Abstract
This study aimed to retrospectively investigate the factors related to pediatric obstructive sleep apnea–hypopnea syndrome (OSAHS) with attention deficit hyperactivity disorder (ADHD) in children younger than 6 years and those older than 6 years.
A total of 437 children who were hospitalized due to OSAHS between January 2014 and December 2014 were retrospectively reviewed. The children were further divided into OSAHS group and OSAHS + ADHD group. The general characteristics, OSA-18 quality of life, intention-hyperactivity score, and polysomnographic parameters (apnea–hypopnea index and the lowest oxygen saturation) were collected and compared between groups.
There were 298 boys and 139 girls with the male to female ratio of 2.14:1. ADHD was found in 146 children including 105 boys and 41 girls with the male to female ratio of 2.56:1. Of these children, 31.62% and 35.46% had concomitant ADHD in children aged 4 to 5 years and those aged 6 to 11 years, respectively. In children aged 4 to 5 years, the incidence of allergic rhinitis was significantly higher (P = .016) and the adenoid hypertrophy was more severe (P = .001) in those with concomitant ADHD. In children aged 6 to 11 years, the tonsil hypertrophy was more severe in those with concomitant ADHD (P = .019). In children with concomitant ADHD, OSA-18 score was higher than in those with OSAHS alone (P < .001). Higher frequency of respiratory events (P < .001) and more severe hypoxia (P < .001) were found in children with concomitant ADHD than in those with OSAHS alone.
As high as 30% of OSAHS children have concomitant ADHD, and the incidence of ADHD in OSAHS children is increasing over age. Boys are more likely to develop OSAHS and incidence of ADHD in OSAHS boys is higher than in OSAHS girls. In addition, risk factors of ADHD also vary between age groups. The ADHD is related to the severity of allergic rhinitis and adenoid hypertrophy in children aged 4 to 5 years, and to the severity of tonsil hypertrophy in children aged 6 to 11 years. Hypoxia may be an important factor causing ADHD. OSAHS should be treated as early as possible to reduce the incidence of ADHD in children.
Keywords: adenoid, allergic rhinitis, hypoxia, obstructive sleep apnea–hypopnea syndrome, pediatric attention deficit hyperactivity disorder, tonsil
1. Introduction
Attention deficit hyperactivity disorder (ADHD) is the most common developmental behavioral disorder in childhood and characterized by attention deficit, hyperactivity, irritability, and impulsivity. The main features of ADHD include the lack of active attention and passive attention hyperactivity with learning difficulty, conduct disorder, tic disorder, and emotional disorder.[1]
Obstructive sleep apnea–hypopnea syndrome (OSAHS) refers to the pathophysiology caused by the repetitive partial or complete collapse of the upper airway, which may significantly disturb the normal ventilation and sleep structure. OSAHS children usually have the impairment of cognition, executive, and emotional functions. In addition, the daytime drowsiness, nocturnal hypoxia, and sleep disorder will aggravate with the OSAHS deterioration, leading to hyperactivity and attention deficit.[2] In 81% of ADHD children with OSAHS, treatment of OSAHS to alleviate or abolish snoring may lead to the spontaneous disappearance of ADHD symptoms.[3] Available studies have shown the close relationship between OSAHS and ADHD, but whether some characteristics of OSAHS induce ADHD is still poorly understood.[4]
In children, tonsil hypertrophy and adenoid hypertrophy are the 2 major factors causing OSAHS-5.[5] Several hypotheses have been proposed for the pathogenesis of ADHD. Currently, some investigators accept that the metabolism of neurotransmitters (monoamine oxidase: dopamine, norepinephrine, and serotonin) plays an important role in the pathogenesis of ADHD.[6]
Previous studies have revealed that the ADHD is related to the nighttime hypoxia in OSAHS children, but sleeping monitor is costly and time consuming and requires hospitalization, and the analysis of parameters should be done by specialist. Thus, it is imperative to investigate the factors related to ADHD (such as sleep disorder, tonsil size, adenoid size, and allergic rhinitis) which may be used for the prediction of ADHD in OSAHS children. In the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV),[7] the diagnosis criteria for ADHD are recommended for children older than 6 years. In the Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents,[8] the diagnosis criteria are applicable in children older than 4 years. However, little is known about the relationship of ADHD with adenoid hypertrophy, tonsil hypertrophy, and allergic rhinitis, few studies report the ADHD in children younger than 6 years, and the characteristics of OSAHS children with ADHD are also unclear among different age groups. It is well known that adenoid hypertrophy and tonsil hypertrophy are 2 major causes of OSAHS in children. The rapid growth of adenoid occurs in 3 to 6 years, and the tonsil enlarges significantly in 3 to 5 years and shrinks until adolescence due to the reduction of immune function. Thus, the adenoid hypertrophy and tonsil hypertrophy generally peak in 6 years old. Our previous study showed that the incidence of allergic rhinitis was different between OSAHS children older than 6 years and those younger than 6 years.[9] Thus, whether the factors related to ADHD in OSAHS children are also different between 2 age groups is warranted to be elucidated.
