qian W, et al. Impact of flexion versus extension of knee position on outcomes after total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg 2017;137:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim JL, Park JH, Han SB, et al. Allogeneic blood transfusion is a significant risk factor for surgical-site infection following total hip and knee arthroplasty: a meta-analysis. J Arthroplasty 2017;32:320–5. [DOI] [PubMed] [Google Scholar]
- [5].Pawaskar A, Salunke AA, Kekatpure A, et al. Do autologous blood transfusion systems reduce allogeneic blood transfusion in total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [6].Zhang P, Liang Y, Chen P, et al. Intravenous versus topical tranexamic acid in primary total hip replacement: a meta-analysis. Medicine (Baltimore) 2016;95:e5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fu Y, Shi Z, Han B, et al. Comparing efficacy and safety of 2 methods of tranexamic acid administration in reducing blood loss following total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2016;95:e5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang S, Gao X, An Y. Topical versus intravenous tranexamic acid in total knee arthroplasty: a meta-analysis of randomized controlled trials. Int Orthop 2017;41:739–48. [DOI] [PubMed] [Google Scholar]
- [9].Higgins JPT, G. S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011. Available at: http://www.cochrane-handbook.org. Accessed date: 2011. [Google Scholar]
- [10].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang Z, Ma J, Shen B, et al. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty 2014;29:2342–6. [DOI] [PubMed] [Google Scholar]
- [13].Jain NP, Nisthane PP, Shah NA. Combined administration of systemic and topical tranexamic acid for total knee arthroplasty: can it be a better regimen and yet safe? A randomized controlled trial. J Arthroplasty 2016;31:542–7. [DOI] [PubMed] [Google Scholar]
- [14].Karaaslan F, Karaoglu S, Mermerkaya MU, et al. Reducing blood loss in simultaneous bilateral total knee arthroplasty: combined intravenous-intra-articular tranexamic acid administration. A prospective randomized controlled trial. Knee 2015;22:131–5. [DOI] [PubMed] [Google Scholar]
- [15].Lee SY, Chong S, Balasubramanian D, et al. What is the ideal route of administration of tranexamic acid in TKA? A randomized controlled trial. Clin Orthop Relat Res 2017;475:1987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lin SY, Chen CH, Fu YC, et al. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty 2015;30:776–80. [DOI] [PubMed] [Google Scholar]
- [17].Nielsen CS, Jans O, Orsnes T, et al. Combined intra-articular and intravenous tranexamic acid reduces blood loss in total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am 2016;98:835–41. [DOI] [PubMed] [Google Scholar]
- [18].Song EK, Seon JK, Prakash J, et al. Combined administration of IV and topical tranexamic acid is not superior to either individually in primary navigated TKA. J Arthroplasty 2017;32:37–42. [DOI] [PubMed] [Google Scholar]
- [19].Zhang P, Liang Y, Chen P, et al. Combined application versus topical and intravenous application of tranexamic acid following primary total hip arthroplasty: a meta-analysis. BMC Musculoskelet Disord 2017;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li JF, Li H, Zhao H, et al. Combined use of intravenous and topical versus intravenous tranexamic acid in primary total knee and hip arthroplasty: a meta-analysis of randomised controlled trials. J Orthop Surg Res 2017;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lin C, Qi Y, Jie L, et al. Is combined topical with intravenous tranexamic acid superior than topical, intravenous tranexamic acid alone and control groups for blood loss controlling after total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2016;95:e5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang XQ, Ni J, Ge WH. Combined use of intravenous and topical versus intravenous tranexamic acid in primary total joint arthroplasty: a meta-analysis of randomized controlled trials. Int J Surg 2017;38:15–20. [DOI] [PubMed] [Google Scholar]
- [23].Astedt B, Liedholm P, Wingerup L. The effect of tranexamic acid on the fibrinolytic activity of vein walls. Ann Chir Gynaecol 1978;67:203–5. [PubMed] [Google Scholar]
- [24].Moskal JT, Capps SG. Intra-articular tranexamic acid in primary total knee arthroplasty: meta-analysis. J Knee Surg 2017;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [25].Mi B, Liu G, Zhou W, et al. Intra-articular versus intravenous tranexamic acid application in total knee arthroplasty: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 2017;137:997–1009. [DOI] [PubMed] [Google Scholar]
- [26].Xie J, Hu Q, Huang Q, et al. Comparison of intravenous versus topical tranexamic acid in primary total hip and knee arthroplasty: an updated meta-analysis. Thromb Res 2017;153:28–36. [DOI] [PubMed] [Google Scholar]
- [27].Meena S, Benazzo F, Dwivedi S, et al. Topical versus intravenous tranexamic acid in total knee arthroplasty. J Orthop Surg (Hong Kong) 2017;25:2309499016684300. [DOI] [PubMed] [Google Scholar]


