Abstract
Nonspecific low back pain (LBP) is a common musculoskeletal problem that is intensified during physical activity. Patients with LBP have been reported to change their abdominal muscle activity during walking; however, the effects of pain intensity, disability level, and fear-avoidance belief on this relationship have not been evaluated. Thus, we compared abdominal muscle activity in patients with LBP and asymptomatic controls, and assessed the impact of pain intensity, disability level, and fear-avoidance belief.
Thirty patients with LBP divided into groups reporting low (LLBP) and high-pain intensity low back pain (HLBP), and 15 participants without LBP were recruited. LBP patients’ self-reported pain intensity, disability, and fear-avoidance belief were recorded. To examine abdominal muscle activity (rectus abdominis [RA], internal [IO], and external oblique [EO] muscles) during walking, all subjects walked at a self-selected speed. Abdominal muscle activity (RA, IO, and EO) was compared among groups (LLBP, HLBP, and controls) in different phases of walking (double support vs swing). Relationships between abdominal muscle activity and clinical measures (pain intensity, disability, fear-avoidance belief) were analyzed using partial correlation analysis.
Right IO muscle activity during walking was significantly decreased in LLBP and HLBP compared with controls in certain walking phase. Partial correlation coefficients showed significant correlations between fear-avoidance belief and right EO activity (r = .377, P < .05) and between disability index and left IO activity (r = .377, P < .05) in patients with LBP. No significant difference was found in abdominal muscle activity in walking between patients with LLBP and HLBP (P > .05).
This study demonstrated decreased IO muscle activity during certain walking phases in LLBP and HLBP compared with asymptomatic participants. Although altered IO muscle activity during walking was observed in patients with LBP, no changes were found with other abdominal muscles (EO, RA). Thus, these results provide useful information about abdominal muscle activity during walking in patients with LBP.
Keywords: abdominal muscles, disability, fear, low back pain, walking
1. Introduction
Low back pain (LBP) is a major musculoskeletal disorder, and managing patients with LBP is a significant economic burden in many countries.[1,2] Previous studies have reported changed patterns of abdominal muscle activity in patients with LBP compared with asymptomatic controls.[3–6] Increased or changed abdominal muscle patterns during physical activities can lead to impaired spinal motion.[5–7]
Investigators and clinicians have focused on the relationship between abdominal muscle activity in walking and LBP.[3–6] Some studies have reported that patients with LBP appear to show increased muscle activity of the superficial abdominal muscles during walking, thereby stiffening the spine compared with asymptomatic controls.[4–6] However, other studies have demonstrated that the rectus abdominis (RA) and internal oblique (IO) muscles are activated less in LBP during certain phases of walking.[3] Although findings from these studies suggest that abdominal muscle activity in walking may display differing patterns in LBP patients compared with healthy controls, whether any particular abdominal muscle activation pattern may be related to pain intensity in LBP patients remains unknown.
One plausible mechanism for back pain's effects on motor control via trunk muscle activity is the fear-avoidance model.[8] Fear of back pain can disrupt normal control of the trunk muscles, causing difficulty in relaxing the trunk muscles during walking.[5,6,9] Anticipation of pain may lead to activity avoidance and activity restriction, resulting in disability during functional activity.[8] Thus, it is important to understand whether such disability or fear and avoidance influence changes in abdominal muscle activity in LBP during walking.
A graded classification of pain can reflect disease or disability severity in LBP.[10] High-severity chronic pain would be expected to contribute to a high level of disability in walking tasks. To date, no study has evaluated the influence of pain intensity on abdominal muscle activity during walking in patients with LBP. Thus, in this study, we assessed abdominal muscle activity during walking in patients with low- (LLBP) and high-pain intensity low back pain (HLBP) and in healthy controls. We also investigated relationships among pain intensity, disability, fear-avoidance belief, and abdominal muscle activity during walking in patients with LBP.
