Abstract
Background:
Breast cancer (BC) is considered a systemic disease with a primarily locoregional component. The accumulation of basic researches and clinical studies related to cytokine-induced killer (CIK) cells has confirmed their safety and feasibility in treating BC. By searching the PubMed, Embase, CNKI, and Wanfang databases, we conducted a meta-analysis to assess the efficacy and safety of DC/CIK plus chemotherapy regimen (Exp) compared with chemotherapy (Con) alone regimen for breast carcinoma. Studies were pooled, and the relative risk (RR) and its corresponding 95% confidence interval (CI) were calculated.
Methods:
Eleven relevant articles were included in this meta-analysis. We observed that complete response (CR) (RR = 1.54, 95% CI: 1.09–2.19, Pheterogeneity = .994, I2 = 0%), partial response (PR) (RR = 1.33, 95% CI: 1.11–1.59, Pheterogeneity = .802, I2 = 0%) and overall response rate (ORR) (RR = 1.37, 95% CI: 1.20–1.57, Pheterogeneity = .619, I2 = 0%) in BC patients treatment with DC/CIK plus chemotherapy regimen was improved than that with chemotherapy alone. There was no difference in the incidence of leukopenia, thrombocytopenia, hair loss, nausea/vomiting, hepatic complications, and neurologic complications in BC patient's treatment with DC/CIK plus chemotherapy regimen and with chemotherapy alone.
Results:
Compared to chemotherapy alone, DC/CIK plus chemotherapy treatment significantly increased CR, PR, and ORR; however, there was no difference between the safeties.
Conclusion:
DC/CIK plus chemotherapy treatment may be a valuable new option for the treatment of breast carcinoma in women. The present study, therefore, provides valuable information to help physicians make treatment decisions for their patients with BC.
Keywords: breast cancer, cytokine-induced killer, immunotherapy, meta-analysis
1. Introduction
Breast cancer (BC) is one of the most common cancers in women; and about 521,900 women die each year from BC.[1] BC has become the most common cancer in women in China, accounting for 12.2% of all newly diagnosed BCs and 9.6% of all mortalities from BC worldwide.[2] BC is a systemic disease, mainly of local components.[3] Besides surgical removal and irradiation of the local tumor setting, central therapeutic purpose is the elimination of the diffuse micro metastatic tumor cells using cell growth inhibition and/or hormone therapy. However, in the course of time, most of the patients suffered from systemic relapse in the form of distant metastases.[4]
BC is immunogenic, and in the primary breast tumor, infiltrating immune cells communicate important clinical prognostic and predictive information. In addition, the immune system is critically involved in some of the clinical response to standard cancer therapy.[5] BC immunotherapy primarily enables the immune system to recognize tumor growth and prevent cancer, and may eliminate the malignant cells become transformed cells.[6] In recent years, with in-depth exploration of rapid development mechanism of tumorigenesis and modern biotechnology, autologous immune cell therapy has played a major role in the treatment of tumor, and found some application prospect in clinic. Currently, common immune effector cells applied in immunotherapy are cytokine-induced killer (CIK) cells and dendritic cells (DCs).[7,8] DC/CIK infusion was related with BC survival and improvement of the body's immune function. Ren et al reported that combination therapy with chemotherapy and DC/CIK immunotherapy improved progression-free and overall survival and metastasis of BC.[9]
In recent years, several randomized controlled trials (RCTs) have been conducted to evaluate the efficacy and safety of DC/CIK therapy for the treatment of patients with BC.[9,10–19] However, the results were not consistent. As DC/CIK therapy is being increasingly used for BC, its effectiveness must be scrutinized. In this study, we conducted the present meta-analysis of RCTs to explore the efficacy and safety of DC/CIK therapy for breast carcinoma.
2. Materials and methods
2.1. Search strategy
We are looking for relevant research to June 2016 with the following terms and their combinations through PubMed, Embase, China National Knowledge Infrastructure (CNKI), and Wanfang databases: “cytokine-induced killer,” “immunotherapy,” and “breast cancer.” All scan summary, research, and references were reviewed. In addition, reference is also retrieved, and the manuscript is manually searched for further relevant publications.
