Supplemental Digital Content is available in the text
Keywords: cardiovascular events, inflammatory process, rheumatoid arthritis, subclinical atherosclerosis, traditional cardiovascular risk factor
Abstract
Several studies have pointed out a significant association between rheumatoid arthritis (RA) and accelerated atherosclerosis. At the best of our knowledge, no such study has been carried out in a large Italian series and, in this study, we aimed to investigate the prevalence of both subclinical atherosclerosis and history of cardiovascular events (CVEs), in patients consecutively admitted from January 1, 2015 to December 31, 2015 to Rheumatology Units throughout the whole Italy.
Centers members of GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) were invited to enrol patients consecutively admitted from January 1, 2015 to December 31, 2015 and satisfying American College of Rheumatology/ European League Against Rheumatism criteria for RA and to investigate each of them for: traditional cardiovascular risk factors: sex, age, smoking habit, total cholesterol, triglycerides, glycaemia, high blood pressure, metabolic syndrome (MS), type 2 diabetes (T2D); RA features: disease duration as assessed from the first symptom, disease activity as evaluated by DAS28, radiographic damage as assessed by hands and feet x-ray, and previous joint surgery; prevalence of both subclinical atherosclerosis and history of CVEs.
Eight centers participated to the study. From January 1, 2015 to December 31, 2015, the 1176 patients, who had been investigated for all the items, were enrolled in the study. They were mostly women (80.52%), with a median age of 60 years (range, 18–91 years), a median disease duration of 12 years (range, 0.8–25 years), seropositive in 69.21%. Nineteen percent were in remission; 17.51% presented low disease activity; 39.45% moderate disease activity; 22.61% high disease activity.
Eighty-two patients (6.9%) had a history for CVEs (58 myocardial infarction, 38 heart failure, 10 ischemic transitory attack, and 7 stroke). This figure appears to be lower than that reported worldwide (8.5%). After excluding the 82 patients with a history of CV events, subclinical atherosclerosis was detected in 16% of our patients, (176 patients), a figure lower than that reported worldwide (32.7%) and in previous Italian studies.
This is the first Italian multicenter study on subclinical and clinical atherosclerosis in patients with RA. We pointed out a low prevalence of both subclinical atherosclerosis and history of CV events.
1. Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by an increased risk of mortality, as compared with general population, mainly due to cardiovascular events (CVEs).[1–3] In fact, a large percentage of RA patients experience myocardial infarction (MI), cerebrovascular accidents (CVAs), and congestive heart failure (CHF) during their life.[4–6] Furthermore, an increased prevalence of subclinical atherosclerosis as evidenced by carotid artery plaques, has been observed in these patients, and this subclinical condition may allow to identify those RA patients with a higher risk to develop CVEs.[4,7] In addition, it has been pointed out that “traditional” cardiovascular (CV) risk factors, such as high blood pressure (HBP), type 2 diabetes (T2D), and metabolic syndrome (MS) are underestimated in RA patients and therefore untreated,[8–11] thus contributing to the increased CV risk, despite specific international recommendations were developed.[12,13] To date, the causes of accelerated atherosclerosis have not been fully elucidated. In fact, specific features of RA, including systemic inflammatory process and disease activity, seem to be involved in promoting CV risk in addition to traditional risk factors.[14–16]
It has been shown that CV mortality in RA has distinct patterns among different countries; RA has been reported to present both a lower prevalence and a milder disease course in Italy and in other Mediterranean countries with respect to North European countries.[17–19] At the best of our knowledge, no study has been carried out in a large Italian series of RA patients in order to evaluate the risk of CVEs and subclinical atherosclerosis in Italian RA patients. In this work, we investigated the presence of CVEs and subclinical atherosclerosis in a cohort of RA patients enrolled in the multicenter GIRRCS (Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale) observational study (RA-GIRRCS Study), evaluating the possible associations among both traditional CV risk factors and disease specific features and the evidence of CVEs and subclinical atherosclerosis. Furthermore, due to the differences pointed out in RA populations across different Worldwide nations, mainly in comorbidities and long-term outcome,[20] an evaluation between our data of prevalence of both subclinical atherosclerosis and history of CVEs, and the available RA literature was performed in order to discuss possible differences.
