Abstract
This study aimed to investigate the efficacy and safety of celecoxib 24 hours preoperative, 1 hour preoperative, and 4 hours postoperative administration in patients with arthroscopic knee surgery (AKS).
In all, 206 patients who underwent AKS were consecutively recruited and randomized into 3 groups: (1) early preoperative analgesia group (EPEA), celecoxib 400 mg 24 hours preoperative administration; (2) preoperative analgesia group (PEA), celecoxib 400 mg 1 hour preoperative administration; (3) postoperative analgesia group (POA), celecoxib 400 mg 4 hours postoperative administration. Pain visual analog scale (VAS) scores (at rest and at 90o flexion) and patient global assessment (PGA) score were evaluated before and after operation, and also pethidine consumption and adverse events (AEs).
The pain-rest VAS score, percentage of patients with moderate-severe pain at rest, and PGA score in the EPEA and PEA groups were decreased compared with POA group at 8 and 12 hours postoperation. Besides, pain-flexion to 90o VAS score in EPEA and PEA groups were also reduced compared with POA group at 8 hours postsurgery. Interestingly, the percentage of patients with moderate-severe pain at 90o flexion at 8 hours postsurgery in PEA group was fewer compared with POA group, whereas at 4 hours postoperation it was reduced in EPEA group compared with PEA and POA groups. As to consumption of pethidine, it was numerically decreased in EPEA and PEA groups compared with POA group. No difference between each 2 groups was found in AEs.
Celecoxib was effective and safe as pre-emptive analgesia in AKS, and 1 hour administration before operation might be an optimal choice.
Keywords: arthroscopic knee surgery, celecoxib, postoperative analgesia, preoperative analgesia
1. Introduction
Patients with end-stage knee diseases are always eager to receive a clinical treatment for the severe pain. In these cases, arthroscopic knee surgery (AKS) is generally accepted as an efficient and long-lasting therapy.[1,2] To acquire a satisfactory postoperative physical function, and also a high quality of life, early rehabilitation protocol as soon as possible after surgery is necessary.[3,4] However, the overwhelming fear of severe pain from surgery would make patients, especially those sensitive to or less able to tolerate pain, reluctant to initiate early rehabilitative exercise postoperatively, resulting in a delayed recovery or an inferior knee function.[5,6]
Celecoxib, a novel selective cyclooxygenase (COX)-2 inhibitor, has inhibitory effects on prostaglandins synthesis, both in the spinal cord and peripheral nervous system, and reduces hyperalgesia status after surgical traumas.[7] It is illuminated that compared with conventional nonsteroidal anti-inflammatory drugs, celecoxib has less gastrointestinal (GI) side effects and less influence on antiplatelet function with long-term use.[8–11]
The benefits of pain relief by celecoxib for preoperative analgesia have been observed in patients with hip arthroscopy.[6,12,13] To date, a few studies also illustrate that preoperative administration of celecoxib in AKS decreases patients’ pain score and lowers adverse effects.[7,14] However, few studies about the most appropriate time of celecoxib initiation before surgery have been explored in patients with AKS. Therefore, the purpose of this study was to compare the efficacy and safety of celecoxib 24 hours preoperative, 1 hour preoperative, and 4 hours postoperative administration in patients with AKS.
2. Methods
2.1. Patients
In all, 206 patients about to undergo arthroscopic knee surgery at Department of Orthopedics in Dongyang People's Hospital, Wenzhou Medical University, from Junuary 2014 to February 2017, were consecutively recruited in this randomized, controlled study. The inclusion criteria were: age above 18 and below 65 years; meniscal disease about to receive meniscectomy and partial meniscectomy by arthroscopy; and American Society of Anesthesiology physical status as I or II. The exclusion criteria were: about to receive repair of the meniscus, reconstructive procedures for concomitant knee injuries, or internal fixation of osteochondrosis dissecans; analgesic use within 1 week before the enrollment; intra-articular hyaluronic acid injections within 9 months or corticosteroid within 3 months before the enrollment; history of knee surgery; history of coagulopathy or thromboembolic disease; history of chronic pain and/or consumption of daily analgesics; history with GI disease, perforation, ulceration, obstruction, or bleeding; history with severe renal or hepatic disease; history with malignance tumor; known to be allergic to COX-2 selective inhibitors or pethidine; and women with lactating or pregnancy. This study was approved by Ethics Committee of Dongyang People's Hospital, Wenzhou Medical University. All the patients provided written informed consents. Moreover, this study was carried out in accordance with Helsinki statement.
