Abstract
Paclitaxel has been shown to have clinical activity in the treatment of small cell lung cancer (SCLC). However, its role as third-line chemotherapy for SCLC after both etoposide- and camptothecin-based regimens has not been clarified.
All patients with refractory SCLC who were treated with paclitaxel-based regimen as third-line chemotherapy between 2005 and 2011 in Seoul National University Bundang Hospital were reviewed retrospectively. Forty patients previously treated with both etoposide- and camptothecin-based chemotherapy were included.
The median age of the enrolled patients was 67 years (range, 35–86 years). Most patients (77.5%) received cisplatin plus etoposide as first-line therapy, and camptothecins such as irinotecan or topotecan as second-line therapy. Of 34 patients with measurable lesions, 8 patients (23.5%) achieved partial response and 9 (26.5%) had stable disease. The median progression-free survival (PFS) and overall survival (OS) were 2.5 and 5.9 months, respectively. Predictive factors for OS were performance status (PS) (PS <2 vs ≥2; P = .001), the presence of liver metastasis (P < .001), and number of metastatic sites (<3 vs ≥3; P = .047) in univariate analysis. PS and liver metastasis also remained statistically significant in multivariate analysis. Grade 3 or 4 hematologic toxicity was 20% for neutropenia, and 10% for thrombocytopenia. Other common non-hematological toxicities were peripheral neuropathy and mild liver enzyme elevation.
Paclitaxel-based chemotherapy showed modest activity in SCLC patients refractory to both etoposide- and camptothecin-based chemotherapy. PS and presence of liver metastasis were predictive of survival after paclitaxel chemotherapy.
Keywords: paclitaxel, refractory, small cell lung cancer, third-line chemotherapy
1. Introduction
Small cell lung cancer (SCLC) is a very aggressive disease with dismal prognosis although it constitutes <20% of all lung cancer.[1] It is considered to be initially a chemosensitive and radiosensitive disease, but most patients eventually relapse. Long-term survivors who survive >5 years are approximately 10% and 2% in patients with limited disease (LD) and extensive disease (ED), respectively.[2]
The main cause of relapse or treatment failure in SCLC is thought to be the rapid development of chemoresistance. Therefore, non-cross-resistant chemotherapeutic agents have been introduced for SCLC patients after failure of first-line or second-line chemotherapy. Topotecan is the only approved drug for which survival benefit was shown especially in patients with sensitive disease when compared with best supportive care in randomized phase III trials.[3] In addition, irinotecan, paclitaxel, docetaxel, gemcitabine, vinorelbine, and ifosfamide alone or as combination regimens with other agents have been investigated as salvage therapy.[4–9] Amrubicin, a third-generation anthracycline and potent topoisomerase II inhibitor, showed around 40% response rate (RR) in the second-line setting in recent phase II trials.[10,11] However, in phase III trial, amrubicin did not improve survival, although a higher RR was observed than topotecan.[12]
Paclitaxel has been evaluated as a single agent or in combination with other drugs including carboplatin, etoposide, and ifosfamide in untreated or previously treated patients with SCLC. Two phase II trials for paclitaxel as a single agent were conducted in untreated patients with SCLC with 34% to 53% RR, in which paclitaxel was given at a dose of 250 mg/m2 by a 24-hour infusion.[13,14] Another phase II study was conducted in refractory SCLC patients with 29% RR for paclitaxel 175 mg/m2 given over a 3-hour infusion.[15] In addition, combination regimens of paclitaxel and other chemotherapeutics, most frequently carboplatin, with or without etoposide were investigated in previously untreated or treated patients with SCLC, which showed improved RR, but more increased toxicity than paclitaxel monotherapy.[16–19] However, there are few studies which evaluated the efficacy of paclitaxel in SCLC patients after both etoposide- and camptothecin-based regimens.
We intended to evaluate the efficacy and toxicity of paclitaxel-containing regimens as third-line chemotherapy in SCLC patients previously treated with both etoposide- and camptothecin-based regimens.