2.2. Selection criteria
Controlled clinical trials to assess the efficacy and safety of DC/CIK immunotherapy for breast carcinoma were included if they met the following criteria: eligibility is limited to RCTs of BC; study the efficacy and safety of DC/CIK regimen for breast carcinoma; research report-specific data related response rate (WHO Criteria) and adverse events (AEs); and only DC/CIK regimen RCTs may be included.
2.3. Data extraction
All the available data were extracted from each study by 2 investigators independently according to the inclusion criteria listed previously. The efficacy outcomes were: complete response (CR); partial response (PR); and overall response rate (ORR). The safety outcomes included: leukopenia; thrombocytopenia; hair loss; nausea/vomiting; hepatic complications; and neurologic complications.
2.4. Statistical analysis
All results were summarized using STATA Software (version 12, StataCorp, College Station, TX). We calculated the risk ratio (RR) and 95% confidence intervals (CIs) for dichotomous data. Preliminary analysis was done using a fixed-effect model (Mantel-Haenszel method); if there are study heterogeneity (P < .1), using a random-effects model. Using Begg funnel plot and Egger test to assess publication bias symmetry was visually evaluated (P < .05 was considered statistically significant).
3. Results
3.1. Characteristics of the studies
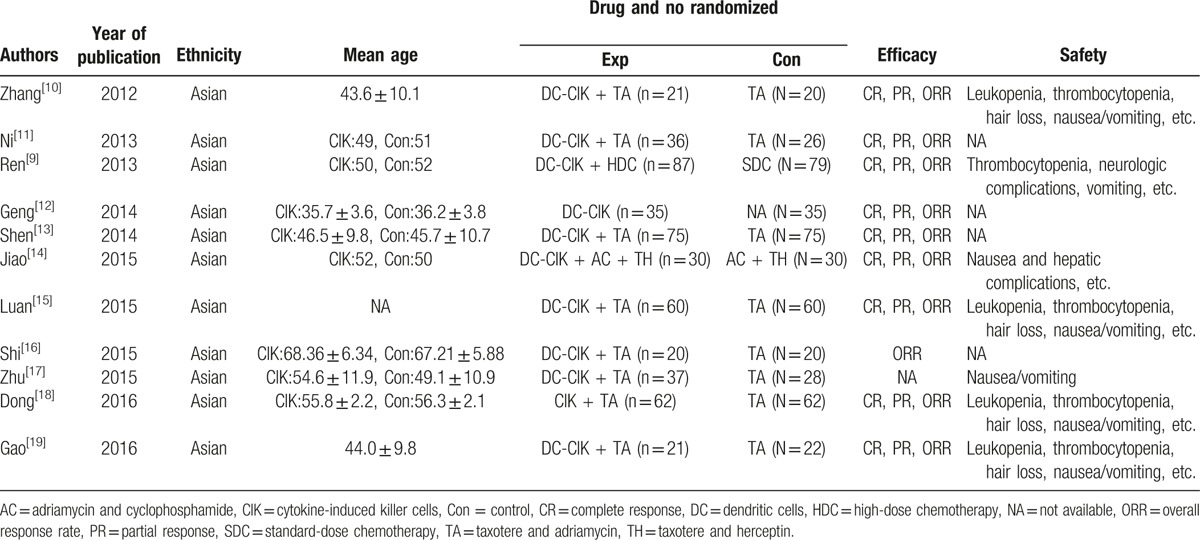
The initial literature search identified 154 articles. Through screening of titles and abstracts, the studies such as conference abstracts, redundant publications, reviews, and case reports were excluded. The remaining studies were subjected to full-text screening of which 11 articles that did not satisfy the selection criteria were removed. Eventually, a total of 11 trials including 941 patients were eligible for the analysis. The reasons for the exclusion of studies are illustrated in Figure 1. The main characteristics of the included studies are shown in Table 1.
Figure 1.