2. Methods
2.1. Study design and setting
This study was designed as a case–control, cross-sectional study. Subjects were recruited from a college campus and a community in South Korea. All experimental procedures were conducted in the motion analysis laboratory at Yonsei University. Patients with LBP were divided into 2 groups based on self-reported back pain intensity measured using a 100-mm visual analog scale (VAS). LLBP was defined as VAS < 5/10, and HLBP as VAS ≥ 5/10, where a VAS score of 0 meant no pain, and a score of 10 meant the most severe back pain possible. This cutoff value was chosen based on a previous study.[10] After all subjects completed a clinical questionnaire, the walking test was performed. Before participating in the study, all participants were provided with information sheets, and all signed an informed consent statement. This study was approved by the Yonsei University Wonju institutional review board (1041849-201701-BM-069-01).
2.2. Participants
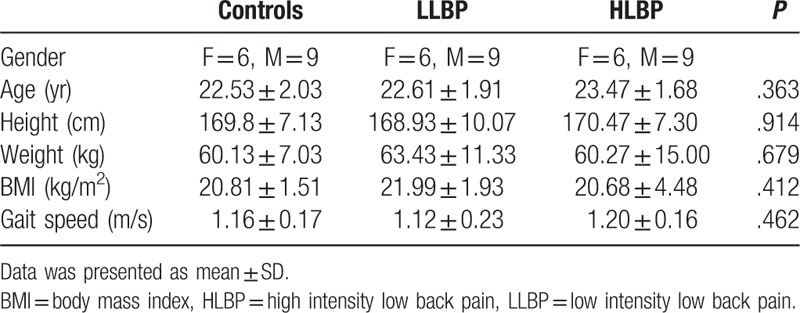
In total, 30 patients with LBP and 15 age- and gender-matched controls with no history of LBP within the past year participated (Table 1). A sample size of 15 for each group was determined based on an effect size of .7 at 80% power and a significance level of .05 (5% chance of type 1 error).[11] Inclusion criteria for patients with LBP were current or recurrent nonspecific LBP lasting more than 7 weeks and pain under the costal margin and above the inferior gluteal folds, without radiating pain. Exclusion criteria were as follows: back pain with a history of surgery in the back or lower extremities; spinal fracture; structural deformity; and specific diseases such as radicular syndrome, cauda equine syndrome, tumor, or ankylosing spondylitis (according to Waddell definition of “nonspecific”).[12] Subjects were also excluded if they had difficulty walking due to a neurological or musculoskeletal disorder or a lower extremity injury.
Table 1.
Characteristics of the study participants.
2.3. Questionnaires
Questionnaires were completed by patients who reported LBP. To measure disability, modified Oswestry Disability Index (ODI) and Roland Morris Disability Questionnaire (RMDQ) scales were used. Modified ODI scores range from 0 (no disability) to 100 (maximum disability).[13] The RMDQ consists of 24 items related to limitations in activities of daily living. RMDQ scores range from 0 (no disability) to 24 (maximum disability).[14] The Fear-Avoidance Beliefs Questionnaire (FABQ) was used to determine how much fear and avoidance of pain affected patients with LBP.[15]
2.4. Walking test
To collect abdominal muscle activity during walking, all participants walked in an experimental room at a comfortable speed. A warm-up time of 5 minutes was provided for participants to become familiar with the experimental walkway. After accommodation trials, subjects were asked to walk at a self-selected speed. Averaged results of 12 gait cycles were used for statistical analyses.
2.5. EMG analysis and gait events
A 3-dimensional system (Vicon MX system, Oxford Metrics, Oxford, UK) for kinematic data and the Noraxon TeleMyo 2400T instrument (Noraxon, Scottsdale, AZ) for EMG data were used simultaneously. All data were recorded using Nexus 1.4 software. A 3-dimensional system was used to detect gait cycles. Reflective markers were attached to the heel and 2nd metatarsal head.[16] A 1-stride cycle was defined as extending from a right heel strike (HS) to the next right HS. HS was defined as the time of minimum vertical velocity of the 2nd metatarsal head marker. Toe-off (TO) was defined as the time of maximum vertical velocity of the heel marker.[16] Gait was divided into 4 phases: 1st double support (from right HS to left TO), left swing (from left TO to left HS), 2nd double support (from left HS to right TO), and right swing (from right TO and right HS).