2.2. Treatments
This was a randomized, controlled study. After the eligibility, all the patients were randomized into 3 groups: (1) early preoperative analgesia group (EPEA group); (2) preoperative analgesia group (PEA group); (3) postoperative analgesia group (POA group). In EPEA group, patients were assigned to receive celecoxib 400 mg at 24 hours before the operation, and then 200 mg every 12 hours (12 hours preoperation, 1 and 13 hours postoperation). In PEA group, patients were required to receive celecoxib 400 mg at 1 hour before the operation, and then 200 mg every 12 hours (10 and 22 hours postoperation). In the POA group, patients received celecoxib 400 mg at 4 hours after the operation, and then 200 mg every 12 hours (16 hours postoperation). The treatment duration was until 24 hours postoperation, whereas the observation duration was until 36 hours postoperation. Pethidine injection (5 mg/kg) was given if required during the entire observation duration.
2.3. Randomization
The randomization was conducted by a medical and statistic service company (Shanghai Qeejen, China) as follows: the randomization code was generated by a statistician using blocked randomization method (block length: 6) due to the need of allocation balance among 3 groups. The randomization documents were subsequently sent to the Department of Orthopedics in Dongyang People's Hospital kept by a doctor separately and a copy was kept in Shanghai Qeejen for backup. When a patient was eligible for the study, an unique subject identification number was provided from the randomized module and the patient was assigned to the identified group.
2.4. Data collection
Patient characteristics were recorded before the operation including age, sex, and body mass index (BMI). Operative time was collected during the surgery. Pain visual analog scale (VAS) score at rest, pain VAS score at flexion to 90°, patient global assessment (PGA) score were evaluated preoperation, at 4, 8, 12, 24, and 36 hours postoperation. Pethidine consumption during 36 hours was collected. Pain VAS score was graded as: 0, no pain; 1 to 3, light pain; 4 to 6, moderate pain; 7 to 10, severe pain.
2.5. Primary endpoints
The primary endpoints were the differences of pain VAS score at rest and pain VAS score at flexion to 90° among 3 groups after 12 and 24 hours postoperation.
2.6. Secondary endpoints
The secondary endpoints were: the difference of PGA score among 3 groups after 12 and 24 hours postoperation; the difference of pethidine consumption among 3 groups after 24 hours postoperation; the difference of common adverse events (AEs) among 3 groups within 36 hours observational period.
2.7. Statistics
Statistical analysis was performed using SAS 9.4 software and GraphPad Prism 6 software. Data were mainly presented as mean value and standard deviation (SD), count, and percentage. Comparison between 2 groups was determined by t test and chi-square test. P < 0.05 was considered significant.
3. Results
3.1. Study flow
In all, 292 patients were screened, among which 86 patients were excluded (31 patients exclusions and 55 patients disagreed to participate). Also, the remaining 206 patients were randomly assigned to EPEA group, PEA group, and POA group at the ratio of 1:1:1. In the POA group, there were 9 withdrawn patients (5 protocol violations, 3 insufficient efficacy, and 1 patient decision), and the remaining 60 (87%) patients completed the study. In the PEA group, there were 7 withdrawn patients, in which 4 were protocol violations, 2 insufficient efficacy, and 1 patient decision, and the remaining 62 (90%) patients fulfilled the study. As to EPEA group, 8 patients withdrawed (6 protocol violations, 2 insufficnent efficancy), and the remaining 60 patients (88%) finishd the study (Fig. 1).
Figure 1.
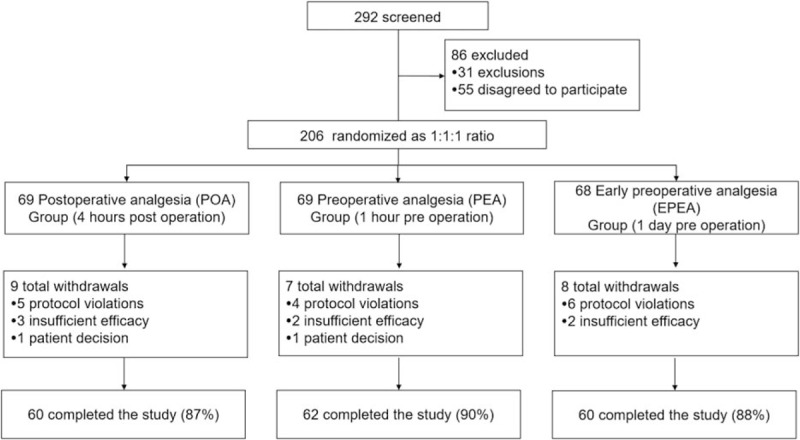
Study flow.