2. Patients and methods
2.1. Study design, inclusion criteria, and data collection
Eight tertiary Rheumatologic units, throughout the whole Italy, with high experience in management of RA patients as well as in observational studies were involved. The reports of the first scheduled visit of each patient, satisfying the American College of Rheumatology/ European League Against Rheumatism criteria for RA,[21,22] and visited from January 1, 2015 to December 31, 2015 were reviewed. The patients were evaluated for traditional CV risk factors, history of CVEs and subclinical atherosclerosis. CVEs were defined as history of MI and/or CHF and/or CVAs. The prevalence of each condition and their cumulative prevalence was compared with those registered in Italian general population matched for sex and age by the Italian National Institute of Statistics (ISTAT, www.istat.it). Subclinical atherosclerosis was defined as the presence of carotid plaque as assessed by carotid and/or peripheral arteries ultrasound imaging. Its prevalence was compared with that registered in control subjects from 3 studies on subclinical atherosclerosis in Italian patients with RA, Sjogren syndrome, or systemic lupus erythematosus.[23–26] Smoking habit, evidence of HBP, T2D, and MS as well as serum levels of total cholesterol and triglycerides were recorded, at the first visit. Disease activity was assessed by Disease Activity Score in 28 joints (DAS28).[27] Furthermore, disease duration from the first disease symptom, radiographic damage evaluated as the presence of erosions/joint narrowing at hands or feet, history of joint surgery, C reactive protein (CRP) and rheumatoid factor (RF), and/or anti-citrullinated protein antibody (ACPA) were also registered. Extra-articular features were assessed, the diagnosis of these disease manifestations was based on those used in previous studies.[19] The extra-articular manifestations considered were: secondary Sjögren syndrome with clinical evidence of dry eyes/mouth, rheumatoid nodules, vasculitis, pulmonary involvement, cutaneous, and neurologic manifestations. The patients were divided in 5 groups, according with their therapeutic regimen: single conventional synthetic disease-modifying anti-rheumatic drug (csDMARD) + low dose corticosteroids (ldCCSs); combination of ≥2 csDMARDs without CCSs; csDMARD(s) + ldCCSs + Biologic DMARD (bDMARD); sDMARD(s) + DMARD (bDMARD) without corticosteroids (CCSs); other therapeutic regimens.
Both international and national recommendations for the management of RA patients were strictly followed. The local ethics committee (Comitato Etico Azienda Sanitaria Locale 1 Avezzano/Sulmona/L’Aquila, L’Aquila, Italy; protocol number 000331/17) approved the study, that was performed according to the Good Clinical Practice guidelines and the Declaration of Helsinki. Written informed consent was obtained from all patients.
2.2. Statistical analysis
Associations between subclinical atherosclerosis and CVEs and any other demographic or clinical feature were assessed by calculating the respective ORS. Covariates were selected from 2 main areas: traditional CV risk factors: sex, age, smoking habit, serum levels of total cholesterol and triglycerides presence of MS, T2D, and HBP; RA-related risk factors: DAS28 values, duration of the disease, presence of extra-articular disease, evidence of radiographic damage, and history of joint surgery. We modeled different statistical analyses, adjusted for sex and age, by performing a logistic regression, to evaluate the possible associations between each of these among these covariates and both history of CVEs and presence of subclinical atherosclerosis. Furthermore, we performed the same analysis in order to evaluate the possible associations between the same set of variables and the evidence of MI, CHF, and CVAs singularly considered. Models fitting has been assessed carrying out a log-likelihood ratio Sidak test, adjusted for multiple tests, with type I error set at 0.05. Due to the relative simple design and the expertise of the recruiting centers, we had a low percentage of missing data, that could impair our analyses. The analysis was performed using SPSS software (SPSS for Windows, version 17.0, SPSS Inc., Chicago, IL).
3. Results
3.1. Baseline characteristics of evaluated RA patients
From January 1, 2015 to December 31, 2015, 1176, admitted to 8 GIRRCS centers, were enrolled in the study; the demographic features of our cohort are shown in Table 1. They were mostly women (80.52%), with a median age of 60 years (range, 18–91 years), a median disease duration of 12 years (range, 0.8–25 years), seropositive in 69.21%. Nineteen percent were in remission; 17.51% presented low disease activity; 39.45% moderate disease activity; 22.61% high disease activity. The most common RA therapeutic strategies were csDMARD + ldCCSs (24.60%) and sDMARD(s) + ldCCSs + bDMARD (34.70%). Methotrexate (MTX) and TNF inhibitors were the most common sDMARD and bDMARD prescribed, respectively.
Table 1.
Demographic and clinical features of the evaluated patients.

As far as the traditional CV risk factors are concerned, 45.20% presented a HBP, 32% reported smoking habit, 24.30% were affected by MS, and 13.10% by T2D. Serum total cholesterol resulted 196.31 ± 48.82 mg/dL (mean ± SD), serum triglycerides 117.25 ± 51.79 mg/dL; blood glucose 92.60 ± 33.36 mg/dL. In particular, 49.54% displayed a serum total cholesterol >200 mg/dL; 31.46% triglycerides >130 mg/dL; 13.43% out of patients displayed a glycaemia >110 mg/dL. Furthermore, 5.4% were affected by osteoporosis, 4.3% by thyroid diseases, 1.1% by HBV infection, and 0.9% by HCV infection.
3.2. History of CVEs and related risk factors
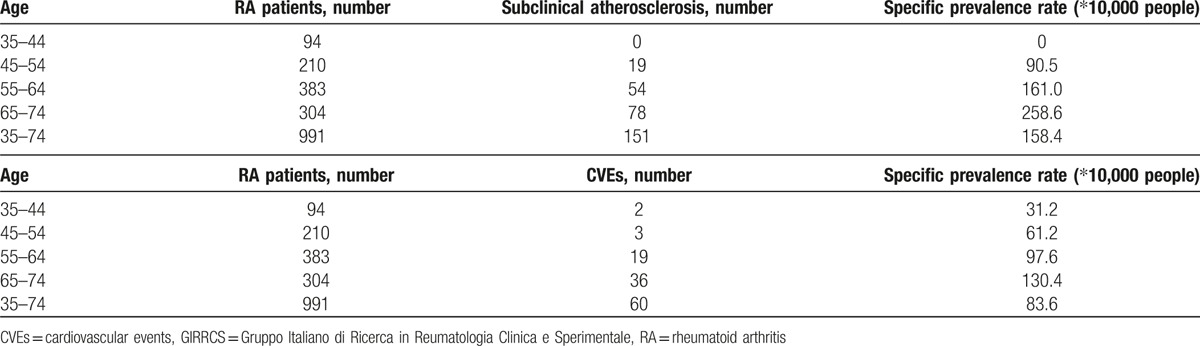
Eighty-two patients (6.9%) had a history for CVEs (58 patients myocardial infarction, 38 patients heart failure, 10 patients ischemic transitory attack, 7 patients stroke). This prevalence is higher than that reported in Italian National Institute of Statistics (ISTAT, www.istat.it) (Table 2).