To measure RA activation, an electrode was attached 2 cm laterally from the umbilicus on the RA belly.[17] The electrode for the external oblique (EO) was placed slightly obliquely and anterior to the halfway point on a line between the iliac crest and the ribs, positioned laterally to the RA.[17] For the IO, the electrode was attached superior to the inguinal ligament on the lateral border of the RA sheath, medial, and anterior to the superior iliac spine.[18] Muscle activity of the RA, EO, and IO were measured during walking and normalized by sub-maximal voluntary isometric contraction (MVIC) according to Dankaerts et al.[19] Averaged muscle activity for the 12-gait cycle is presented as percentages of sub-MVIC. Sub-MVIC was more reliable between days than was MVIC in patients with LBP.[19]
2.6. Statistical analysis
All statistical analyses were performed using the SPSS software (ver. 24.0). The Kolmogorov–Smirnov test was conducted to ensure a normal distribution of the variables. One-way ANOVA was used to compare subject characteristics (age, height, weight, BMI, and gait speed) among LLBP, HLBP, and controls. The independent t test for parametric variables and Mann–Whitney U test for nonparametric variables were used to compare pain duration, VAS, modified ODI, RMDQ, and FABQ between LLBP and HLBP. EMG activities (EO, IO, and RA) for each muscle during each phase of walking were compared among LLBP, HLBP, and controls using the Kruskal–Wallis test. If a significant group difference was identified, post hoc comparisons with the Mann–Whitney U test were conducted. The effect size, Cohen d, was calculated to determine the standardized mean difference among groups for each muscle. An effect size of d = .20 was considered “small,” d = .50 was “medium,” and d = .80 was “large.” Linear correlation analysis was used to assess relationships among the clinical measures (VAS, ODI, RMDQ, and FABQ) in patients with LBP. Partial correlation analysis was used to determine relationships between clinical measures (VAS, ODI, RMDQ, and FABQ) and the averaged abdominal muscle activity (EO, IO, and RA) across the whole gait cycle, controlling for gait speed, in the LBP patients. A correlation coefficient ≥.75 was considered “good to excellent,” .50 to .75 was “moderate to good,” .25 to .50 was “fair,” and .00 to .25 indicated “little or no relationship.”[20] The level of statistical significance was set at P < .05.
3. Results
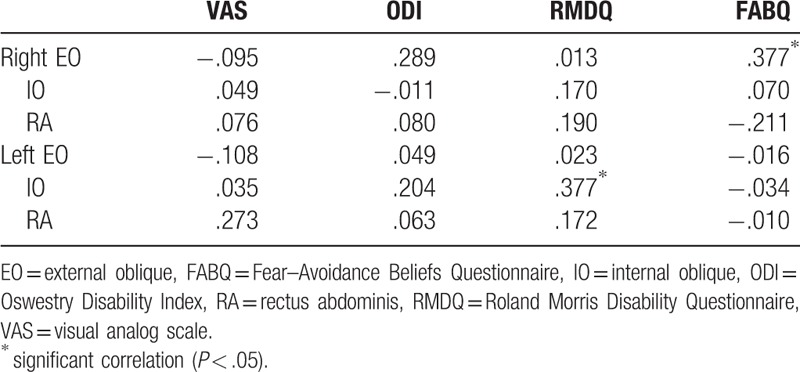
Pain duration and clinical measures (VAS, modified ODI, RMDQ, and FABQ) in patients with LBP are presented in Table 2. The HLBP group had significantly higher VAS (P < .001) and RMDQ (P = .004) scores than the LLBP group. A significant group difference was found for right IO muscle activity during left swing (χ2 = 6.150, P = .046) and during the 2nd double support phase (χ2 = 9.323, P = .009; Fig. 1). Compared to the control group, HLBP had significantly lower right IO muscle activity during left swing (P = .019) and the 2nd double support phase (P = .015), and LLP had significantly lower right IO muscle activity during the 2nd double support phase (P = .006). Table 3 presents the effect size among groups for each muscle. Results of the linear correlation analysis are shown in Table 4. Moderate to good correlations were observed between VAS and RMDQ (r = .534), between ODI and RMDQ (r = .507), and between ODI and FABQ (r = .606). A fair correlation was observed between VAS and ODI (r = .378). Partial correlation analysis revealed significant correlations between FABQ and right EO (r = .377, P < .05) and between RMDQ and left IO activity (r = .377, P < .05; Table 5).