3.2. Baseline characteristic
Sixty-eight patients aged 34.69 ± 7.05 years, with 44 males and 24 females, were included in EPEA group; and 69 patients aged 36.04 ± 6.06 years, with 34 males and 35 females, were included in PEA group; as to POA group, 69 patients aged 35.86 ± 6.64 years, with 41 males and 28 females, were included. There was no difference in age, sex, BMI, and operative time between each 2 groups (EPEA group vs PEA group; EPEA group vs POA group; PEA group vs POA group; all P > .05), neither did pain at rest, pain at flexion of 90°, and PGA score at baseline (all P > .05). The detailed information is present in Table 1.
Table 1.
Baseline characteristics.
3.3. The VAS scores of pain-rest in EPEA group, PEA group, and POA group
The pain-rest VAS scores increased after the operation in all 3 groups and then back to the level of preoperation gradually. At 8 and 12 hours postoperation, the pain-rest VAS score of EPEA group (both P < .05) and PEA group (both P < .05) were lowered than that of POA group, and no difference between EPEA and PEA group was found (P > .05) (Fig. 2A). As shown in Fig. 2B, the percentage of patients with moderate-severe pain at rest and at 8 and 12 hours postoperation in EPEA (both P < .05) and PEA groups (both P < .05) were also decreased compared with POA group, whereas no difference was found between the EPEA and PEA groups (P > .05). No difference of pain-rest VAS score nor pain severity between each 2 groups at other observational time was discovered.
Figure 2.
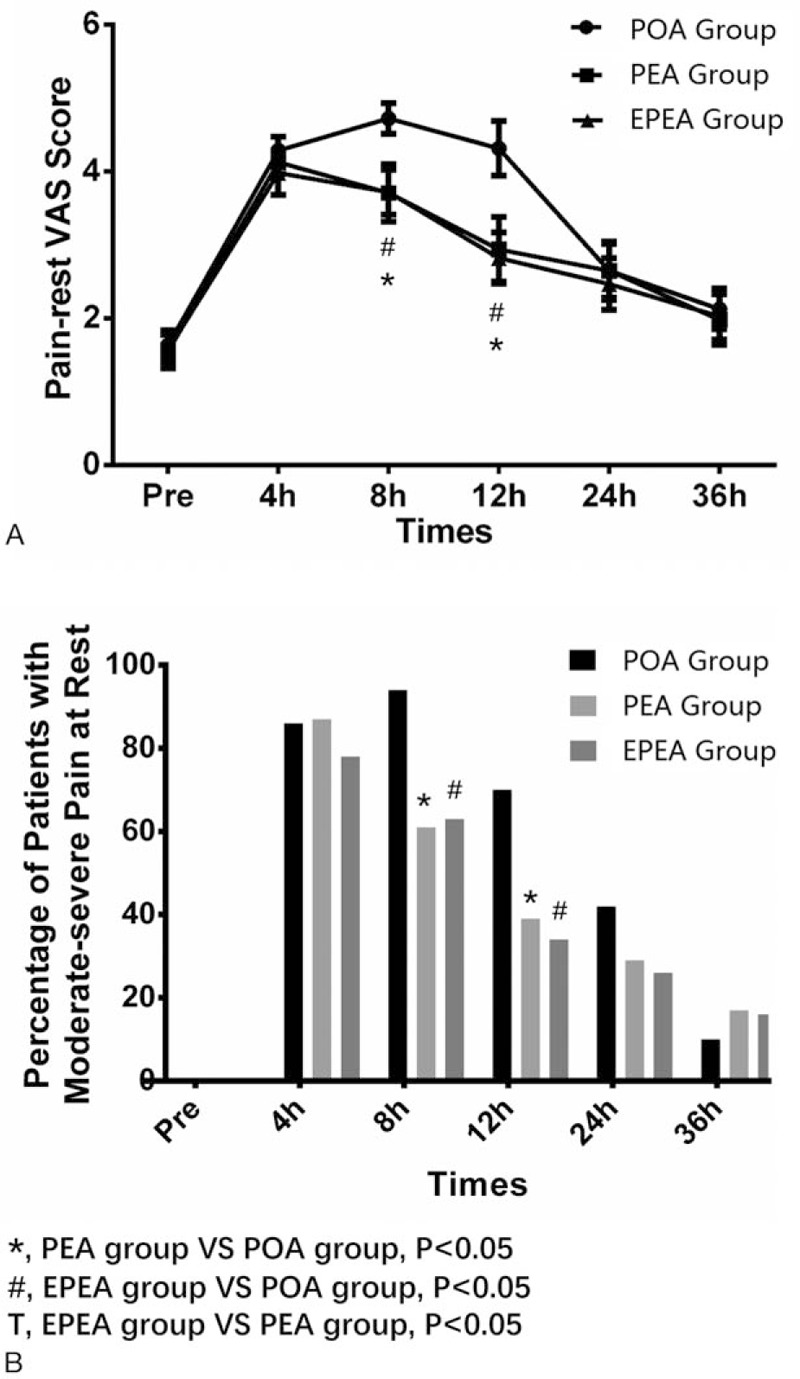
Comparison of the pain-rest VAS scores and the percentage of patients with moderate-severe pain at rest among EPEA group, PEA group, and POA group. (A) Comparison of the pain-rest VAS scores among EPEA group, PEA group, and POA group. (B) Comparison of the percentage of patients with moderate-severe pain at rest among EPEA group, PEA group, and POA group. Comparison between groups was performed by t test. P < .05 was considered significant. EPEA = early preoperative analgesia, PEA = preoperative analgesia, POA = postoperative analgesia, VAS = visual analog scale.