In this study, OSAHS children with and without concomitant ADHD who were hospitalized in Shanghai Children's Hospital were retrospectively reviewed, and factors related to the occurrence of ADHD were further analyzed and compared in OSAHS children older than 6 years and those younger than 6 years. Our findings may provide evidence on the pathogenesis and prevention of ADHD.
2. Materials and methods
2.1. Study population
This study was approved by the Ethics Committee of Shanghai Children's Hospital. A total of 437 children were diagnosed with OSAHS between January 2014 and December 2014. Fiberoptic nasopharyngoscopy showed adenoid or tonsil hypertrophy. OSAHS was diagnosed by polysomnography (PSG). The inclusion criteria were as follows: children were aged 4 to 11 years; PSG confirmed the diagnosis of OSAHS; exclusion criteria were as follows: children with central sleep apnea or hypopnea syndrome were excluded; medical history reviewing and clinical examinations excluded OSAHS related to other diseases (Down syndrome, maxillofacial disorders, neuromuscular diseases, chronic lung disease, sickle cell disease, metabolic diseases, and laryngeal softening); children with other nervous and mental diseases (such as schizophrenia, affective disorder, autism, epilepsy, hepatolenticular degeneration, rheumatic chorea, and hyperthyroidism) and organic diseases (such as hyperthyroidism) were excluded; children with recurrent limb movement disorders were excluded; children treated with psychotropic drugs for ADHD were excluded; children who took coffee, cola, and food rich in amines within prior 3 days were excluded; and children or their parents unable to complete the questionnaire were excluded. These patients were further divided into OSAHS group and OSAHS + ADHD group according to whether concomitant ADHD was diagnosed by a specialist. In addition, all the children were further subdivided into 4 to 6 years group and 6 to 11 years group according to the age of these children. The general characteristics, tonsil size, adenoid size, history of allergy, OSA-18 quality of life (QoL), ADHD score, and PSG (apnea–hypopnea index and lowest oxygen saturation [SaO2]) were compared between 2 groups.
2.2. Collection of clinical characteristics
The gender, age, height, body weight, allergic rhinitis, and severity of airway obstruction evaluated by the degree of tonsil and/or adenoid hypertrophy, ADHD score, and PSG parameters (apnea–hypopnea index [AHI] and the lowest SaO2) were recorded.
2.3. PSG
Polysmith 36 leads sleep system (4.0 version) was employed to monitor the sleep at night for consecutive 7 h. Children had no upper respiratory infection within prior 2 weeks, and sleeping pills, alcohol, tea, and coffee were not used within 24 h before PSG. The parameters were recorded and analyzed.