Table 2.
Comparison of the pain duration, VAS, ODI, RMDQ, and FABQ between patients with LLBP and HLBP.
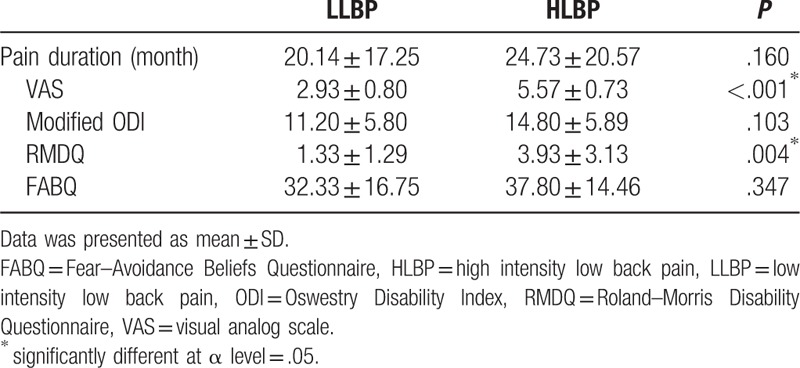
Figure 1.
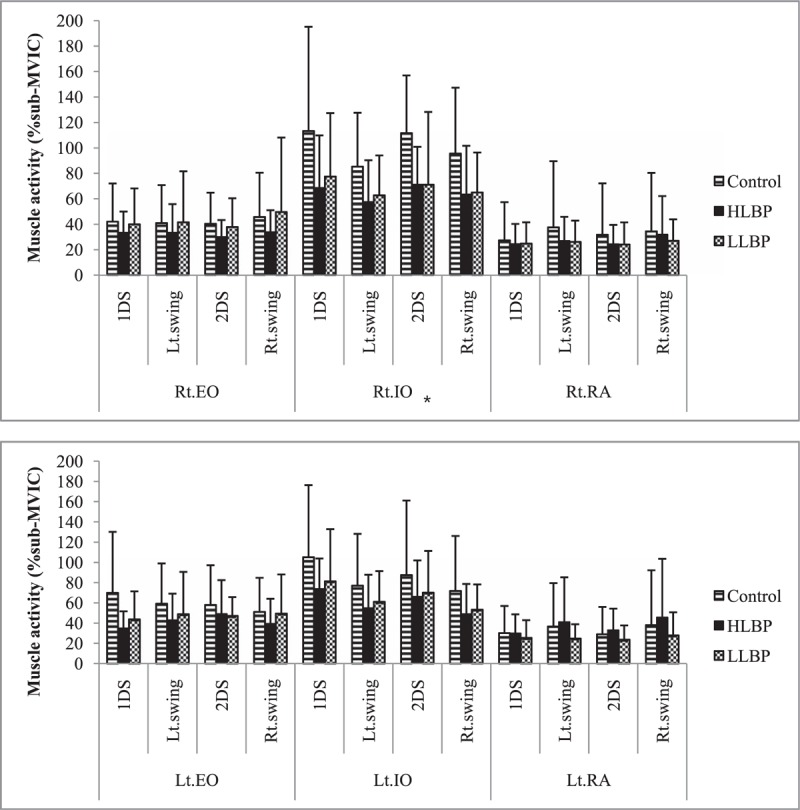
Muscle activity for each group and phase of gait, for right and left abdominal muscles. ∗Significant difference of group (P < .05). EO = external oblique, HLBP = high intensity low back pain, IO = internal oblique, LLBP = low intensity low back pain, MVIC = maximal voluntary isometric contraction, RA = rectus abdominis.
Table 3.
Effect size among groups for each muscle.