2. Methods
2.1. Patients
We retrospectively reviewed 40 patients who had given diagnoses of histologically or cytologically confirmed SCLC and treated with paclitaxel-based chemotherapy in addition as third-line chemotherapy at our hospital between January 2005 and February 2011. All patients received 2 prior chemotherapy including both etoposide- and camptothecin (irinotecan or topotecan)-based regimens, irrespective of sequence, before paclitaxel-based chemotherapy. These patients all had at least 1 measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.0[20] or an evaluable lesion, assessed using computed tomography (CT) or magnetic resonance imaging (MRI) at the initiation of paclitaxel-based chemotherapy. Other exclusion criteria were Eastern Cooperative Oncology Group (ECOG) performance status (PS) >3 or inadequate hematopoietic (absolute neutrophil count [ANC] < 1.5 × 109 L−1, platelet count <100 × 109 L−1), renal, or hepatic dysfunction. All patients gave written informed consent before initiating chemotherapy and this study was approved by the institutional review board of SNUBH.
2.2. Study treatment
Paclitaxel 175 mg/m2 was dissolved in 500 mL of 5% dextrose or 0.9% normal saline, and administered intravenously over 3 hours every 21 days. Treatment by either paclitaxel alone or combination regimen with platinum agents such as carboplatin and cisplatin was given according to the physician's choice. Cisplatin or carboplatin were given intravenously on day 1 of each cycle at a dose of 60 mg/m2 or AUC 5 mg/mL/min, respectively. Chemotherapy was continued until documentation of disease progression, unacceptable toxic effects, or patient refusal with a maximum of 6 cycles. The dose from the second cycle was modified according to toxicity in the previous cycle for each patient. To avoid acute paclitaxel allergic reactions for paclitaxel, patients were premedicated with 10 mg dexamethasone, 4 mg chlorpheniramine, and 300 mg cimetidine or 50 mg ranitidine, intravenously, 30 minutes before paclitaxel.
2.3. Evaluation of treatment efficacy and toxicity
Before each cycle of chemotherapy, we performed physical examination, laboratory tests, and chest radiography. The response to chemotherapy was evaluated in each patient according to the RECIST 1.0. Appropriate imaging studies, usually CT scans, were performed every 2 cycles. Toxicities were assessed using the National Cancer Institute-Common Toxicity Criteria (NCI-CTC, version 3.0) before each cycle of chemotherapy. Treatment delays and dose modifications were determined according to the degree of toxicities.
2.4. Statistical analysis
Overall survival (OS) was calculated from the first day of chemotherapy to the day of death or the last follow-up visit. Progression-free survival (PFS) was calculated from the first day of chemotherapy until the time of the first documentation of progression, death from any cause or to the date of last follow-up visit if no events had occurred. Confidence intervals (95% CI) for RR and disease control rate (DCR) were estimated by binomial distribution.[21] Survival curves were estimated by the Kaplan-Meier method for OS and PFS, and 95% CI for the median time to event was calculated by Greenwood formula.[21] The associations between RR or DCR and clinicopathologic variables were assessed by the chi-square or Fisher exact test. The log-rank test was used to compare the distribution of survival between groups in univariate analysis. Multivariate analyses were carried out using the Cox proportional hazard regression model including variables with P < .10 in univariate analysis. Two-sided P values of <.05 were considered to indicate statistical significant. All analyses were performed using SPSS for Windows, version 17.0 (SPSS Inc, Chicago, IL).
The dose intensity (DI) was calculated as the ratio of the total dose (expressed in milligram) per square meter of the patient, divided by the total treatment duration expressed in days. The end of treatment was considered to be 21 days after day 1 of the last cycle of chemotherapy. The relative DI was calculated as the ratio of the DI actually delivered to the DI planned by the protocol.