2.4. Diagnosis of OSAHS
OSAHS was diagnosed according to the Chinese Guideline for the Diagnosis and Treatment of OSAHS (Urumqi, Xinjiang, China).[10] Obstructive sleep apnea (OSA) refers to the persistent nasal/oral airflow obstruction for a specific duration (≥2 respiratory cycles), in which there is chest and/or abdominal movement during dyspnea. In the hypopnea sleep, the airflow intensity reduces by 50% with reduction of SaO2 by 0.03 and/or awakening. OSAHS is diagnosed if obstructive apnea index is >1/h or AHI is >5/h and the lowest SaO2 is <92%.
2.5. Diagnosis of ADHD
ADHD was diagnosed by the clinicians specialized on ADHD according to the DSM-IV and 2011 Expert Consensus on the ADHD in Children.[8] The guardians were trained, and then scored their children according both guidelines. ADHD was diagnosed by the experienced specialist on ADHD. There are main 3 subtypes of ADHD: Inattention subtype (ADHD- I): Six or more symptoms are present among 9 symptoms of inattention; Hyperactivity and Impulsivity subtype (ADHD-HI): Six or more symptoms are present among 9 symptoms of Hyperactivity and Impulsivity; and Combined subtype (ADHD-C): there are at least 6 symptoms of Inattention subtype and 6 symptoms of Hyperactivity and Impulsivity subtype, the course of disease is longer than 6 months and disease onset was found before 7 years old. The first 9 symptoms are those of Inattention subtype and the second 9 symptoms are those of Hyperactivity and Impulsivity subtype; the higher the score, the more severe the ADHD is.
2.6. Evaluation of tonsil and adenoid hypertrophy
Severity of tonsil hypertrophy[11]: Oropharyngeal examination was done in all the patients on admission and the severity of tonsil hypertrophy was determined. The tonsil hypertrophy is classified into 3 types: Degree I—the enlarged tonsil exceeds the pharyngeal arch palate; Degree II—the enlarged tonsil exceeds the pharyngeal arch palate; and Degree III—the enlarged tonsil reaches the posterior pharyngeal midline.
Severity of adenoid hypertrophy[12]: Fibrous nasopharyngoscopy was employed for the diagnosis of adenoid hypertrophy and the severity of adenoid hypertrophy was classified according to the degree of postnaris obstruction: degree 1, 25% obstruction; degree 2, 50% obstruction; degree 3, 75% obstruction; degree 4, 100% degree.
2.7. Evaluation of QoL
OSA-18 scale was employed to evaluate the QoL in OSAHS children.[13] There are 5 domains in the OSA-18 scale: sleep disturbance, physical suffering, emotional distress, daytime problems, and caregiver concerns. There are 3 to 4 items in each domain, and a total of 18 items in this scale. Items are scored in an ordinal 7-point classification: 1—none of the time, 2—hardly any of the time, 3—a little of the time, 4—some of the time, 5—a good bit of the time, 6—most of the time, and 7—all of the time. The higher the score, the most significant influence on the QoL is. Total score (range: 18–126) and score of each domain were calculated in each child for the evaluation of the impact on the QoL of OSAHS children.[14]
2.8. Statistical analysis
Data were collected with epidata 3.1 software, and the data input were double-checked. Statistical analysis was performed with SPSS version 20.0. Qualitative data are expressed as percentages or rates, and compared with Chi-squared test. Quantitative data were subjected to K–S normality test and those with normal distribution are expressed as mean ± standard deviation and compared with t test between 2 groups and 1-way analysis of variance among groups, followed by paired comparison with least-significant difference test. Quantitative data with abnormal distribution are expressed as medians (quartile range) and compared with rank sum test. A value of P < .05 was considered statistically significant.