3.4. The VAS scores of pain-flexion to 90o in EPEA group, PEA group, and POA group
As to pain-flexion to 90o VAS score, it ascended postsurgery in all 3 groups and descended to preoperative level gradually as well. Pain VAS score at flexion to 90° was found to be decreased in EPEA group (P < .05) and PEA group (P < .05) compared with POA group at 8 hours postoperation (Fig. 3A). Interestingly, at 4 hours postsurgery, percentage of patients with moderate-severe pain-flexion at 90o in EPEA group was lower than that in PEA (P < .05) and POA groups (P < .05). Besides, percentage of patients with moderate-severe pain-flexion at 90o in PEA group (P < .05) was lower than that of POA group at 8 hours postoperation (Fig. 3B). No difference of pain-flexion to 90° VAS score nor pain severity between each 2 groups at other observational time was observed.
Figure 3.
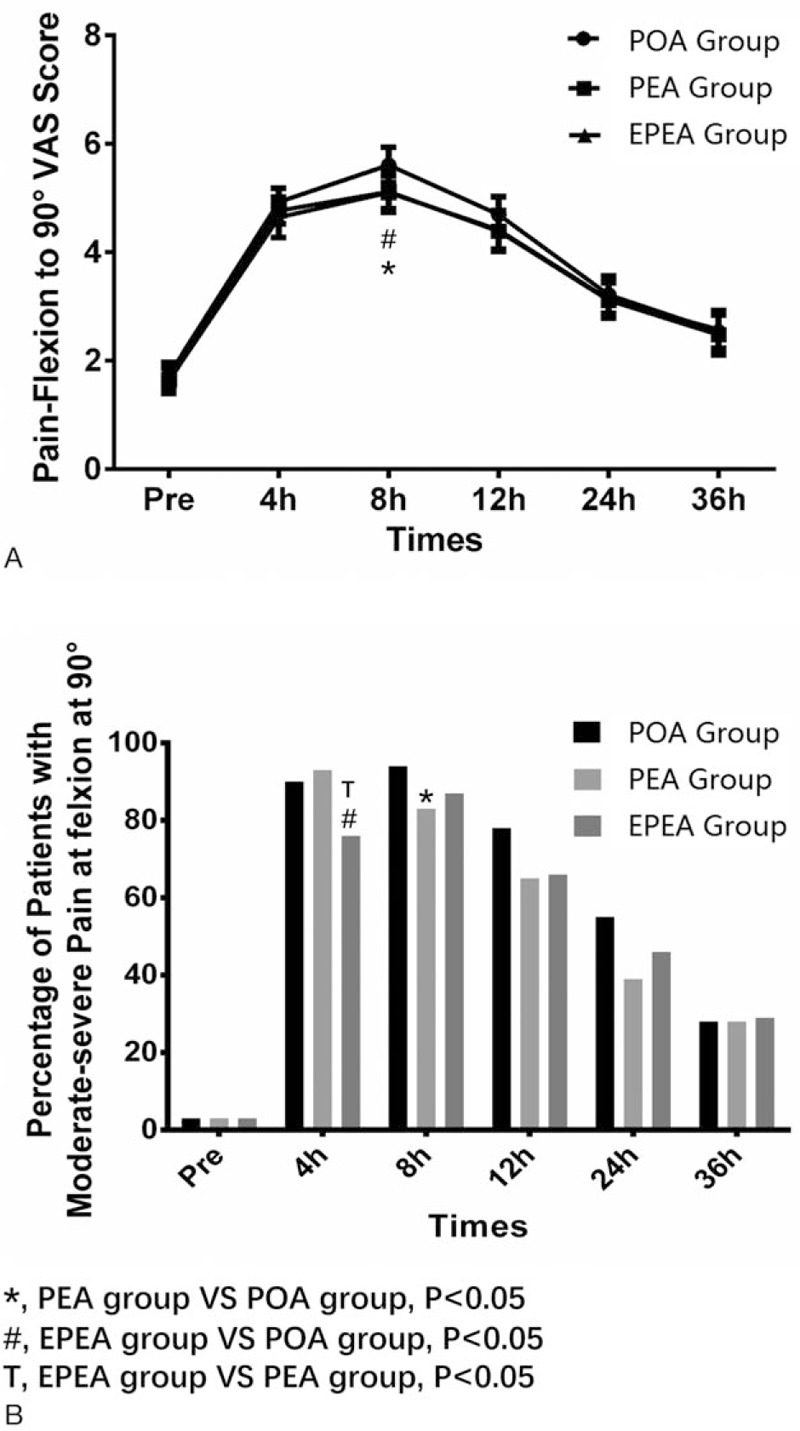
Comparison of the pain-flexion to 90o VAS scores and the percentage of patients with moderate-severe pain at flexion to 90o among EPEA group, PEA group, and POA group. (A) Comparison of the pain-flexion to 90o VAS scores among EPEA group, PEA group, and POA group. (B) Comparison of the percentage of patients with moderate-severe pain at flexion to 90o among EPEA group, PEA group, and POA group. Comparison between groups was performed by t test. P < .05 was considered significant. EPEA = early preoperative analgesia, PEA = preoperative analgesia, POA = postoperative analgesia, VAS = visual analogue scale.