3. Results
3.1. Baseline characteristics
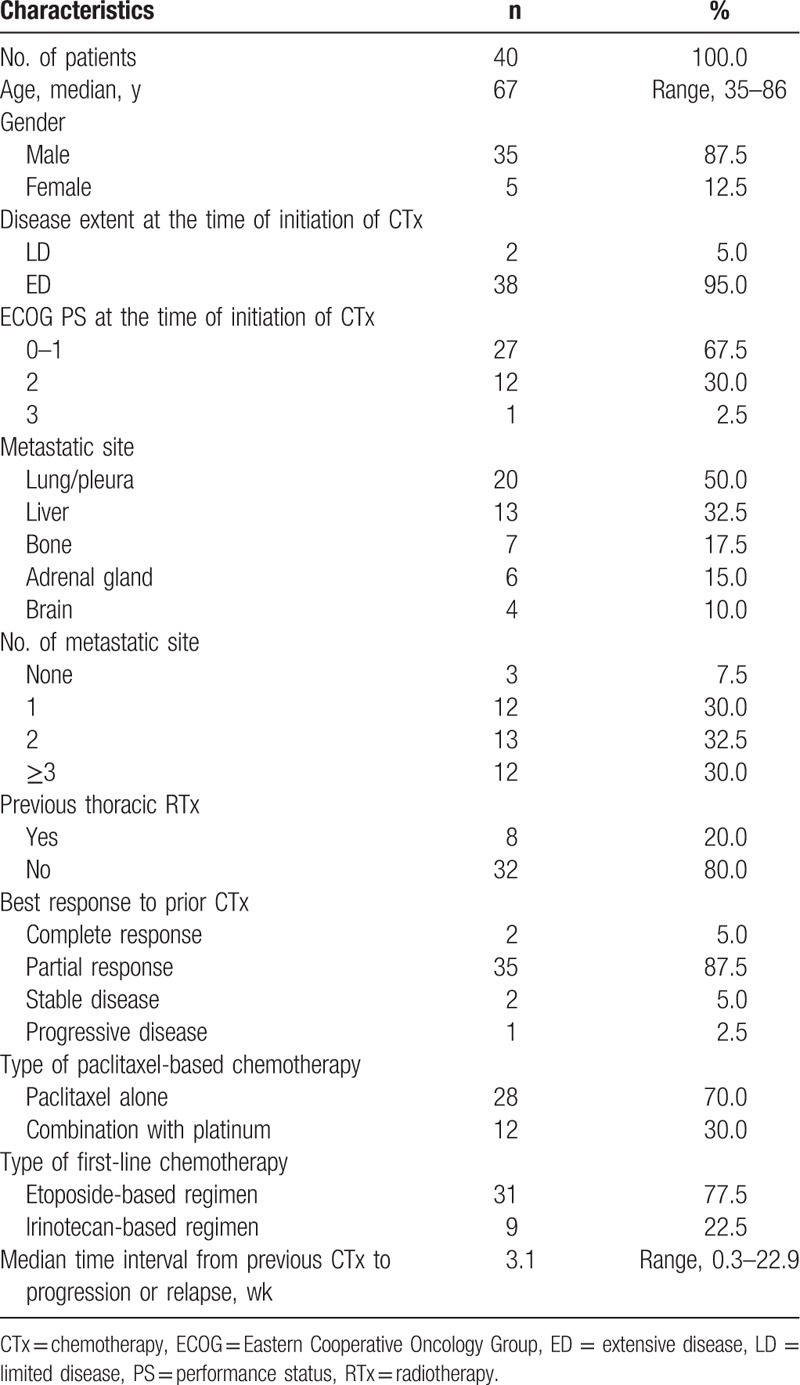
Patient characteristics are shown in Table 1. The median age of enrolled patients was 67 (range, 35–86 years). The majority of patients were men (87.5%). Thirty-eight patients (95.0%) had ED at the start time of chemotherapy. Thirteen patients (32.5%) had an ECOG PS ≥2. All patients received both etoposide- and camptothecin-based chemotherapy, irrespective of the sequence of administration. Etoposide-based chemotherapy as first-line treatment was given in 31 patients (77.5%) and they all received camptothecin (irinotecan or topotecan)-based chemotherapy as second-line treatment. Irinotecan-based chemotherapy as first-line treatment was given in 9 patients (22.5%), and they all received etoposide-based chemotherapy as second-line treatment. All patients were exposed to platinum such as cisplatin or carboplatin on first-line and/or second-line chemotherapy.
Table 1.
Baseline characteristics.