3. Results
3.1. General characteristics of OSAHS children
A total of 437 children were diagnosed with OSAHS by PSG. There were 298 boys and 139 girls with the mean age of 5.71 ± 1.45 years (range: 4–11). Among them, ADHD was found in 146 children. There were 105 boys and 41 girls with the male to female ratio of 2.56:1. The mean age was 5.97 ± 2.04 years (range: 4–11) in ADHD children. In 291 children with OSAHS alone, there were 193 boys and 98 girls with the male to female ratio of 1.97:1, and the mean age was 5.80 ± 1.68 years (range: 4–11). There were no significant differences in the gender and age between OSAHS group and OSAHS + ADHD group (t = 1.535, χ2 = 1.403, all P > .05).
3.2. Comparisons of different factors
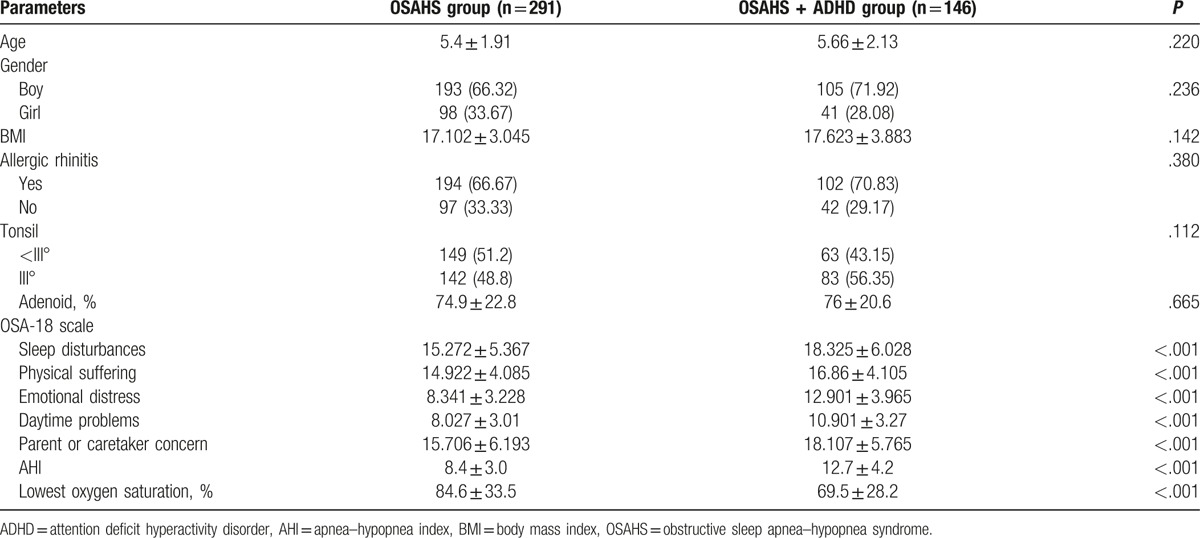
There were no marked differences in the tonsil size, adenoid size, allergic rhinitis, and body mass index (BMI) between OSAHS group and OSAHS + ADHD group. The scores of 5 domains in OSAHS + ADHD group were significantly higher than in OSAHS group (P < .001) and the score of each domain of OSA-18 in OSAHS + ADHD group was significantly higher than in OSAHS group regardless the age (P < .05). The sleep disorder was more severe, AHI was significantly higher (P < .001) and the lowest SaO2 was markedly lower in OSAHS + ADHD group as compared with OSAHS group (Table 1).
Table 1.
Comparisons of characteristics between OSAHS group and OSAHS + ADHD group.
3.3. Factors in different age groups
In 291 children with OSAHS alone, 160 were aged 4 to 5 years, and 131 aged 6 to 11 years. In 146 children with both OSAHS and ADHD, 74 were aged 4 to 5 years and 72 aged 6 to 11 years. Concomitant ADHD was found in 74 OSAHS children (31.62%) of those aged 4 to 5 years and in 72 OSAHS children (35.46%) of those aged 6 to 11 years. There was no difference in the age between OSAHS group and OSAHS + ADHD group (χ2 = 0.922, P > .05).