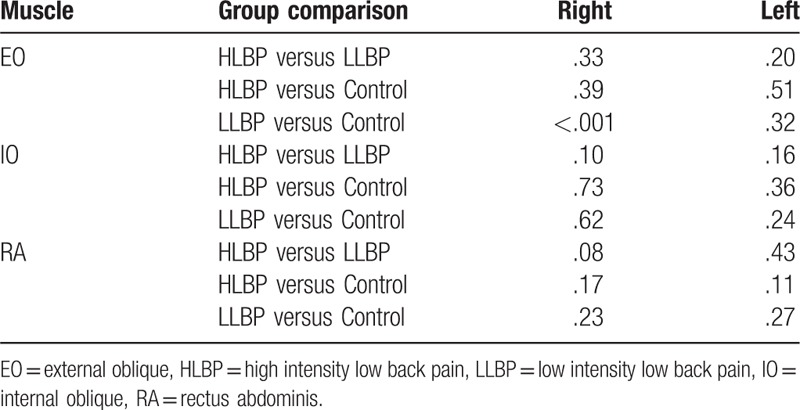
Table 4.
Linear correlation analyses between four clinical measurements.
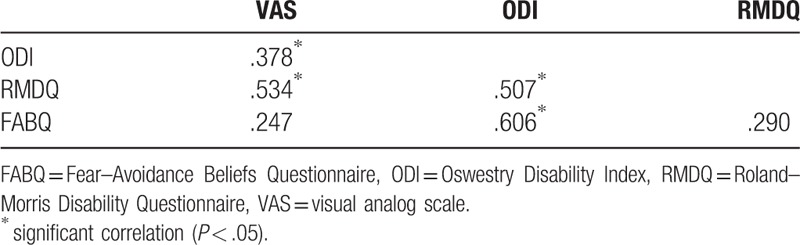
Table 5.
Partial correlation coefficients between clinical measures and muscle activities during walking.
4. Discussion
The primary purpose of this study was to compare abdominal muscle activation during walking in patients with LLBP and HLBP, and asymptomatic controls. This study provides evidence of abdominal muscle activation during walking in LBP according to pain intensity (low vs high). The results showed no significant difference in abdominal muscle activation between patients with LLBP and HLBP in walking. However, the right IO muscle showed low muscle activation during walking in patients with LLBP and HLBP compared with controls in certain phase. Also, we identified a correlation between abdominal muscle activation during walking and clinical measures (VAS, RMDQ, ODI, and FABQ); certain activation of the abdominal muscles was significantly correlated with disability and fear-avoidance belief. These findings enhance our knowledge of abdominal muscle activity during walking in patients with different pain scale values and of the association between clinical and averaged normalized muscle activation. Altered abdominal muscle activity may be a factor in the recurrence or persistence of LBP, and it should be considered in treatment and rehabilitation of LBP.
Our study showed that only right IO muscle activity was significantly different between patients with LBP and controls by post hoc analysis. No significant differences in EO and RA muscle activity were observed between LBP and controls during walking; however, a significant decrease in right IO muscle activity was seen in LLBP and HLBP patients compared with controls. Similar findings were observed in a small study comparing IO muscle activity during walking in LBP patients and controls.[3] Hanada et al[3] reported consistently lower muscle activity of the IO during gait in older adults. The IO muscle, which attaches directly to the lumbar spine, is a global stabilizer during dynamic activities.[21,22] Additionally, the IO is activated along with the transverse abdominalis to control lumbar segmental movement.[23] This effect may be explained by the pain-adaptation model, which proposes that musculoskeletal pain results in reduced muscle activity when the muscle is active as an agonist.[8,24,25] However, it is not clear whether abdominal muscles, particularly the IO muscle, act as antagonists or agonists during walking. The presence of LBP may contribute to alterations in motion control and IO muscle activation during walking.