3.2. Drug delivery
Patients received a median chemotherapy of 2 cycles (range, 1–6) per patient. The average relative DI was 86.9% for paclitaxel (58.3 mg/m2 per week for planned dose; 50.7 mg/m2 per week for median delivered dose; range 24.8–75.0). Paclitaxel was administered with a reduced dose in 15 patients (37.5%). Of them, only 2 patients were given a 20% to 25% dose reduction from the second cycle of chemotherapy because of grade 3 febrile neutropenia and grade 3 infection without neutropenia, respectively. The rest was administered with a reduced dose from the first cycle, mainly due to poor PS or old age. The chemotherapy schedule was delayed in 4 patients (10.0%) because of asthenia, mucositis, and grade 2–3 infection.
3.3. Efficacy
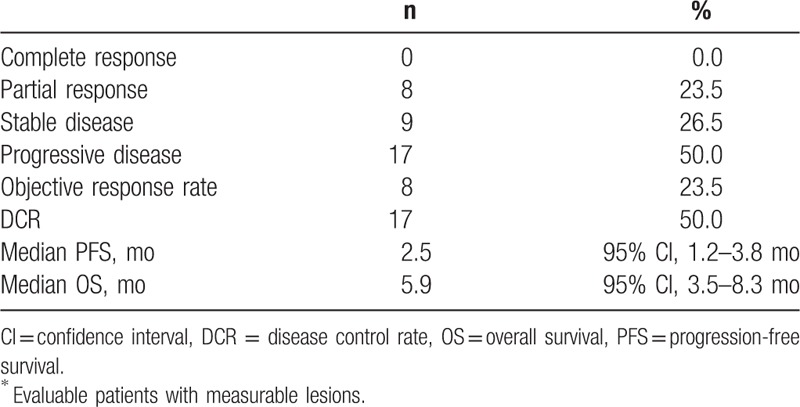
Of 40 patients, 34 were evaluable for response evaluation. Six patients were excluded because 3 patients died of infection or drug-induced pneumonitis before response evaluation, 2 refused further chemotherapy after 1 cycle of chemotherapy due to toxicity, and the remaining patient received other chemotherapy without response evaluation after paclitaxel chemotherapy. The overall RR was 23.5% (95% CI, 9.2–37.8%), and none achieved complete response. Nine patients had stable disease (26.5%), and 17 patients had progressive disease (50.0%), showing a DCR of 50.0% (95% CI, 33.2–66.8%). Median duration of response was 3.8 months (range, 2.5–6.5 months) (Table 2).
Table 2.
Treatment outcomes (n = 34∗).
In particular, patients with time interval >8 weeks from the last chemotherapy to progression before paclitaxel-based chemotherapy had higher RR than patients with time interval ≤8 weeks (75.0% vs 16.7%; P = .033). In addition, patients with good PS (ECOG PS 0–1) had a higher DCR than patients with poor PS (ECOG PS 2–3) (62.5% vs 20.0%; P = .024). However, age, gender, metastatic site(s), and response to previous chemotherapy had no significant effects on RR and DCR after paclitaxel-based chemotherapy.
3.4. Toxicity
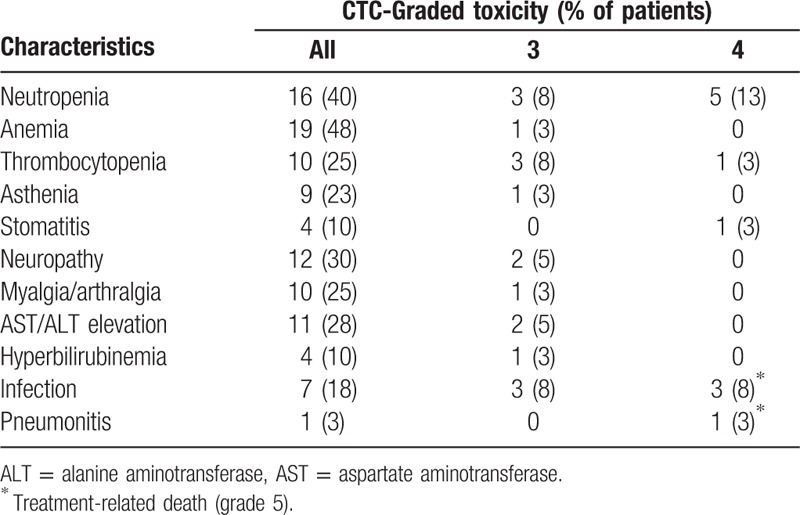
Forty patients were assessable for toxicity evaluation. Adverse events that developed during treatment were predominantly grade 1 or 2. The most common grade 3 or 4 hematologic toxicity was neutropenia, which occurred in 8 patients (20.0%). Grade 3 or 4 thrombocytopenia occurred in 4 patients (10.0%). Six patients experienced severe pulmonary infection (≥grade 3) and 3 of them subsequently died due to accompanying the septic shock. Grade 4 neutropenia was observed in 3 of 7 patients who had infection complications, and 2 of them were fatal. Other common non-hematological toxicities were peripheral neuropathy, mild aspartate aminotransferase/alanine aminotransferase (AST/ALT) elevation and myalgia/arthralgia. One patient died of life-threatening pneumonitis which is expected to be paclitaxel induced (Table 3).