In children aged 4 to 5 years, the incidence of allergic rhinitis in OSAHS group was markedly lower than in OSAHS + ADHD group (P < .05). In children aged 6 to 11 years, there was no significant difference in the incidence of allergic rhinitis between OSAHS group and OSAHS + ADHD group (P > .05) (Table 2).
Table 2.
Comparisons of characteristics between OSAHS group and OSAHS + ADHD group in different age groups.
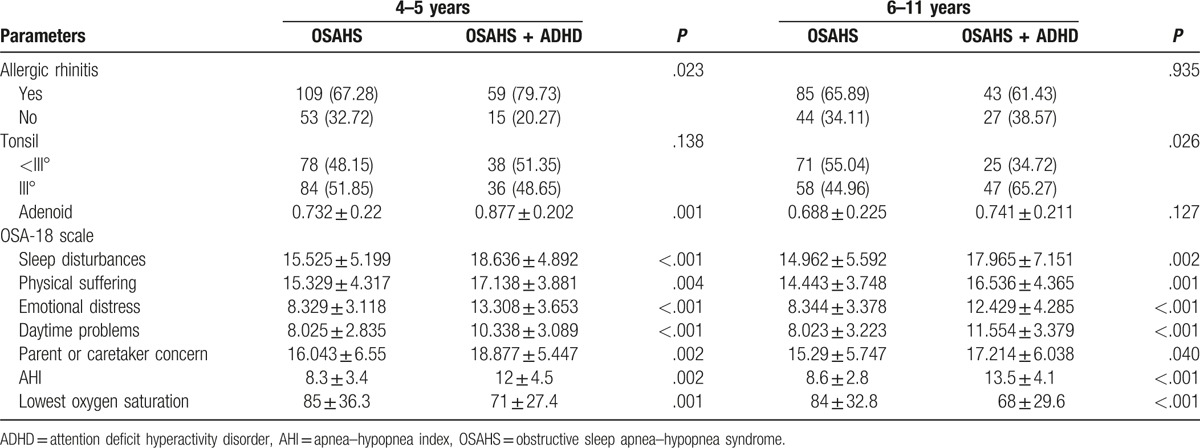
In children aged 4 to 5 years, the tonsil size was comparable between OSAHS group and OSAHS + ADHD group (P > .05). In children aged 6 to 11 years, the tonsil was markedly larger in OSAHS + ADHD group than in OSAHS group (P < .05) (Table 2).
There was no marked difference in BMI between OSAHS group and OSAHS + ADHD group regardless the age (P > .05). In children aged 4 to 5 years, the adenoid size in OSAHS + ADHD group was significantly larger than in OSAHS group (P < .01). In children aged 6 to 11 years, the adenoid size was comparable between OSAHS group and OSAHS + ADHD group (P > .05). The sleep disorder was more severe, AHI was significantly higher (P < .01), and the lowest SaO2 was markedly lower in OSAHS + ADHD group than in OSAHS group regardless the age.
4. Discussion
ADHD is the most common developmental behavioral disorder. The prevalence of ADHD is about 3% worldwide, and the incidence of ADHD in boys is about 4 to 9 times that in girls. ADHD children usually go to bed later, have longer time to falling asleep and easily aroused sleep, and are difficult to fall asleep after being awakened. Goraya et al found that sleep-disordered breathing was the most common symptom of ADHD.[6] Several studies have shown that the incidence of OSAHS is higher in ADHD patients, and sleep apnea may cause attention deficit and hyperactivity in ADHD children.[15]
It was reported that the prevalence of OSAHS was 2% to 4% in children.[16] Uncontrolled OSAHS may cause adverse effects on the physical and mental development of children.[17] A study from the National Institutes of Health of United States indicates that mild ADHD is found in 26% of OSAHS children aged 5 to 7 years.[18] The sleep apnea may aggravate rapid eye movement ratio and further lower the nocturnal SaO2 with the deterioration of OSAHS, which may impair the brain function and aggravate the cognitive dysfunction, executive dysfunction, and emotional disorder. This plays a role in the pathogenesis of ADHD in OSAHS children.