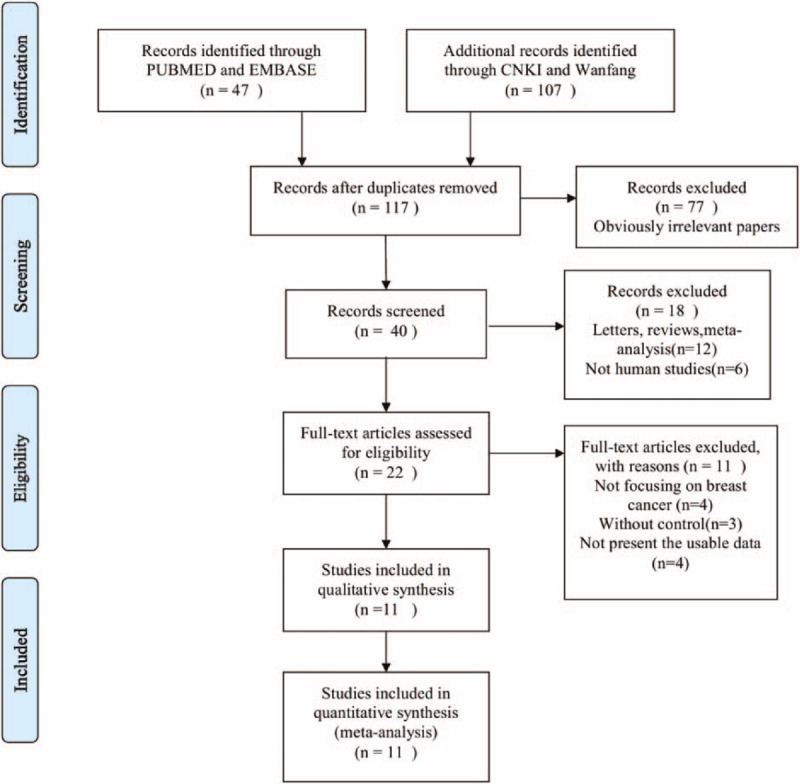
Flow diagram of the study selection process.
Table 1.
Clinical information of the eligible trails for the meta-analysis.
3.2. Quantitative synthesis
All 11 studies including 941 BC patients explored the efficacy and safety of DC/CIK plus chemotherapy regimen (Exp) compared with chemotherapy alone (Con) regimen for breast carcinoma.
CR: This outcome was reported in 9 trials, all comparing Exp with Con. There were 747 cases of patients, 386 cases in Exp group, 361 cases in Con group. The heterogeneity was not statistically significant (P = .994, I2 = 0%), the fixed-effect model was used. The difference in the CR was significant (RR = 1.54, 95% CI: 1.09–2.19), as shown in Figure 2A.
Figure 2.
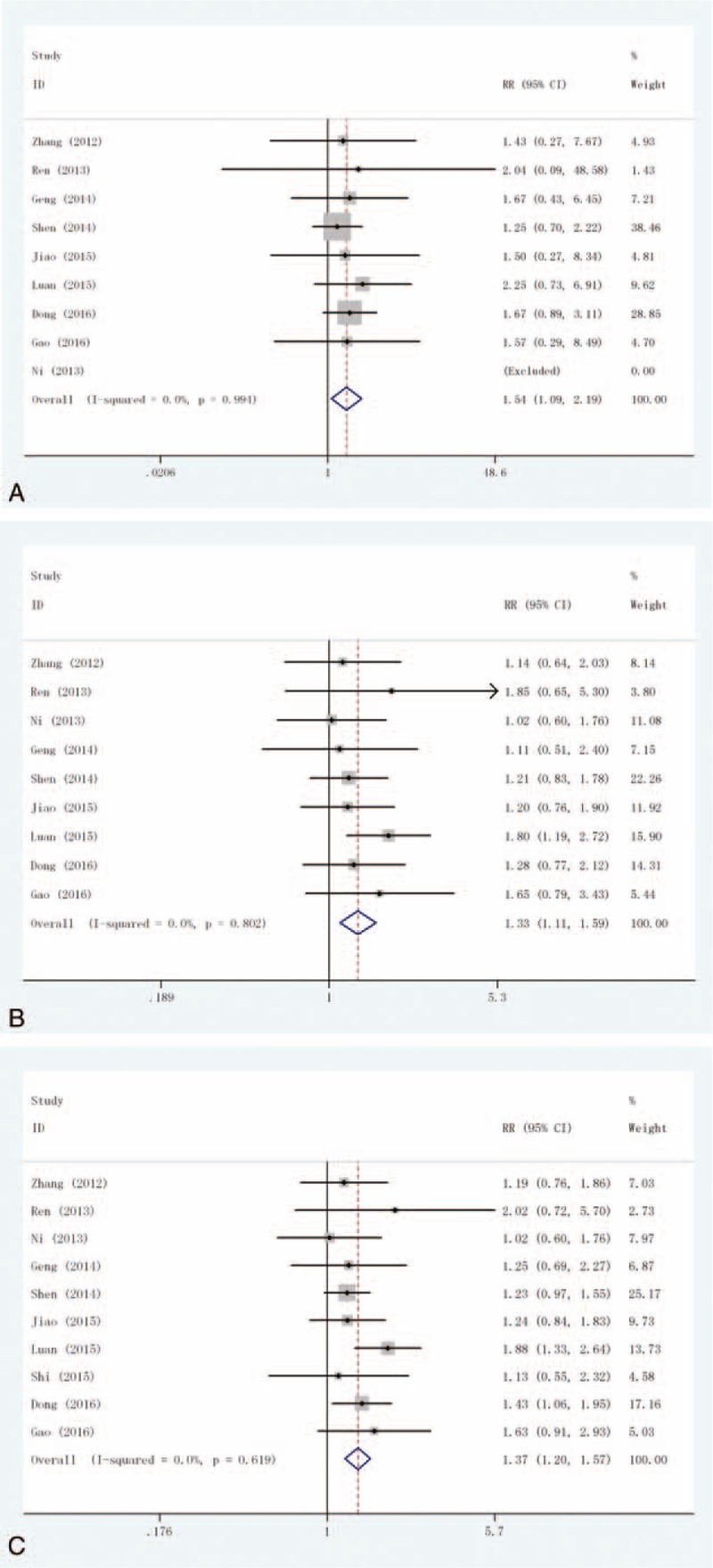
Forest plot of the comparison of efficacy outcomes: (A) complete response (CR); (B) partial response (PR); and (C) overall response rate (ORR).
PR: This outcome was reported in 9 trials, all comparing Exp with Con. There were 747 cases of patients, 386 cases in Exp group, 361 cases in Con group, the heterogeneity was not statistically significant, the fixed-effect model was used (P = .802, I2 = 0%). The difference in the PR was significant (RR = 1.33, 95% CI: 1.11–1.59), as shown in Figure 2B.