Table 3.
Major toxicities.
3.5. Survival
With a median follow-up of 4.8 months (range, 0.1–39.7 months), the median OS was 5.9 months (95% CI, 3.5–8.3 months) and median PFS was 2.5 months (95% CI, 1.2–3.8 months) (Fig. 1). The median OS in responder was 7.3 months (95% CI, 7.1–7.6 months) and the median PFS in this group was 3.1 months (95% CI, 0.62–5.5 months). However, the median OS in nonresponder was 4.6 months (95% CI, 4.3–4.9 months) and the median PFS in this group was 1.4 months (95% CI, 0.89–1.8 months) (P values based on log-rank test; .514 for OS and .003 for PFS, respectively) (Fig. 2).
Figure 1.
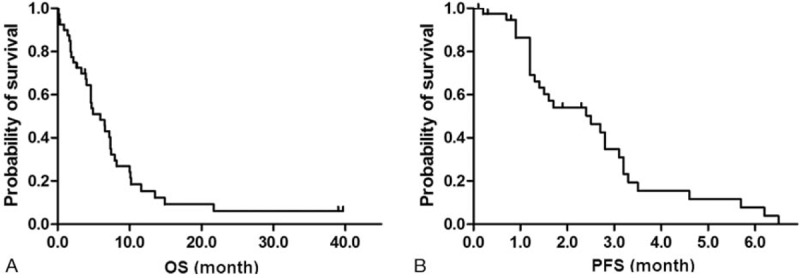
Kaplan-Meier survival curves for OS (A) and PFS (B) in a total of patients. The median OS and PFS were 5.9 mo (95% CI, 3.5–8.3 mo) and 2.5 mo (95% CI, 1.2–3.8 mo), respectively. OS = overall survival, PFS = progression-free survival.
Figure 2.
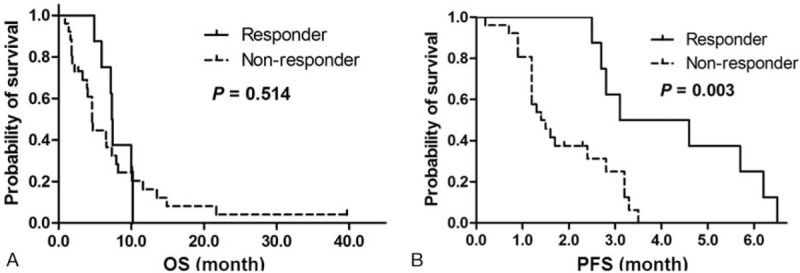
Kaplan-Meier survival curves of OS (A) and PFS (B) in comparisons with respect to the response to paclitaxel-based chemotherapy. The median OS in responder versus nonresponder was 7.3 mo (95% CI, 7.1–7.6) versus 4.6 mo (95% CI, 4.3–4.9) and the median PFS in the same analysis of this group was 3.1 mo (95% CI, 0.62–5.5) versus 1.4 mo (95% CI, 0.89–1.8). Responders had significantly prolonged PFS compared with nonresponders. OS = overall survival, PFS = progression-free survival.
In univariate analysis of OS for clinicopathologic features, good ECOG PS (<2 vs ≥2; 7.3 months [95% CI, 5.5–9.2 months] vs 1.9 months [95% CI, 1.2–2.6 months]; P = .001), the presence of hepatic metastasis (yes vs no; 7.3 months [95% CI, 6.3–8.3 months] vs 1.8 months [95% CI, 1.3–2.3 months]; P < .001), and smaller numbers of metastatic sites (<3 vs ≥3; 7.3 months [95% CI, 5.0–9.7 months] vs 3.3 months [95% CI, 0.0–6.6 months]; P = .047) were associated with longer OS. PS and the presence of hepatic metastasis remained significant predictors for OS in multivariate analysis (Table 4).