The incidence of ADHD is about 3 to 7% in United States[19] and 8.4% to 11.7% in Taiwan, China.[20] In China mainland, the incidence of ADHD is 5.7% (95% confidence interval [CI]: 4.9–6.6) in general population, 7.5% (95% CI: 6.4–8.8) in boys and 3.4% (95% CI: 2.7–4.4) in girls.[21] The prevalence of ADHD tends to reduce over age.[22] In the present study, results also showed a higher incidence of ADHD in boys than in girls. In OSAHS children with ADHD, the male to female ratio was 2.56:1 in children aged 4 to 5 years and 1.97:1 in children aged 6 to 11 years. The incidence of ADHD was higher than 30% in both groups and significantly higher than that in general population. Moreover, the incidence of ADHD in OSAHS children increased over age, which might be related to the long course of OSAHS, the long-term hypoxia, and the significant adverse effect on the brain function.
Previous studies have shown that the hypoxia in OSAHS is related to ADHD,[23] and symptoms of ADHD are improved after treatment for OSAHS, but little is known about the relationship of adenoid hypertrophy, tonsil hypertrophy, and allergic rhinitis with ADHD. In the present study, results showed that there were no marked differences in the tonsil size, adenoid size, and allergic rhinitis between OSAHS group and OSAHS + ADHD group, but significant difference was observed between 2 groups after stratification according to age. This indicates that age is an important factor related to the pathogenesis of ADHD.
In children aged 4 to 5 years, the incidence of allergic rhinitis in OSAHS group was significantly lower than in OSAHS + ADHD group (P = .016). The tonsil size did not increase the risk for ADHD. The adenoid grows rapidly at 4 to 5 years. In our study, more children developed sleep disorder and the incidence of ADHD was higher (P = .001) in children with more severe adenoid hypertrophy. In children aged 6 to 11 years, allergic rhinitis (P = .898) and adenoid size (P = .098) did not increase the risk for ADHD. More children developed sleep disorder and the incidence of ADHD was higher (P = .019) in children with more severe tonsil hypertrophy. This might be explained as that young children have no mouth breathing and the nasal and nasopharyngeal obstruction may cause more severe hypoxia, increasing the incidence of ADHD; in old children, the adenoid becomes atrophy and nasal obstruction is improved, or they accommodate to the long-term nasal obstruction and then establish mouth breathing under which the contribution of nasal and nasopharyngeal obstruction is compromised. Thus, the more severe the tonsil hypertrophy, the more severe the sleep disorder is in children and the higher the incidence of ADHD is.
In all the children, the BMI was comparable between OSAHS group and OSAHS + ADHD group. The score of each domain of OSA-18 scale in OSAHS + ADHD group was markedly higher than in OSAHS group. This indicates that OSAHS children with concomitant ADHD have more severe sleep disorder and poorer QoL.
In addition, the AHI was markedly higher and the lowest SaO2 was significantly lower in OSAHS + ADHD group than in OSAHS group. It is well known that the increases in the number and duration of respiratory events may further reduce the SaO2.[24] Available animal experiments have shown that intermittent hypoxia may affect the brain function, causing the symptoms of hyperactivity.[25] Children have active growth and development, and their brain is more sensitive to the hypoxia. Sleep disorder lasting for 1 week or longer may significantly cause emotional disorder or compromise the cognitive function,[26] which might be related to the pathogenesis of ADHD. In children, tonsil hypertrophy and adenoid hypertrophy are the 2 main causes of OSAHS.[5] Tonsillectomy and adenoidectomy are the most effective strategies for the treatment of tonsil hypertrophy and adenoid hypertrophy, respectively. There is evidence showing that treatment of OSAHS and snoring may cause the spontaneous recovery of ADHD in 81% of OSAHS children with concomitant ADHD.[3] Lumeng and Chervin also found the effective treatment of snoring could abolish the symptoms of ADHD in 25% of children.[27] AHI > 1 is regarded abnormal and suggests that AHI > 1 may confer adverse effects on the hyperactivity in children.[28] Even in children with AHI < 1 and snoring alone, tonsillectomy and adenoidectomy are also effective to improve the behaviors and cognition of ADHD children.[18] Thus, hypoxia may play an important role in the pathogenesis of ADHD.