White and McNair[26] reported the pattern of abdominal muscle (EO, IO, and RA) activity during gait and demonstrated 2 patterns of muscle activation in healthy subjects. In most subjects, the RA and EO muscles showed consistently low activity, whereas some subjects showed higher activation level, as well as peaks of activity.[26] IO was observed to consistently have a lower activity level in 64% of subjects; however, 34% showed the biphasic pattern of activity during walking.[26] In this study, there was no significant difference in abdominal muscle activity between LLBP and HLBP during walking. It is possible that individual variability within the groups masked any clear difference in abdominal muscle EMG pattern between the asymptomatic and pain groups.
Previous studies suggest that fear of pain contributes to changes in movement patterns and increases abdominal muscle activity (RA, EO, and erector spinae) during walking in patients with LBP.[4–6,8,24] Similar to our findings showing a correlation between right EO muscle activation and FABQ (r = .377), Pakzad et al[4] demonstrated a significant positive correlation between % sub-MVIC right EO activation during walking and ratings on a pain catastrophizing scale (r = .376). However, the partial correlation analysis showed no significant relationship between EMG activation of the RA, IO, and left EO during walking and FABQ in patients with LBP (P > .05). Thus, the LBP-related increase in abdominal muscle activity could not be explained by FABQ, or vice versa, suggesting that fear-avoidance belief is related only to right EO abdominal muscle activity during walking.
In this study, significant correlations between muscle activation and clinical measurements were observed unilaterally in the right EO and left IO. Patients with unilateral LBP showed selective ipsilateral atrophy of the lumbar stabilizing muscle in the multifidus and psoas muscles.[27,28] Atrophy and muscle reduction on the symptomatic side may be due to ipsilateral muscle disuse or compensatory hypertrophy of the muscle of the opposite side.[28] In this study, EMG activity of the abdominal muscles on both sides was analyzed during walking, and all subjects were right-handed. The inconsistent result within the same muscle group could be explained by differing muscle activities according to the symptomatic side (unilateral vs bilateral or right vs left) in patients with LBP.
Vlaeyen et al[29] reported that individuals with chronic pain showed fear in anticipation of pain as well as avoidance, resulting in reduced physical activity. Fritz et al[9] also demonstrated that fear-avoidance beliefs may be a significant predictor of future disability in patients with acute LBP. In our findings, a significant correlation between disability in daily activities and fear-avoidance measures, and between pain intensity and disability were demonstrated, and future research is needed to identify means to reduce disability and/or pain intensity by reducing fear and avoidance in LBP patients.
Our study has some limitations. First, the study was cross-sectional, so it is unknown whether abdominal muscle change induced LBP and fear-avoidance or whether LBP caused changes in abdominal muscle activity during walking. Further studies are needed to demonstrate cause and effect. Second, we did not consider pain duration, depression scores, age, or the presence of trigger points in patients with LBP in terms of the influence on disability or abdominal muscle activity. Patients with chronic LBP showed greater disability levels, fear, and avoidance in work, and decreased abdominal muscle endurance.[29,30] Depressive symptoms were influenced by age distribution in patients with LBP,[31] and patients with low back myofascial trigger points had greater stiffness and lower pressure pain thresholds in the erector muscle.[32] Future studies should consider these confounding factors when evaluating abdominal muscle activity during walking in patients with LBP. Third, the generalizability of the data may be limited due to characteristics of the sample. The sample size was small and consisted of more males (n = 9) than females (n = 6) in each group. Although some evidence suggests that females have shorter stride length, narrower steps, greater anterior pelvic tilt, and more oblique pelvic motion than males do during gait,[33] subjects in our study were gender-matched in each group. Thus, the results in this study were not influenced by a higher proportion of males. However, studies with larger sample sizes for each gender are required. Finally, this study did not consider the possible effect of LBP treatments in patients. Subjects with LBP complained of pain for more than 7 weeks, and treatments during this period, such as an exercise program, may have influenced abdominal muscle activity during walking.
5. Conclusions
The results of this study provide evidence for differences in abdominal muscle activation among patients with LLBP, HLBP, and asymptomatic groups during the phases of walking. No differences in abdominal muscle activity during walking were observed between the HLBP and LLBP groups; however, the right IO muscle showed decreased activation during certain phases of walking in both LBP groups relative to controls. Also, disability levels related to LBP and fear-avoidance belief were correlated with IO and EO muscle activity during walking, respectively. Although we could not determine whether altered abdominal muscle activation is adaptive or maladaptive, altered muscle activation may contribute to musculoskeletal problems in patients with LBP. Thus, this study provides useful information for managing patients with LBP in walking.