Table 2.
Specific prevalence rate ratios in RA GIRRCS cohort.
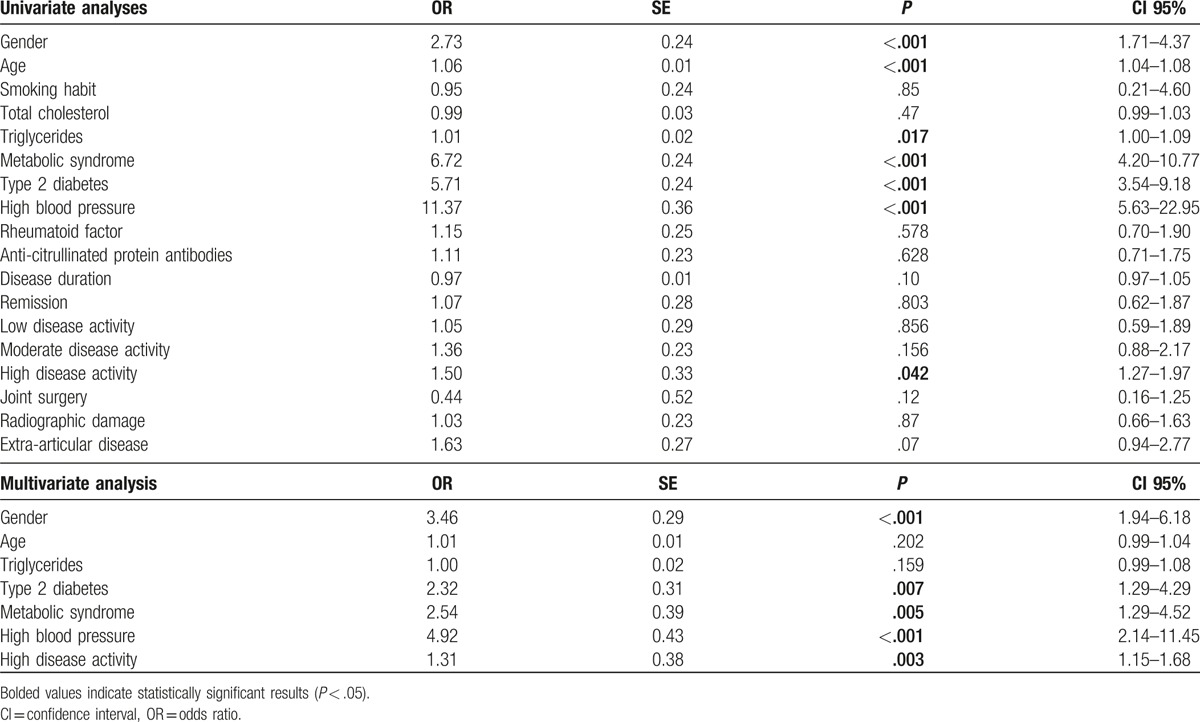
Univariate analyses (Table 3) pointed out significant associations between both some traditional CV risk factors and some RA-related factors and history of CVEs. In particular, among the former, older age (odds ratio [OR]: 1.06, 95% confidence interval [CI]: 1.04–1.08, P < .001), male sex (OR: 2.73, 95% CI: 1.71–4.37, P < .001), MS (OR: 6.72, 95% CI: 4.20–10.77, P < .001), T2D (OR: 5.71, 95% CI: 3.54–9.18, P < .001), HBP (OR: 11.37, 95% CI: 5.63–22.95, P < .001) were each significantly associated with previous CVEs. Among the latter, a high disease activity (DAS28 >5.1) only was significantly associated with the presence of CVEs (OR: 1.50, 95% CI: 1.27–1.97, P = .042). Therefore, smoking habit, total cholesterol values, disease duration, RF, ACPA, extra-articular disease, joint surgery, and radiographic were not statistically associated with history of CVEs. Of note, in multivariate analysis (Table 3), the evidence of MS (OR: 2.54, 95% CI: 1.29–4.52, P = .005), T2D (OR: 2.32, 95% CI: 1.29–4.29, P = .007), and HBP (OR: 4.92, 95% CI: 2.14–11.45, P < .001) as well as the high disease activity (DAS28 >5.1) (OR: 1.31, 95% CI: 1.15–1.68, P = .003) were significantly associated with previous CVEs, in our cohort of RA patients. Furthermore, by univariate and multivariate analyses, we evaluated the possible association among the identified variables and evidence of MI, CHF, and CVAs (additional Table 1 additional Table 2 additional Table 3).
Table 3.