Taken together, the incidence of ADHD is higher than 30% in OSAHS children and increases over age in OSAHS children, which may be related to the long course of OSAHS, the long-term hypoxia and more significant influence on the brain function. In China, less attention has been paid to the OSAHS children and the mental health of these children because of lack of knowledge about OSAHS and ADHD. Some parents speculate that OSA is not important, and the adenoid and tonsil enlargement will resolve over year because some children are afraid of surgery, and thus they also pay little attention to the relevant allergic rhinitis. In addition, they think that the naughty or even aggressive behavior is common for children, and thus they may not notice the symptoms of ADHD. Only when the inattention cause learning problem do the parent pay attention to the harmful behaviors of these children. In our study, results showed that the risk factors varied between age groups. In children aged 4 to 5 years, allergic rhinitis is positively related to the pathogenesis of ADHD, and more children with severe adenoid hypertrophy develop the symptoms of ADHD; in children aged 6 to 11 years, more children with tonsil hypertrophy develop the symptoms of ADHD. In OSAHS children with concomitant ADHD, the sleep disorder is more severe, the number of nocturnal respiratory events is larger, the lowest SaO2 is lower, the hypoxia is more severe, and the QoL is poorer as compared with children with OSAHS alone. Thus, OSAHS should be treated as early as possible to reduce the incidence of ADHD.
Of note, this study investigated that the factors related to ADHD in OSAHS, and children with ADHD alone were not included. The inclusion of these children will further provide evidence on our findings. In addition, this was a retrospective study, and our findings are needed to be validated in more prospective studies.
Footnotes
Abbreviations: ADHD = attention deficit hyperactivity disorder, AHI = apnea–hypopnea index, BMI = body mass index, CI = confidence interval, OSA = obstructive sleep apnea, OSAHS = obstructive sleep apnea–hypopnea syndrome, PSG = polysomnography, QoL = quality of life, SaO2 = oxygen saturation.
This study was supported by the Research Fund of Shanghai Children's Hospital (No. 2012M013).
The authors have no conflicts of interest to disclose.
References
- [1].Sridhar C, Bhat S, Acharya UR, et al. Diagnosis of attention deficit hyperactivity disorder using imaging and signal processing techniques. Comput Biol Med 2017;88:93–9. [DOI] [PubMed] [Google Scholar]
- [2].Wang GF, Li XW. The influence of obstructive sleep apnea hypopnea syndrome on the brain function. J Clin Intern Med 2006;23:226–7. [Google Scholar]
- [3].Chervin RD, Dillon JE, Bassetti C, et al. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep 1997;20:1185–92. [DOI] [PubMed] [Google Scholar]
- [4].Youssef NA, Ege M, Angly SS, et al. Is obstructive sleep apnea associated with ADHD? Ann Clin Psychiatry 2011;23:213–24. [PubMed] [Google Scholar]
- [5].Korcarz CE, Gepner AD, Peppard PE, et al. The effects of sleep-disordered breathing on arterial stiffness are modulated by age. Sleep 2010;33:1081–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goraya JS, Cruz M, Valencia I, et al. Sleep study abnormalities in children with attention deficit hyperactivity disorder. Pediatr Neurol 2009;40:42–6. [DOI] [PubMed] [Google Scholar]
- [7].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed.Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- [8].Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011;128:1007–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wu JL, Chen SM, Tian X, et al. Relationship between allergic rhinitis and secretory otitis media in children with adenoid hypertrophy. J Otolaryngol Ophthalmol Shandong Univ 2013;27:53–6. [Google Scholar]