Table 4.
Multivariate analysis for survival.
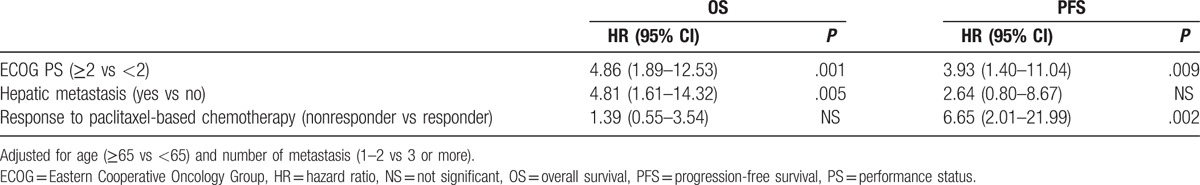
In addition, clinicopathologic factors which affected a longer PFS included PS (<2 vs ≥2; 2.8 months [95% CI, 2.3–3.3 months] vs 1.2 months [95% CI, 0.8–1.6 months]; P = .020) and the presence of brain metastasis (yes vs no; 2.5 months [95% CI, 1.2–3.7 months] vs 1.6 months [95% CI, not available]; P = .080), as well as response to paclitaxel-based chemotherapy in univariate analysis. However, multivariate analysis revealed PS and response to paclitaxel-based chemotherapy alone had significant effects on PFS (Table 4).
4. Discussion
Most SCLC patients develop recurrence or progression after initial chemotherapy, and therefore they have a great demand for salvage chemotherapy. The most extensively studied drugs are camptothecin such as topotecan or irinotecan as second-line chemotherapy in SCLC. Recently, amrubicin, an investigational anthracycline, showed promising activity in refractory or relapsed SCLC. A randomized phase II trial showed that amrubicin has a higher RR than topotecan as second-line therapy in patients with SCLC sensitive to first-line platinum-based chemotherapy.[10,11,22] Although newer agents are under investigations, the probability of responding to second-line chemotherapy is not very high with relatively short duration of response.[23] Therefore, a considerable number of refractory or relapsed patients who are expected to tolerate further chemotherapy with good PS need subsequent chemotherapy. We intended to evaluate the efficacy and safety of paclitaxel-based chemotherapy as third-line chemotherapy for refractory SCLC to both etoposide- and camptothecin-based chemotherapy.
In previous phase II studies, paclitaxel monotherapy showed overall RR ranging from 24% to 29% and median OS ranged from 3.3 to 5.8 months in patients with refractory SCLC[15,24]; however, it showed more increased overall RR (30–40%) in untreated patients with SCLC.[13,16] Consistent with these studies, our study showed comparable overall RR (23.5%) and median survival time (OS 5.9 months, PFS 2.5 months) despite third-line chemotherapy given to patients with refractory SCLC. Furthermore, it is noteworthy that 5 of 8 responders in this study had not responded to first- or second-line chemotherapy. They included 1 patient resistant to front-line etoposide plus cisplatin, and 4 resistant to second-line irinotecan or topotecan chemotherapy, showing a lack of complete cross resistance between previously exposed drugs and paclitaxel.
We gave paclitaxel as a single agent or in combination with platinum (carboplatin or cisplatin). It has been reported that there is synergism between platinum compounds and paclitaxel.[25] Paclitaxel in combination with other agents have shown superior outcome to paclitaxel alone with slightly increased toxicity.[16,19] However, the present study did not verify survival gain with the addition of platinum to paclitaxel (paclitaxel alone vs in combination with platinum; 4.9 vs 7.2 months, P = .474 for OS; 2.4 vs 2.5 months, P = .136 for PFS). There were no significant differences in the baseline characteristics between groups, maybe due to the small sample size. Therefore, further study is needed in terms of the best schedule and combination of paclitaxel-based chemotherapy.