CVEs univariate and multivariate analyses.
3.3. Subclinical atherosclerosis and its associations
After excluding the 82 patients with a history of CV events, subclinical atherosclerosis was detected in 16% of our patients, (176 patients), it resulted higher than those observed in control groups from Italian studies on other diseases reported in those studies.[23–26]
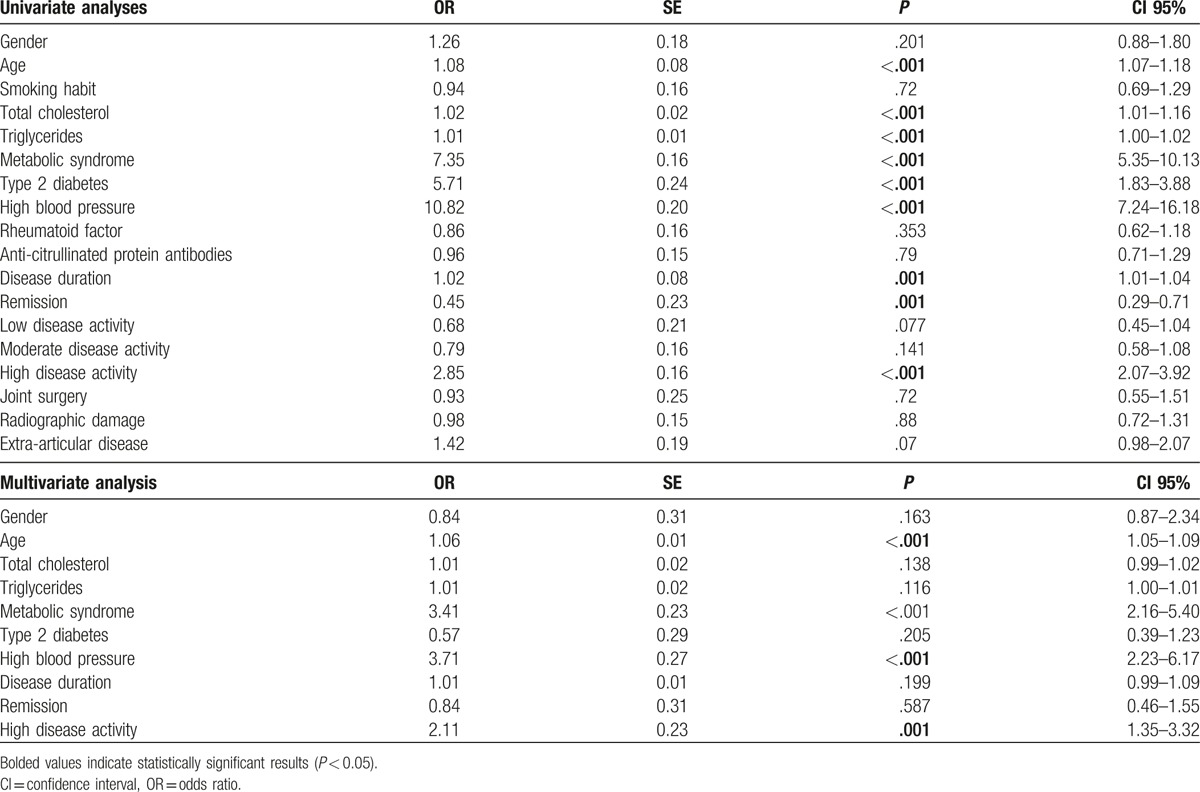
At univariate analyses (Table 4), it was suggested that both some traditional CV risk factors and some RA-related risk factors resulted to be associated with the presence of subclinical atherosclerosis. As far the traditional CV risk factors are concerned, older age (OR: 1.08, 95% CI: 1.07–1.18, P < .001), higher serum values of total cholesterol (OR:1.02, 95% CI: 1.01–1.16, P < .001), and triglycerides (OR: 1.01, 95% CI: 1.00–1.02, P < .001), MS (OR: 7.35, 95% CI: 5.35–10.13, P < .001), T2D (OR: 5.71, 95% CI: 1.83–3.88, P < .001), HBP (OR:10.82, 95% CI: 7.24–16.18, P < .001), were each significantly associated with subclinical atherosclerosis. Concerning the RA related risk factors, high disease activity (DAS28 >5.1) (OR: 2.85, 95% CI: 2.07–3.92, P < .001), and longer disease duration (OR: 1.02, 95% CI: 1.01–1.04, P = .001) were significantly associated with presence of subclinical atherosclerosis. To date, remission (DAS28 <2.6) (OR: 0.45, 95% CI: 0.29–0.714, P = .001) was negatively associated with subclinical atherosclerosis. Therefore, smoking habit, RF, ACPA, extra-articular disease, joint surgery, and radiographic damage were not associated with subclinical atherosclerosis. Interestingly, in multivariate analysis, older age (OR: 1.069, CI 95%: 1.05–1.09, P < .001), MS (OR: 3.41, CI 95%: 2.16–5.40, P < .001), and HBP (OR: 3.71, CI 95%: 2.23–6.17, P < .001) and high disease activity (DAS28 >5.1) (OR: 2.11, CI 95%: 1.35–3.32, P = .001) were all significantly associated with the presence of subclinical atherosclerosis.
Table 4.