Acknowledgments
The authors thank “Brain Korea 21 Project” sponsored by the Korean Research Foundation for the support. The authors also thank the participants who participated in our study for their help.
Footnotes
Abbreviations: EO = external oblique, FABQ = Fear–Avoidance Beliefs Questionnaire, HLBP = high-pain intensity low back pain, HS = heel strike, IO = internal oblique, LBP = low back pain, LLBP = low pain intensity low back pain, MVIC = maximal voluntary isometric contraction, ODI = Oswestry Disability Index, RA = rectus abdominis, RMDQ = Roland Morris Disability Questionnaire, TO = toe-off, VAS = visual analog scale.
Funding/support: This study was partially supported by “Brain Korea 21 Project” sponsored by the Korean Research Foundation.
The authors have no conflicts of interest to disclose.
References
- [1].Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:968–74. [DOI] [PubMed] [Google Scholar]
- [2].Luo X, Pietrobon R, Sun SX, et al. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine (Phila Pa 1976) 2004;29:79–86. [DOI] [PubMed] [Google Scholar]
- [3].Hanada EY, Johnson M, Hubley Kozey C. A comparison of trunk muscle activation amplitudes during gait in older adults with and without chronic low back pain. PM R 2011;3:920–8. [DOI] [PubMed] [Google Scholar]
- [4].Pakzad M, Fung J, Preuss R. Pain catastrophizing and trunk muscle activation during walking in patients with chronic low back pain. Gait Posture 2016;49:73–7. [DOI] [PubMed] [Google Scholar]
- [5].van der Hulst M, Vollenbroek Hutten M, Rietman JS, et al. Lumbar and abdominal muscle activity during walking in subjects with chronic low back pain: support of the “guarding” hypothesis? J Electromyogr Kinesiol 2010;20:31–8. [DOI] [PubMed] [Google Scholar]
- [6].van der Hulst M, Vollenbroek Hutten M, Rietman JS, et al. Back muscle activation patterns in chronic low back pain during walking: a “guarding” hypothesis. Clin J Pain 2010;26:30–7. [DOI] [PubMed] [Google Scholar]
- [7].Vogt L, Pfeifer K, Portscher And M, et al. Influences of nonspecific low back pain on three-dimensional lumbar spine kinematics in locomotion. Spine (Phila Pa 1976) 2001;26:1910–9. [DOI] [PubMed] [Google Scholar]
- [8].Hodges PW, Moseley GL. Pain and motor control of the lumbopelvic region: effect and possible mechanisms. J Electromyogr Kinesiol 2003;13:361–70. [DOI] [PubMed] [Google Scholar]
- [9].Fritz JM, George SZ, Delitto A. The role of fear-avoidance beliefs in acute low back pain: relationships with current and future. disability and work status. Pain 2001;94:7–15. [DOI] [PubMed] [Google Scholar]
- [10].Von Korff M, Ormel J, Keefe FJ, et al. Grading the severity of chronic pain. Pain 1992;50:133–49. [DOI] [PubMed] [Google Scholar]
- [11].Teyhen DS, Miltenberger CE, Deiters HM, et al. The use of ultrasound imaging of the abdominal drawing-in maneuver in subjects with low back pain. J Orthop Sports Phys Ther 2005;35:346–55. [DOI] [PubMed] [Google Scholar]
- [12].Waddell G. The Back Pain Revolution. Edinburgh, Scotland: Churchill Livingstone; 1998. [Google Scholar]
- [13].Kim DY, Lee SH, Lee HY, et al. Validation of the Korean version of the oswestry disability index. Spine (Phila Pa 1976) 2005;30:E123–7. [DOI] [PubMed] [Google Scholar]
- [14].Lee JS, Lee DH, Suh KT, et al. Validation of the Korean version of the Roland-Morris Disability Questionnaire. Eur Spine J 2011;20:2115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Joo MK, Kim TY, Kim JT, et al. Reliability and validity of the Korean version of the Fear-Avoidance Beliefs Questionnaire. Phys Ther Korea 2009;16:24–30. [Google Scholar]