We analyzed the clinicopathologic factors which affected survival on third-line chemotherapy in patients with refractory SCLC. The responders to paclitaxel-based therapy had longer PFS than nonresponders, whereas there was no significant difference in terms of OS (Fig. 2). These results were also confirmed in multivariate analysis in which the response to paclitaxel-based chemotherapy had a significant effect only for PFS, not OS (Table 4). This may be caused by the effect of fourth-line chemotherapy using diverse agents which was administered to 22 patients (55%) of enrolled patients or small sample size of our study. In addition, ECOG PS and hepatic metastasis had significant effects on OS (Table 4), and therefore these factors may be helpful to select optimal patients for third-line chemotherapy. However, the time from the previous chemotherapy (ie, the first- or second-line chemotherapy) to relapse or progression did not affect the outcome of paclitaxel-based chemotherapy in the present study.
The toxicity of paclitaxel-based chemotherapy reported in our study was similar to that addressed in other studies. The major hematologic toxicity was neutropenia, of which grade 3 or 4 were 50% (8/16). Anemia developed in 19 patients (48%), however only 1 patient was grade 3. Remarkably, 6 of 7 patients who had infection complications were grade 3 or more. Three patients who had septic shock died of it and 2 of them had grade 4 neutropenia. The patients all of whom experienced life-threatening infection had ECOG PS 2 to 3 at the initiation time of paclitaxel-based chemotherapy. These toxicity profiles also suggest the importance of baseline PS for the selection of the patients to whom third-line chemotherapy would be given. However, SCLC patients tend to have poor PS due to severe disease-related symptoms such as dyspnea and cough. Therefore, the physician's decision-making for optimal patient selection may be important. The limited size of the retrospective study was a limitation but it is clear that the baseline PS has a strong carry-over effect.
In conclusion, our results show that paclitaxel-based chemotherapy has modest activity and predictable toxicity in SCLC patients refractory to both etoposide and camptothecin-based chemotherapy. For the improvement of clinical outcomes for patients with SCLC, further studies combining paclitaxel with novel agents, such as PD-1 antibody (NCT02551432) or aurora kinase A inhibitor (NCT02038647), are warranted.
Footnotes
Abbreviations: ALT = alanine aminotransferase, ANC = absolute neutrophil count, AST = aspartate aminotransferase, AUC = are under the curve, CI = confidence interval, CT = computed tomography, DCR = disease control rate, DI = dose intensity, ECOG = Eastern Cooperative Oncology Group, ED = extensive disease, LD = limited disease, MRI = magnetic resonance imaging, NCI-CTC = National Cancer Institute-Common Toxicity Criteria, OS = overall survival, PFS = progression-free survival, PS = performance status, RECIST = Response Evaluation Criteria in Solid Tumors, RR = response rate, SCLC = small cell lung cancer, SNUBH = Seoul National University Bundang Hospital.
Se Hyun Kim and Mi-Jung Kim contributed equally to this work.
The authors report no conflicts of interest.