Subclinical atherosclerosis univariate and multivariate analyses.
4. Discussion
CV comorbidities are frequently associated with RA, strongly influencing not only the clinical course and the outcome of these patients, but also the therapeutic strategies in order to prevent the occurrence of severe CVEs.[14,15] On this light, any study exploring this clinical setting may provide useful information about the evolution of clinical picture and the response to treatments. Of interest, taking together our data it is possible to point out a low prevalence of both subclinical atherosclerosis and history of CV events when compared with other available series.[5–7] In addition, in our multicenter GIRRCS observational study, we show that, among the traditional CV risk factors, only some of them were related with a history of CVEs, and, on other hand, highest values of the DAS28 were associated with the presence of both CVEs and/or subclinical atherosclerosis, suggesting that not only the traditional CV risk factors but also the disease activity is independently associated with the development of CV complications.
In our study, we observed a low prevalence of these CV comorbidities, both subclinical atherosclerosis and history of CVEs when compared with other series.[5–7] Subclinical atherosclerosis prevalence is lower than that reported in meta-analysis of observational studies on the topic (16% vs 32.7%).[7] In this study, an increased prevalence of carotid plaques in RA patients has been shown.[7] Specifically, the authors analyzed 59 studies and reported that 32.7% of patients displayed this feature.[7] As far as history of CVEs is concerned, several studies and meta-analyses evaluated the prevalence of CVEs in RA patients, and our prevalence seems lower than that reported in meta-analysis of observational studies (6.9% vs 8.5%).[5,7] Taking together these findings is not possible to address definitive conclusions concerning the reduced prevalence that we observed and future specific-designed studies are surely needed to fully elucidated these possible differences, in RA population, across different nations. In this context, it has been proposed that the iniquity in access to health care system and stricter eligibility criteria for treatments, such observed in countries with lower socioeconomic welfare, may strongly influence the comorbidities and long-term outcome in RA patients.[15]
Despite the well-known role of traditional CV risk factors, in inducing CVEs and subclinical atherosclerosis in general population, our analysis failed to show the expected association among some of these factors and the development of CVEs. In fact, we observed that only MS and HBP were associated with both CVEs and subclinical atherosclerosis while T2D was associated only with the evidence of CVEs. To date, multiple lines of evidence confirmed a significant association between RA and MS, T2D and HBP.[28,29] Furthermore, in this context, meta-analytic data confirmed the role of traditional CV risk factors in accelerating atherosclerosis, and thus CVEs, in RA patients.[30] In addition, it has been shown that traditional CV risk factor may not be optimally identified and treated, in RA patients, thus contributing to the increase of CVEs risk.[31,32] Finally, NSAIDs and steroids, frequently used in RA patients, may rise the systemic blood pressure.[33,34]
Although the well-known role of the smoking habit in RA pathogenesis, its impact on CVEs and subclinical atherosclerosis, during RA, have still not been identified and the “smoking paradox” has been proposed.[35–37] The weaker association between smoking habit and both CVEs and subclinical atherosclerosis might be related with an “index event” bias, in which causal factors appear not to apply to disease complications, during observational and epidemiological studies.[38] We did not observe any role of hypercholesterolemia in RA CVEs. In fact, despite of the increased CV risk observed during this disease, the rheumatoid pro-inflammatory process may induce a decrease of serum total cholesterol, the so called RA lipid paradox.[39] This result confirms that quantitative analyses might not identify the real impact of cholesterol on CV comorbidities in RA. In fact, the systemic inflammation modulates specific qualitative changes in lipoproteins, mainly affecting the HDL fraction, which loses its anti-inflammatory activity and its skill to reverse cholesterol transport function.[40,41] On these bases, further studies are needed to fully clarify the role of lipoproteins in inducing CVEs in RA patients.