3.5. The PGA score in EPEA group, PEA group, and POA group
As presented in Fig. 4, at 8 and 12 hours after surgery, the PGA score in EPEA group (both P < .05) and PEA group (both P < .05) was decreased compared with that in POA group, but no difference between EPEA group and PEA group (both P > .05) was found. No difference of PGA score between each 2 groups at other observational time was found.
Figure 4.
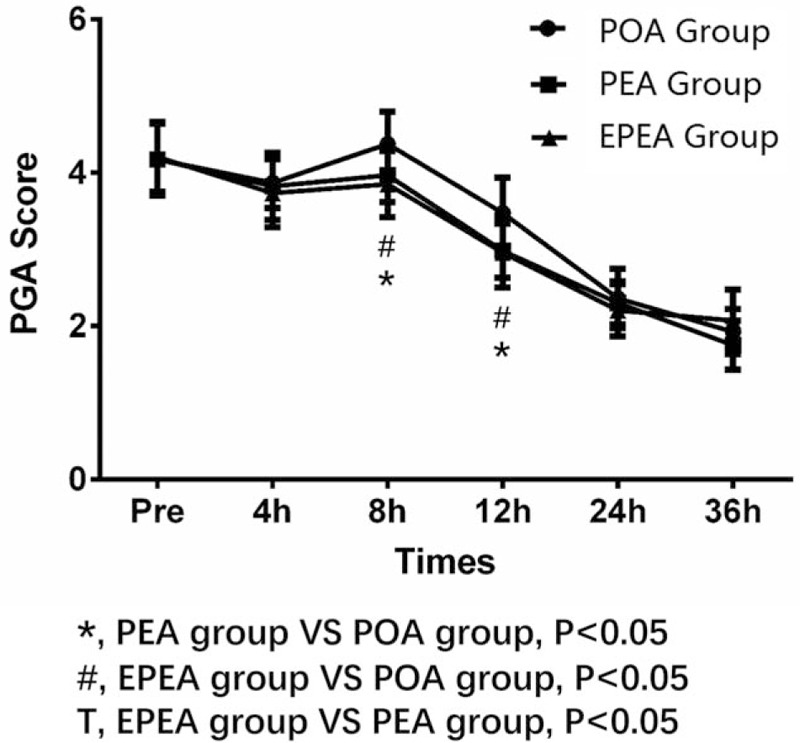
Comparison of the PGA score among EPEA group, PEA group, and POA group. Comparison between groups was performed by t test. P < .05 was considered significant. EPEA = early preoperative analgesia, PEA = preoperative analgesia, PGA = patient global assessment, POA = postoperative analgesia.
3.6. The consumption of pethidine
Pethidine (5 mg/kg) was injected during the study if required. The consumption of pethidine in EPEA group (P = .060) and PEA group (P = .091) was numerically decreased compared with POA group (Fig. 5), but there was no statistical significance. However, there was no difference of pethidine usage between EPEA group and PEA group (P = .809).
Figure 5.