- [10].Editorial Board of Chinese Journal of Otorhinolaryngological Head, Neck Surgery, Branch of Otolaryngology of Chinese Medical Association. Guideline for the diagnosis and surgical treatment of obstructive sleep apnea hypopnea syndrome. Chin J Otorhinolaryngol Head Neck Surg 2009;44:95–6. [PubMed] [Google Scholar]
- [11].Liner LH, Marcus CL. Ventilatory management of sleep-disordered breathing in children. Curr Opin Pediatr 2006;18:272–6. [DOI] [PubMed] [Google Scholar]
- [12].Kindermann CA, Roithmann R, Lubianca Neto JF. Sensitivity and specificity of nasal flexible fiberoptic endoscopy in the diagnosis of adenoid hypertrophy in children. Int J Pediatr Otorhinolaryngol 2008;72:63–7. [DOI] [PubMed] [Google Scholar]
- [13].de Serres LM, Derkay C, Astley S, et al. Measuring quality of life in children with obstructive sleep disorders. Arch Otolaryngol Head Neck Surg 2000;126:1423–9. [DOI] [PubMed] [Google Scholar]
- [14].Long CQ, Yan YY, Rong QF. Assessment of quality of life in children with adenoid hypertrophy by OSA-1 8 questionnaire. J Otolaryngol Ophthalmol Shandong Univ 2015;29:13–9. [Google Scholar]
- [15].Heck T, Zolezzi M. Obstructive sleep apnea: management considerations in psychiatric patients. Neuropsychiatr Dis Treat 2015;11:2691–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu DB, Qiu SY, Zhong JW, et al. Treatment of obstructive sleep apnea-hypopnea with refractory epilepsy in children. Chin J Otorhinolaryngol Head Neck Surg 2009;44:425–6. [PubMed] [Google Scholar]
- [17].Chang L. Early recognition and diagnosis of obstructive sleep apnea hypopnea syndrome in children. Chin J Med 2015;7:7–9. [Google Scholar]
- [18].Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130:e714–55. [DOI] [PubMed] [Google Scholar]
- [19].American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders. DSM-IV-TR (Text Revision). 4th ed.Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- [20].Huang YS, Guilleminault C, Li HY, et al. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: a treatment outcome study. Sleep Med 2007;8:18–30. [DOI] [PubMed] [Google Scholar]
- [21].Tong L, Shi HJ, Zang JJ. Prevalence of ADHD in children of China: a systematic review and meta analysis. Chin J Pub Health 2013;29:1279–83. [Google Scholar]
- [22].Liu J, Zheng Y. Interpretation to the guideline for the prevention and treatment of attention defective hyperactivity disorder in China (2nd edition). Chin J Psych 2016;49:132–5. [Google Scholar]
- [23].Deng JH, Ding XJ, Wang JZ. Effect of surgical treatment of children with obstructive sleep apnea syndrome on attention deficit-hyperactivity disorder. J Nanjing Med Univ 2009;29:1761–82. [Google Scholar]
- [24].Kim SP, Choi MH, Kim JH, et al. Changes of 2-back task performance and physiological signals in ADHD children due to transient increase in oxygen level. Neurosci Lett 2012;511:70–3. [DOI] [PubMed] [Google Scholar]
- [25].Dayyat EA, Zhang SX, Wang Y, et al. Exogenous erythropoietin administration attenuates intermittent hypoxia-induced cognitive deficits in a murine model of sleep apnea. BMC Neurosci 2012;13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Orzel-Gryglewska J. Consequences of sleep deprivation. Int J Occup Med Environ Health 2010;23:95–114. [DOI] [PubMed] [Google Scholar]
- [27].Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Alexander NS, Schroeder JW., Jr Pediatric obstructive sleep apnea syndrome. Pediatr Clin North Am 2013;60:827–40. [DOI] [PubMed] [Google Scholar]