- [16].Pijnappels M, Bobbert MF, van-DieÃn JH. Changes in walking pattern caused by the possibility of a tripping reaction. Gait Posture 2001;14:11–8. [DOI] [PubMed] [Google Scholar]
- [17].Cram JR, Kasman GS, Holtz J. Introduction to Surface Electromyography. Gaithersburg: Aspen; 1998. [Google Scholar]
- [18].McGill S, Juker D, Kropf P. Appropriately placed surface EMG electrodes reflect deep muscle activity (psoas, quadratus lumborum, abdominal wall) in the lumbar spine. J Biomech 1996;29:1503–7. [DOI] [PubMed] [Google Scholar]
- [19].Dankaerts W, O'Sullivan PB, Burnett AF, et al. Reliability of EMG measurements for trunk muscles during maximal and sub-maximal voluntary isometric contractions in healthy controls and CLBP patients. J Electromyogr Kinesiol 2004;14:333–42. [DOI] [PubMed] [Google Scholar]
- [20].Portney LG, Watkins MP. Foundations of Clinical Research, 3rd ed. Upper Saddle River, NJ: Prentice Hall; 2009. [Google Scholar]
- [21].Bergmark A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop Scand 1989;230:20–4. [DOI] [PubMed] [Google Scholar]
- [22].Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord 1992;5:383–9. [DOI] [PubMed] [Google Scholar]
- [23].Cresswell A, Grundstrom H, Thorstensson A. Observations on intraabdominal pressure and patterns of abdominal intramuscular activity in man. Acta Physiol Scand 1992;144:409–18. [DOI] [PubMed] [Google Scholar]
- [24].Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, et al. The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain 1996;64:231–40. [DOI] [PubMed] [Google Scholar]
- [25].Vogt L, Pfeifer K, Banzer W. Neuromuscular control of walking with chronic low-back pain. Man Ther 2003;8:21–8. [DOI] [PubMed] [Google Scholar]
- [26].White SG, McNair PJ. Abdominal and erector spinae muscle activity during gait: the use of cluster analysis to identify patterns of activity. Clin Biomech 2002;17:177–84. [DOI] [PubMed] [Google Scholar]
- [27].Barker KL, Shamley DR, Jackson D. Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain: the relationship to pain and disability. Spine (Phila Pa 1976) 2004;29:E515–9. [DOI] [PubMed] [Google Scholar]
- [28].Dangaria TR, Naesh O. Changes in cross-sectional area of psoas major muscle in unilateral sciatica caused by disc herniation. Spine 1998;23:928–31. [DOI] [PubMed] [Google Scholar]
- [29].Vlaeyen JW, Kole-Snijders AM, Boeren RG, et al. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain 1995;62:363–72. [DOI] [PubMed] [Google Scholar]
- [30].Brox JI, Storheim K, Holm I, et al. Disability, pain, psychological factors and physical performance in healthy controls, patients with sub-acute and chronic low back pain: a case-control study. J Rehabil Med 2005;37:95–9. [DOI] [PubMed] [Google Scholar]
- [31].Calvo-Lobo C, Vilar Fernández JM, Becerro-de-Bengoa-Vallejo R, et al. Relationship of depression in participants with nonspecific acute or subacute low back pain and no-pain by age distribution. J Pain Res 2017;10:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Calvo-Lobo C, Diez-Vega I, Martínez-Pascual B, et al. Tensiomyography, sonoelastography, and mechanosensitivity differences between active, latent, and control low back myofascial trigger points: A cross-sectional study. Medicine (Baltimore) 2017;9:e6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cho SH, Park JM, Kwon OY. Gender differences in three dimensional gait analysis data from 98 healthy Korean adults. Clin Biomech (Bristol, Avon) 2004;19:145–52. [DOI] [PubMed] [Google Scholar]