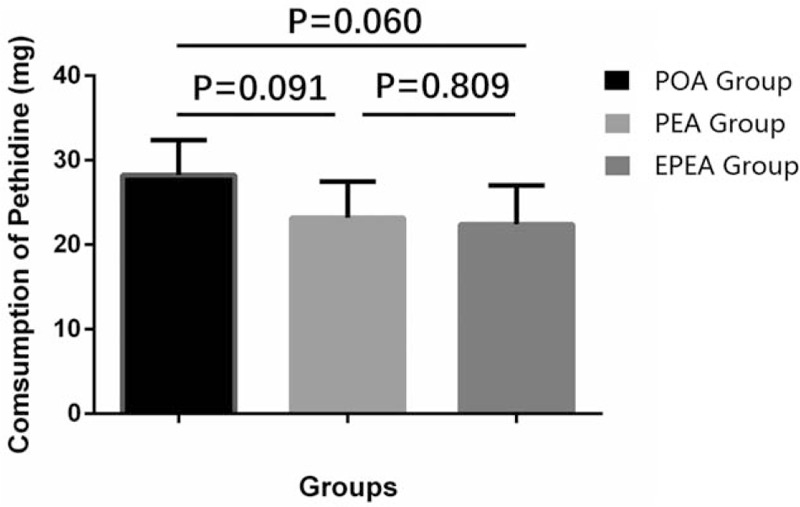
Comparison of the consumption of pethidine among EPEA group, PEA group, and POA group. Comparison between groups was performed by t test. P < .05 was considered significant. EPEA = early preoperative analgesia, PEA = preoperative analgesia, POA = postoperative analgesia.
3.7. AEs
Nausea, vomiting, constipation, drowsiness, and dizziness were the most frequent AEs in all groups. No difference existed between each 2 groups in AEs as showed in Fig. 6 (all P > .05).
Figure 6.
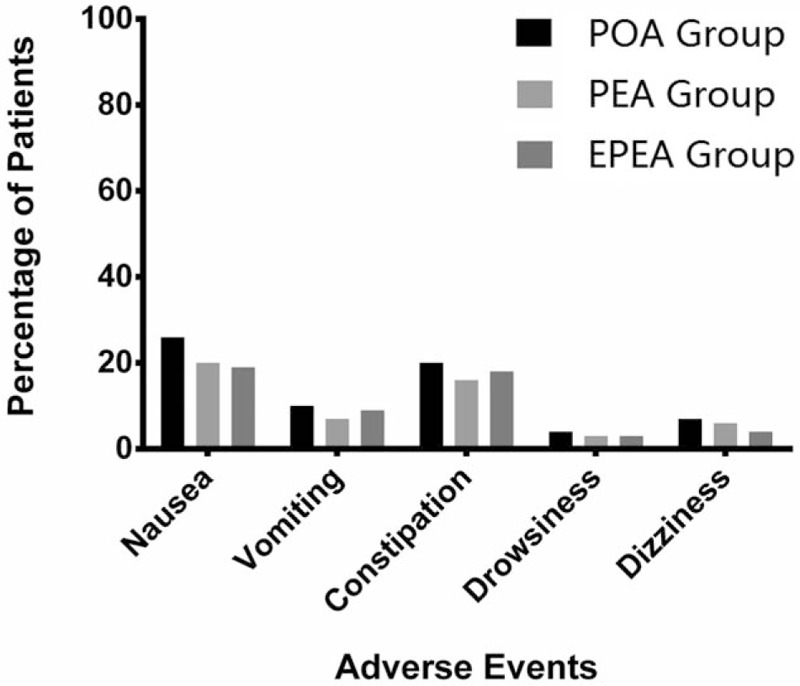
Comparison of the percentage of patients with AEs in EPEA group, PEA group, and POA group. Comparison between groups was performed by t test. P < .05 was considered significant. AEs = adverse events, EPEA = early preoperative analgesia group, PEA = preoperative analgesia group, POA = postoperative analgesia group.
4. Discussion
In the present study, we found: the pain-rest and pain-flexion to 90o in EPEA group and PEA group seem to be decreased compared with those in POA group, which was validated by differences in pain VAS score among 3 groups at 8 and 12 hours; the PGA score in the EPEA group and PEA group was likely be reduced compared with that in POA group, which was validated by difference in PGA score among 3 groups at 8 and 12 hours; the consumptions of pethidine in EPEA group and PEA group were numerically decreased compared with POA group, whereas no difference between each 2 groups was found in AEs. These indicate preoperative analgesia with celecoxib is more effective compared with postoperative use of celecoxib, whereas early preoperative treatment (24 hours before operation) presents with the same outcomes with preoperative treatment (1 hour before operation).
Pain after AKS, 1 of the major causes of patient dissatisfaction, delays the recovery and increases the hospitalization cost.[15] Achieving effective pain control after AKS continues to be a clinically important issue in facilitating the rehabilitation process and enhancing patient satisfaction. Although opioids are an important components of postoperative pain management, they are associated with side-effects,[16] and so, the pre-emptive analgesic approach with the use of celecoxib, which has less adverse effects, has been recommended for relieving postoperative pain.[17,18]
Generally, the use of celecoxib for elders does not need to adjust the dose, but for elders with weight no more than 50 kg, it is better to have minimum recommended dose when starting treatment. Moreover, celecoxib should not be used in patients with glomerular filtration rate no more than 60 mL/min and patients with risk factors for stroke and myocardial infarction.