The inflammatory process strongly modulates all stages of the atherosclerosis, including endothelial damage, plaque formation, destabilization, and, finally, the thrombogenic events leading to occlusion.[42] Many pathogenic pathways are activated in this complex process including, endothelial cell dysfunction, oxidative stress, pro-thrombotic phenotype, and pro-atherogenic metabolic effects.[15,16] Taken together all these data confirm that the pro-inflammatory process, associated with a dysregulation of immune system, is strongly involved in the development of accelerate atherosclerosis in RA.[43–47] In our observational study, we observed that the high disease activity was significantly associated with the evidence of both CVEs and subclinical atherosclerosis. In fact, it has been shown that patients with history of MI had more tender joints, worse fatigue, and higher ESR levels.[37] As far as the relationship between disease duration and CVEs are concerned, our study did not find any statistical association. Although conflicting data are reported in available literature, concerning the association between disease duration and CV risk,[48,49] our results show that inflammatory burden more than duration of the disease, contributes to the development of CVEs. Taking together our data, we may suggest that not only the management of traditional CV risk factor but also a good control of disease activity, may play an important role in preventing the CVEs and thus premature death in RA patients.[43,48,50]
Our multicenter study carries out some limitations. The specific design of our study, in which the different therapeutic strategies of RA patients were not randomized, did not allow us to analyze the possible association between treatments and outcomes, to avoid the risk of a “confounding by indication” bias.[51–53] However, it must be pointed out that a large percentage of our patients were treated with CCSs. Although the available data are conflicting, because of deleterious effects of CCSs, such as disturbances of lipid and glucose metabolism and increase in blood pressure, might be, at least in this setting, counteracted by the anti-inflammatory properties of this family of medications, the possible pro-atherogenic role of such drugs should be taken into account in the management of these patients.[54,55] In fact, it has been reported that a sustained exposure to CCSs was significantly associated with cardiometabolic comorbidities in rheumatic diseases, thus contributing to increased CV risk.[56–60] Although a growing body of evidence suggests new possible therapeutic targets in rheumatic diseases,[61–64] future specific designed studies are needed to entirely elucidate this issue, considering, even today, the central role of CCSs “bridging” therapy in management of these patients.[65] Furthermore, due to a higher value of missing data concerning body mass index (BMI), we could not evaluate its impact on CVEs and subclinical atherosclerosis. However, it has been shown that during RA, in which sarcopenia may alter the body composition, BMI may not be considered a valid predictor of CVEs and subclinical atherosclerosis, as in normal population.[66–68]
In conclusion, in our knowledge, this is the first multicenter study on subclinical and clinical atherosclerosis in patients with RA, throughout the whole country and not limited to some specific geographic area. We pointed out a low prevalence of both subclinical atherosclerosis and history of CV events when compared with other available series. Nonetheless, a high disease activity and presence of cardiovascular risk factors were found to play a role to RA patients similarly from other countries. Our results show that high values of DAS28 are associated with CVEs and subclinical atherosclerosis, confirming the need of optimal control of the disease activity in order to decrease the burden of CV comorbidities. Furthermore, the presence of HBP and MS were significantly associated with CVEs and subclinical atherosclerosis, confirming the some traditional CV risk factors may play a specific role in modulating CV complications, during RA, and showing that the risk for CVEs in Italian population parallels what observed in other countries. Future longitudinal analyses, on larger cohorts of patients, with longer follow-up, are surely needed in order to reinforce these data and suggest new research fields.
Supplementary Material
Acknowledgments
The authors received no specific funding and have no conflict of interest to declare for the present work. After obtaining her permission to be named, the authors thank Mrs. Federica Sensini for her technical assistance.
Footnotes
Abbreviations: ACPA = anti-citrullinated protein antibody, bDMARD = biologic disease-modifying anti-rheumatic drug, CHF = congestive heart failure, CRP = C-reactive protein, csDMARD = conventional synthetic disease-modifying anti-rheumatic drug, CV = cardiovascular, CVAs = cerebrovascular accidents, CVEs = cardiovascular events, DAS28 = disease activity score in 28 joints, GIRRCS = Gruppo Italiano di Ricerca in Reumatologia Clinica e Sperimentale, HBP = high blood pressure, ISTAT = Italian National Institute of Statistics, ldCCSs = low dose corticosteroids, MI = myocardial infarction, MS = metabolic syndrome, RA = rheumatoid arthritis, RF = rheumatoid factor, T2D = type 2 diabetes.
The authors report no conflicts of interest for this study.
Supplemental Digital Content is available for this article.
References
- [1].Giacomelli R, Gorla R, Trotta F, et al. Quality of life and unmet needs in patients with inflammatory arthropathies: results from the multicentre, observational RAPSODIA study. Rheumatology (Oxford) 2015;54:792–7. [DOI] [PubMed] [Google Scholar]
- [2].Ruscitti P, Cipriani P, Masedu F, et al. Increased cardiovascular events and subclinical atherosclerosis in rheumatoid arthritis patients: 1 year prospective single centre study. PLoS One 2017;12:e0170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hansen IM, Emamifar A, Andreasen RA, et al. No further gain can be achieved by calculating Disease Activity Score in 28 joints with high-sensitivity assay of C-reactive protein because of high intraindividual variability of C-reactive protein: a cross-sectional study and theoretical consideration. Medicine (Baltimore) 2017;96:e5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fransen J, Kazemi-Bajestani SM, Bredie SJ, et al. Rheumatoid arthritis disadvantages younger patients for cardiovascular diseases: a meta-Analysis. PLoS One 2016;11:e0157360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–9. [DOI] [PubMed] [Google Scholar]