References
- [1].Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212–36. [DOI] [PubMed] [Google Scholar]
- [2].Bunn PA, Minna JD, Augustyn A, et al. Small cell lung cancer: can recent advances in biology and molecular biology be translated into improved outcomes? J Thorac Oncol 2016;11:453–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441–7. [DOI] [PubMed] [Google Scholar]
- [4].Cheng S, Evans WK, Stys-Norman D, et al. Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol 2007;2:348–54. [DOI] [PubMed] [Google Scholar]
- [5].Masters GA, Declerck L, Blanke C, et al. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer: Eastern Cooperative Oncology Group Trial 1597. J Clin Oncol 2003;21:1550–5. [DOI] [PubMed] [Google Scholar]
- [6].Ettinger DS. New drugs for chemotherapy-naive patients with extensive-disease small cell lung cancer. Semin Oncol 2001;28:27–9. [PubMed] [Google Scholar]
- [7].Kelly K. New chemotherapy agents for small cell lung cancer. Chest 2000;117:156S–62S. [DOI] [PubMed] [Google Scholar]
- [8].Ando M, Kobayashi K, Yoshimura A, et al. Weekly administration of irinotecan (CPT-11) plus cisplatin for refractory or relapsed small cell lung cancer. Lung Cancer 2004;44:121–7. [DOI] [PubMed] [Google Scholar]
- [9].Fennell DA, Steele JP, Shamash J, et al. Phase II trial of irinotecan, cisplatin and mitomycin for relapsed small cell lung cancer. Int J Cancer 2007;121:2575–7. [DOI] [PubMed] [Google Scholar]
- [10].Inoue A, Sugawara S, Yamazaki K, et al. Randomized phase II trial comparing amrubicin with topotecan in patients with previously treated small-cell lung cancer: North Japan Lung Cancer Study Group Trial 0402. J Clin Oncol 2008;26:5401–6. [DOI] [PubMed] [Google Scholar]
- [11].Jotte R, Conkling P, Reynolds C, et al. Randomized phase II trial of single-agent amrubicin or topotecan as second-line treatment in patients with small-cell lung cancer sensitive to first-line platinum-based chemotherapy. J Clin Oncol 2011;29:287–93. [DOI] [PubMed] [Google Scholar]
- [12].von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 2014;32:4012–9. [DOI] [PubMed] [Google Scholar]
- [13].Ettinger DS, Finkelstein DM, Sarma RP, et al. Phase II study of paclitaxel in patients with extensive-disease small-cell lung cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 1995;13:1430–5. [DOI] [PubMed] [Google Scholar]
- [14].Kirschling RJ, Grill JP, Marks RS, et al. Paclitaxel and G-CSF in previously untreated patients with extensive stage small-cell lung cancer: a phase II study of the North Central Cancer Treatment Group. Am J Clin Oncol 1999;22:517–22. [DOI] [PubMed] [Google Scholar]
- [15].Smit EF, Fokkema E, Biesma B, et al. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer 1998;77:347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Thomas P, Castelnau O, Paillotin D, et al. Phase II trial of paclitaxel and carboplatin in metastatic small-cell lung cancer: a Groupe Francais de Pneumo-Cancerologie study. J Clin Oncol 2001;19:1320–5. [DOI] [PubMed] [Google Scholar]
- [17].Reck M, von Pawel J, Macha HN, et al. Randomized phase III trial of paclitaxel, etoposide, and carboplatin versus carboplatin, etoposide, and vincristine in patients with small-cell lung cancer. J Natl Cancer Inst 2003;95:1118–27. [DOI] [PubMed] [Google Scholar]
- [18].Niell HB, Herndon JE, II, Miller AA, et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony-stimulating factor in patients with extensive-stage small-cell lung cancer: Cancer and Leukemia Group B Trial 9732. J Clin Oncol 2005;23:3752–9. [DOI] [PubMed] [Google Scholar]
- [19].Groen HJ, Fokkema E, Biesma B, et al. Paclitaxel and carboplatin in the treatment of small-cell lung cancer patients resistant to cyclophosphamide, doxorubicin, and etoposide: a non-cross-resistant schedule. J Clin Oncol 1999;17:927–32. [DOI] [PubMed] [Google Scholar]
- [20].Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- [21].Anderson JR, Bernstein L, Pike MC. Approximate confidence intervals for probabilities of survival and quantiles in life-table analysis. Biometrics 1982;38:407–16. [PubMed] [Google Scholar]
- [22].Ettinger DS, Jotte R, Lorigan P, et al. Phase II study of amrubicin as second-line therapy in patients with platinum-refractory small-cell lung cancer. J Clin Oncol 2010;28:2598–603. [DOI] [PubMed] [Google Scholar]
- [23].Albain KS, Crowley JJ, Hutchins L, et al. Predictors of survival following relapse or progression of small cell lung cancer. Southwest Oncology Group Study 8605 report and analysis of recurrent disease data base. Cancer 1993;72:1184–91. [DOI] [PubMed] [Google Scholar]
- [24].Yamamoto N, Tsurutani J, Yoshimura N, et al. Phase II study of weekly paclitaxel for relapsed and refractory small cell lung cancer. Anticancer Res 2006;26:777–81. [PubMed] [Google Scholar]
- [25].Jekunen AP, Christen RD, Shalinsky DR, et al. Synergistic interaction between cisplatin and taxol in human ovarian carcinoma cells in vitro. Br J Cancer 1994;69:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]