A randomized, double-blind, prospective clinical trial reveals that oral celecoxib 200 mg 2 hours before the operation is better than placebo for controlling of postoperative pain in patients who underwent lower-extremity orthopedic surgery under general anesthesia.[19] Another triple-blinded, randomized, placebo-controlled clinical trial suggested that celecoxib as a pre-emptive analgesic agent is efficient in decreasing acute postoperative pain and 24 hours opioid consumption in patients who underwent AKS.[7] Besides, a cohort study illuminates that pre-emptive analgesia by 3-day administration of celecoxib is an efficient and safe therapy regarding patients with total knee arthroplasty in alleviating postoperative pain. These studies prove that celecoxib as medicine of pre-emptive analgesia is effective and safe, which is consistent with our results that pain VAS score and the PGA score postoperative in EPEA and PEA groups were lower than those of POA group. The reason might be that: celecoxib could inhibit the synthesis of prostaglandins, which played a prominent role in inflammation and pain after AKS, both in the spinal cord and peripheral nervous system[20,21]; celecoxib is a concentration-dependent analgesic drug, the area under the plasma concentration-time curve in EPEA group and PEA group was larger than POA group at 8 and 12 hours, which makes the analgesia effect of EPEA and PEA groups superior to POA group.
As to pethidine consumption, we found it declined in EPEA group and PEA group compared with POA group, which may result from the less need of salvage pethidine treatment due to decreaded pain score in EPEA and PEA groups than POA group. Besides, no difference was found among 3 groups in AEs, which was in accordance with the study performed by Mardani-KiviM that AEs are not significantly different between celecoxib pre-emptive treatment group and placebo group such as nausea and vomiting.[7] Similarly, a randomized controlled trial illuminates that incidence of AEs also presented with no difference among 1 hour preoperative etoricoxib, celecoxib and placebo groups for acute postoperative pain in patients with arthroscopic anterior cruciate ligament reconstruction.[22]
Interestingly, no remarkable difference was found between EPEA and PEA groups in terms of pain VAS score and the PGA score postoperative, neither did pethidine consumption and AEs. But for the consideration of cost-saving and toxicity of medicine itself, we thought pre-emptive analgesia in PEA group (1 hour before operation) is the optimal choice for AKS.[12,19,23]
This was the first study comparing the efficacy and safety of celecoxib 24 hours preoperative, 1 hour preoperative, and 4 hours postoperative administration in patients with AKS. However, there were some limitations which existed in this study. Firstly, this was an open-labeled randomized controlled study, thus the influence of bias of doctors’ assessments and patients’ self-reported evaluations exist. Secondly, we evaluated efficacy of pre-emptive use of routine dose of celecoxib (200 mg, initial dose doubling), but this dose was based on the recommended dosage of the drug description which might not be the most suitable dose for pre-emptive analgesia for AKS operation. Thirdly, functional score was not assessed, resulting in the absence of direct evidence in favor of pre-emptive analgesia, improving the postoperative functional recovery. Fourthly, patients who are allergic to sulfonamide did not included in the exclusion criteria, even though no patient was allergic to sulfonamide. Thus, a further blinded randomized controlled trial study with multiple assessment of pain, function, satisfaction, and quality of life is needed in the future.
5. Conclusions
In conclusion, this study observed that celecoxib was effective and safe as pre-emptive analgesia in AKS, and 1 hour administration before operation might be an optimal choice.
Footnotes
Abbreviations: AEs = adverse events, AKS = arthroscopic knee surgery, BMI = body mass index, COX = cyclooxygenase, EPEA = early preoperative analgesia group, GI = gastrointestinal, PEA = preoperative analgesia group, PGA = patient global assessment, POA = postoperative analgesia group, VAS = celecoxib visual analogue scale.
The authors of this work have nothing to disclose.