ORR: This outcome was reported in 10 trials, all comparing Exp with Con. There were 787 cases of patients, 406 cases in Exp group, 381 cases in Con group, the heterogeneity was not statistically significant, the fixed-effect model was used (P = .619, I2 = 0%). The difference in the ORR was significant (RR = 1.37, 95% CI: 1.20–1.57), as shown in Figure 2C.
Seven studies were included in the meta-analysis of AEs.
Leukopenia: This outcome was reported in 4 trials, all comparing Exp with Con. There was no heterogeneity between the study (P = .280, I2 = 21.8%), the fixed-effect model was used. There was no significant difference in the incidence of leukopenia (RR = 0.97, 95% CI: 0.86–1.09), as shown in Figure 3A.
Figure 3.
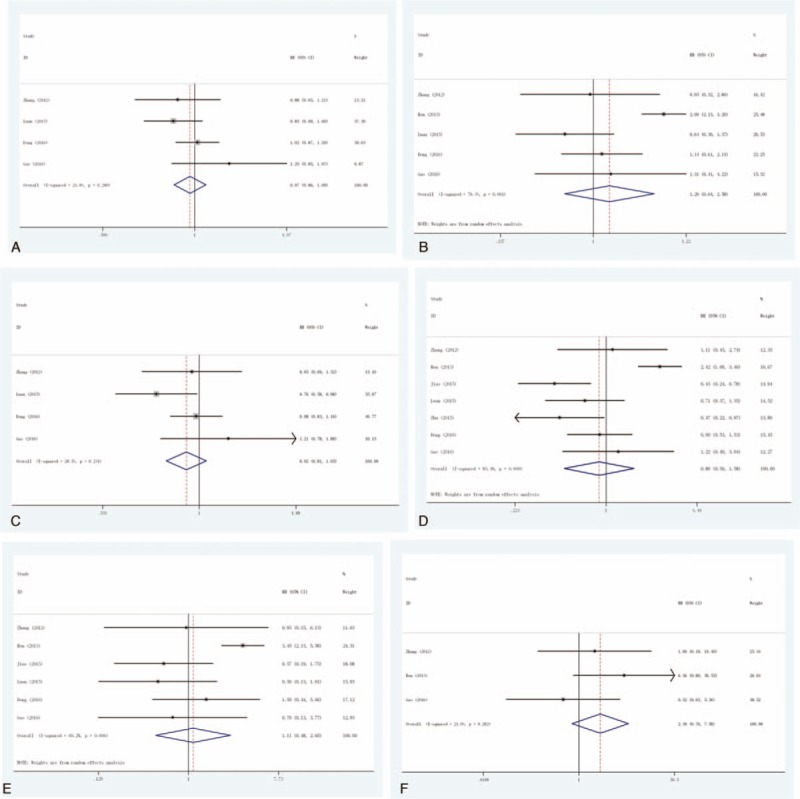
Forest plot of the comparison of adverse effects: (A) leukopenia; (B) thrombocytopenia; (C) hair loss; (D) nausea/vomiting; (E) hepatic complications; and (F) neurologic complications.