- [6].Aviña-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690–7. [DOI] [PubMed] [Google Scholar]
- [7].Ambrosino P, Lupoli R, Di Minno A, et al. Subclinical atherosclerosis in patients with rheumatoid arthritis. A meta-analysis of literature studies. Thromb Haemost 2015;113:916–30. [DOI] [PubMed] [Google Scholar]
- [8].Dong Q, Liu H, Yang D, et al. Diabetes mellitus and arthritis: is it a risk factor or comorbidity? A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Boo S, Froelicher ES, Yun JH, et al. Perceived and actual risk of cardiovascular disease in patients with rheumatoid arthritis in Korea: a cross-sectional study. Medicine (Baltimore) 2016;95:e5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ursini F, Naty S, Mazzei V, et al. Kaposi's sarcoma in a psoriatic arthritis patient treated with infliximab. Int Immunopharmacol 2010;10:827–8. [DOI] [PubMed] [Google Scholar]
- [11].Ruscitti P, Ursini F, Cipriani P, et al. Poor clinical response in rheumatoid arthritis is the main risk factor for diabetes development in the short-term: a 1-year, single-centre, longitudinal study. PLoS One 2017;12:e0181203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010;69:325–31. [DOI] [PubMed] [Google Scholar]
- [13].Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. [DOI] [PubMed] [Google Scholar]
- [14].Hollan I, Meroni PL, Ahearn JM, et al. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev 2013;12:1004–15. [DOI] [PubMed] [Google Scholar]
- [15].Nurmohamed MT, Heslinga M, Kitas GD. Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol 2015;11:693–704. [DOI] [PubMed] [Google Scholar]
- [16].Ruscitti P, Cipriani P, Di Benedetto P, et al. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1β via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3(NLRP3)-inflammasome activation: a possible implication for therapeutic decision in these patients. Clin Exp Immunol 2015;182:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cimmino MA, Parisi M, Moggiana G, et al. Prevalence of rheumatoid arthritis in Italy: the Chiavari Study. Ann Rheum Dis 1998;57:315–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int 2014;34:659–64. [DOI] [PubMed] [Google Scholar]
- [19].Drosos AA, Lanchbury JS, Panayi GS, et al. Rheumatoid arthritis in Greek and British patients. A comparative clinical, radiologic, and serologic study. Arthritis Rheum 1992;35:745–8. [DOI] [PubMed] [Google Scholar]
- [20].Sokka T, Kautiainen H, Pincus T, et al. Disparities in rheumatoid arthritis disease activity according to gross domestic product in 25 countries in the QUEST-RA database. Ann Rheum Dis 2009;68:1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- [22].Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- [23].Cuomo G, Di Micco P, Niglio A, et al. Atherosclerosis and rheumatoid arthritis: relationships between intima-media thickness of the common carotid arteries and disease activity and disability. Reumatismo 2004;56:242–6. [DOI] [PubMed] [Google Scholar]
- [24].Doria A, Shoenfeld Y, Wu R, et al. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis 2003;62:1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vaudo G, Bocci EB, Shoenfeld Y, et al. Precocious intima-media thickening in patients with primary Sjögren's syndrome. Arthritis Rheum 2005;52:3890–7. [DOI] [PubMed] [Google Scholar]
- [26].Bartoloni Bocci E, Marchesi S, Delle Monache F, et al. Subclinical atherosclerosis in young patients with rheumatoid arthritis and low disease activity. Reumatismo 2005;57:16–21. [DOI] [PubMed] [Google Scholar]
- [27].van Gestel AM, Prevoo ML, van ’t Hof MA, et al. Development and validation of the European League against Rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League against Rheumatism Criteria. Arthritis Rheum 1996;39:34–40. [DOI] [PubMed] [Google Scholar]
- [28].Dougados M, Soubrier M, Antunez A, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis 2014;73:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang J, Fu L, Shi J, et al. The risk of metabolic syndrome in patients with rheumatoid arthritis: a meta-analysis of observational studies. PLoS One 2013;8:e78151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Baghdadi LR, Woodman RJ, Shanahan EM, et al. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One 2015;10:e0117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford) 2016;55:1210–6. [DOI] [PubMed] [Google Scholar]
- [32].del Rincón I, Haas RW, Pogosian S, et al. Lower limb arterial incompressibility and obstruction in rheumatoid arthritis. Ann Rheum Dis 2005;64:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Panoulas VF, Douglas KM, Milionis HJ, et al. Long-term exposure to medium-dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology (Oxford) 2008;47:72–5. [DOI] [PubMed] [Google Scholar]
- [34].Del Rincón I, Battafarano DF, Restrepo JF, et al. Glucocorticoid dose thresholds associatedwith all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol 2014;66:264–72. [DOI] [PubMed] [Google Scholar]
- [35].Gonzalez A, Maradit Kremers H, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in nonrheumatoid arthritis patients? Ann Rheum Dis 2008;67:64–9. [DOI] [PubMed] [Google Scholar]
- [36].Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. [DOI] [PubMed] [Google Scholar]
- [37].Crepaldi G, Scirè CA, Carrara G, et al. Cardiovascular comorbidities relate more than others with disease activity in rheumatoid arthritis. PLoS One 2016;11:e0146991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Choi HK, Nguyen US, Niu J, et al. Selection bias in rheumatic disease research. Nat Rev Rheumatol 2014;10:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Choy E, Ganeshalingam K, Semb AG, et al. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology (Oxford) 2014;53:2143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Watanabe J, Charles-Schoeman C, Miao Y, et al. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory highdensity lipoprotein in rheumatoid arthritis. Arthritis Rheum 2012;64:1828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Raterman HG, Levels H, Voskuyl AE, et al. HDL protein composition alters from proatherogenic into less atherogenic and proinflammatory in rheumatoid arthritis patients responding to rituximab. Ann Rheum Dis 2013;72:560–5. [DOI] [PubMed] [Google Scholar]