References
- [1].Koh JL, Zwahlen BA, Altchek DW, et al. Arthroscopic treatment successfully treats posterior elbow impingement in an athletic population. Knee Surg Sports Traumatol Arthrosc 2017;doi: 10.1007/s00167-017-4563-1 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lui TH. Complete arthroscopic synovectomy in management of recalcitrant septic arthritis of the knee joint. Arthrosc Tech 2017;6:e467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McGrory B, Weber K, Lynott JA, et al. The American Academy of Orthopaedic Surgeons Evidence-Based Clinical Practice Guideline on Surgical Management of Osteoarthritis of the Knee. J Bone Joint Surg Am 2016;98:688–92. [DOI] [PubMed] [Google Scholar]
- [4].Sporer SM, Rogers T. Postoperative pain management after primary total knee arthroplasty: the value of liposomal bupivacaine. J Arthroplasty 2016;31:2603–7. [DOI] [PubMed] [Google Scholar]
- [5].Hayashi K, Kako M, Suzuki K, et al. Associations among pain catastrophizing, muscle strength, and physical performance after total knee and hip arthroplasty. World J Orthop 2017;8:336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang Z, Zhu W, Zhu L, et al. Efficacy of celecoxib for pain management after arthroscopic surgery of hip: a prospective randomized placebo-controlled study. Eur J Orthop Surg Traumatol 2014;24:919–23. [DOI] [PubMed] [Google Scholar]
- [7].Mardani-Kivi M, Karimi Mobarakeh M, Haghighi M, et al. Celecoxib as a pre-emptive analgesia after arthroscopic knee surgery; a triple-blinded randomized controlled trial. Arch Orthop Trauma Surg 2013;133:1561–6. [DOI] [PubMed] [Google Scholar]
- [8].Chan FKL, Ching JYL, Tse YK, et al. Gastrointestinal safety of celecoxib versus naproxen in patients with cardiothrombotic diseases and arthritis after upper gastrointestinal bleeding (CONCERN): an industry-independent, double-blind, double-dummy, randomised trial. Lancet 2017;389:2375–82. [DOI] [PubMed] [Google Scholar]
- [9].Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005;352:1092–102. [DOI] [PubMed] [Google Scholar]
- [10].Masclee GM, Valkhoff VE, Coloma PM, et al. Risk of upper gastrointestinal bleeding from different drug combinations. Gastroenterology 2014;147:784–92 e789. [quiz e713–784]. [DOI] [PubMed] [Google Scholar]
- [11].Onuora S. Therapy: celecoxib reduces risk of ulcer bleeding. Nat Rev Rheumatol 2017;13:324. [DOI] [PubMed] [Google Scholar]
- [12].Kahlenberg CA, Patel RM, Knesek M, et al. Efficacy of celecoxib for early postoperative pain management in hip arthroscopy: a prospective randomized placebo-controlled study. Arthroscopy 2017;33:1180–5. [DOI] [PubMed] [Google Scholar]
- [13].Gordo AC, Walker C, Armada B, et al. Efficacy of celecoxib versus ibuprofen for the treatment of patients with osteoarthritis of the knee: a randomized double-blind, non-inferiority trial. J Int Med Res 2017;45:59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ekman EF, Berger M, Bhadra P. Determine whether perioperative celecoxib administration would reduce opioid consumption and provide effective pain relief in patients undergoing arthroscopic knee surgery. Arthroscopy 2006;22:804. [DOI] [PubMed] [Google Scholar]
- [15].Gan TJ, Joshi GP, Viscusi E, et al. Preoperative parenteral parecoxib and follow-up oral valdecoxib reduce length of stay and improve quality of patient recovery after laparoscopic cholecystectomy surgery. Anesth Analg 2004;98:1665–73. [DOI] [PubMed] [Google Scholar]
- [16].Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630–41. [DOI] [PubMed] [Google Scholar]
- [17].Kaufman E, Epstein JB, Gorsky M, et al. Preemptive analgesia and local anesthesia as a supplement to general anesthesia: a review. Anesth Prog 2005;52:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen JQ, Wu Z, Wen LY, et al. Preoperative and postoperative analgesic techniques in the treatment of patients undergoing transabdominal hysterectomy: a preliminary randomized trial. BMC Anesthesiol 2015;15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kashefi P, Honarmand A, Safavi M. Effects of preemptive analgesia with celecoxib or acetaminophen on postoperative pain relief following lower extremity orthopedic surgery. Adv Biomed Res 2012;1:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dutta N, Sarotra P, Gupta S, et al. Mechanism of action of celecoxib on normal and acid-challenged gastric mucosa. Exp Toxicol Pathol 2009;61:353–61. [DOI] [PubMed] [Google Scholar]
- [21].Dabhi JK, Solanki JK, Mehta A. Antiatherosclerotic activity of ibuprofen, a non-selective COX inhibitor–an animal study. Indian J Exp Biol 2008;46:476–81. [PubMed] [Google Scholar]
- [22].Boonriong T, Tangtrakulwanich B, Glabglay P, et al. Comparing etoricoxib and celecoxib for preemptive analgesia for acute postoperative pain in patients undergoing arthroscopic anterior cruciate ligament reconstruction: a randomized controlled trial. BMC Musculoskelet Disord 2010;11:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Steffens JP, Santos FA, Pilatti GL. The use of etoricoxib and celecoxib for pain prevention after periodontal surgery: a double-masked, parallel-group, placebo-controlled, randomized clinical trial. J Periodontol 2011;82:1238–44. [DOI] [PubMed] [Google Scholar]