- [42].Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95. [DOI] [PubMed] [Google Scholar]
- [43].Solomon DH, Reed GW, Kremer JM, et al. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol 2015;67:1449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ruscitti P, Cipriani P, Carubbi F, et al. The role of IL-1β in the bone loss during rheumatic diseases. Mediators Inflamm 2015;2015:782382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ruscitti P, Cipriani P, Cantarini L, et al. Efficacy of inhibition of IL-1 in patients with rheumatoid arthritis and type 2 diabetes mellitus: two case reports and review of the literature. J Med Case Rep 2015;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fernández-Gutiérrez B, Perrotti PP, Gisbert JP, et al. Cardiovascular disease in immune-mediated inflammatory diseases: A cross-sectional analysis of 6 cohorts. Medicine (Baltimore) 2017;96:e7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Giacomelli R, Ruscitti P, Alvaro S, et al. IL-1β at the crossroad between rheumatoid arthritis and type 2 diabetes: may we kill two birds with one stone? Expert Rev Clin Immunol 2016;12:849–55. [DOI] [PubMed] [Google Scholar]
- [48].Arts EE, Fransen J, den Broeder AA, et al. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Ann Rheum Dis 2015;74:998–1003. [DOI] [PubMed] [Google Scholar]
- [49].Franklin J, Farragher TM, Lunt M, et al. Excess risk of hospital admission for cardiovascular disease within the first 7 years from onset of inflammatory polyarthritis. Ann Rheum Dis 2010;69:1660–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Innala L, Möller B, Ljung L, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther 2011;13:R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Landewé R, van der Heijde D. Follow up studies in rheumatoid arthritis. Ann Rheum Dis 2002;61:479–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Salas M, Hofman A, Stricker BH. Confounding by indication: an example of variation in the use of epidemiologic terminology. Am J Epidemiol 1999;149:981–3. [DOI] [PubMed] [Google Scholar]
- [53].Signorello LB, McLaughlin JK, Lipworth L, et al. Confounding by indication in epidemiologic studies of commonly used analgesics. Am J Ther 2002;9:199–205. [DOI] [PubMed] [Google Scholar]
- [54].Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med 2004;141:764–70. [DOI] [PubMed] [Google Scholar]
- [55].Avina-Zubieta JA, Abrahamowicz M, De Vera MA, et al. Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: a population-based study. Rheumatology (Oxford) 2013;52:68–75. [DOI] [PubMed] [Google Scholar]
- [56].Ruscitti P, Ursini F, Cipriani P, et al. Prevalence of type 2 diabetes and impaired fasting glucose in patients affected by rheumatoid arthritis: results from a cross-sectional study. Medicine (Baltimore) 2017;96:e7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ursini F, D’Angelo S, Padula A, et al. Retrospective analysis of type 2 diabetes prevalence in a systemic sclerosis cohort from southern Italy: comment on “Reduced incidence of Type 1 diabetes and Type 2 diabetes in systemic sclerosis: a nationwide cohort study” by Tseng et al. Joint Bone Spine 2016;83:611–2. [DOI] [PubMed] [Google Scholar]
- [58].Ursini F, Grembiale A, Naty S, et al. Serum complement C3 correlates with insulin resistance in never treated psoriatic arthritis patients. Clin Rheumatol 2014;33:1759–64. [DOI] [PubMed] [Google Scholar]
- [59].Giacomelli R, Ruscitti P, Bombardieri S, et al. What could we learn from the sub-analysis of a single nation cohort in a worldwide study? Lessons from the results observed in the Italian cohort of the GO-MORE trial. Clin Exp Rheumatol 2017;35:623–9. [PubMed] [Google Scholar]
- [60].Cipriani P, Berardicurti O, Masedu F, et al. Biologic therapies and infections in the daily practice of three Italian rheumatologic units: a prospective, observational study. Clin Rheumatol 2017;36:251–60. [DOI] [PubMed] [Google Scholar]
- [61].Leblond A, Allanore Y, Avouac J. Targeting synovial neoangiogenesis in rheumatoid arthritis. Autoimmun Rev 2017;16:594–601. [DOI] [PubMed] [Google Scholar]
- [62].Cheung TT, McInnes IB. Future therapeutic targets in rheumatoid arthritis? Semin Immunopathol 2017;39:487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ciccia F, Guggino G, Ferrante A, et al. Interleukin-9 overexpression and Th9 polarization characterize the inflamed gut, the synovial tissue, and the peripheral blood of patients with psoriatic arthritis. Arthritis Rheumatol 2016;68:1922–31. [DOI] [PubMed] [Google Scholar]
- [64].Trouw LA, Pickering MC, Blom AM. The complement system as a potential therapeutic target in rheumatic disease. Nat Rev Rheumatol 2017;13:538–47. [DOI] [PubMed] [Google Scholar]
- [65].Caporali R, Todoerti M, Scirè CA, et al. Oral low-dose glucocorticoids should be included in any recommendation for the use of non-biologic and biologic disease-modifying antirheumatic drugs in the treatment of rheumatoid arthritis. Neuroimmunomodulation 2015;22:104–11. [DOI] [PubMed] [Google Scholar]
- [66].Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, et al. Obesity in rheumatoid arthritis. Rheumatology (Oxford) 2011;50:450–62. [DOI] [PubMed] [Google Scholar]
- [67].Challal S, Minichiello E, Boissier MC, et al. Cachexia and adiposity in rheumatoid arthritis. Relevance for disease management and clinical outcomes. Joint Bone Spine 2016;83:127–33. [DOI] [PubMed] [Google Scholar]
- [68].Giacomelli R, Afeltra A, Alunno A, et al. International consensus: What else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren's syndrome)?: The unmet needs and the clinical grey zone in autoimmune disease management. Autoimmun Rev 2017;16:911–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