Thrombocytopenia: This outcome was reported in 5 trials, all comparing Exp with Con. There was significant heterogeneity between the study (P = .001, I2 = 79.4%), the random-effect model was used. There was no significant difference in the incidence of thrombocytopenia (RR = 1.29, 95% CI: 0.64–2.58), as shown in Figure 3B.
Hair loss: This outcome was reported in 4 trials, all comparing Exp with Con. There was no heterogeneity between the study (P = .241, I2 = 28.5%), the fixed-effect model was used. There was no significant difference in the incidence of hair loss (RR = 0.92, 95% CI: 0.81–1.05), as shown in Figure 3C.
Nausea/vomiting: This outcome was reported in 7 trials, all comparing Exp with Con. There was significant heterogeneity between the study (P < .001, I2 = 83.4%), the random-effect model was used. However, there was no significant difference in the incidence of nausea/vomiting (RR = 0.89, 95% CI: 0.50–1.58), as shown in Figure 3D.
Hepatic complications: This outcome was reported in 6 trials, all comparing Exp with Con. There was significant heterogeneity between the study (P = .006, I2 = 69.2%), the random-effect model was used. However, there was no significant difference in the incidence of hepatic complications (RR = 1.11, 95% CI: 0.48–2.60), as shown in Figure 3E.
Neurologic complications: This outcome was reported in 3 trials, all comparing Exp with Con. There was no heterogeneity between the study (P = .282, I2 = 21%), the fixed-effect model was used. However, there was no significant difference in the incidence of neurologic complications (RR = 2.39, 95% CI: 0.76–7.58), as shown in Figure 3F.
3.3. Publication bias
Finally, the Egger regression test showed no evidence of asymmetrical distribution in the funnel plot in CR (Begg test P = 1.000; Egger test P = .343) and ORR (Begg test P = .721; Egger test P = .888) (Fig. 4A and B).
Figure 4.
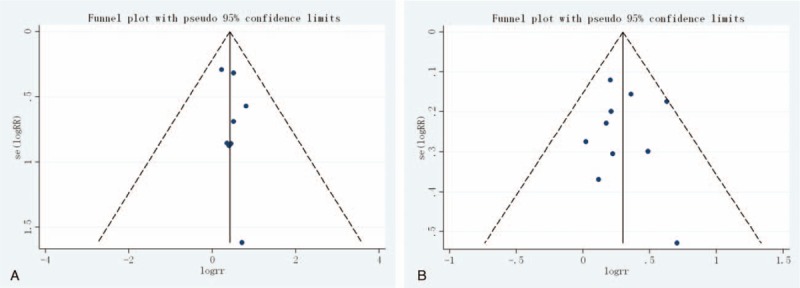
Begg funnel plot for publication bias test. Each point represents a separate study for the indicated association. (A) Complete response (CR); (B) overall response rate (ORR).
4. Discussion
Over the past few decades, many innovations in the development of anticancer drugs, especially those with significant progress targeted therapies and surgical techniques, chemotherapy, and radiation significantly improve the treatment of cancer overall. However, despite these significant advances, the majority of patients may relapse, and bear the serious side effects caused by chemotherapy and radiation, and even targeted therapy. Indeed, the failure of conventional therapy and relapse therapy is often present in cancer treatment, and the more effective treatment strategy is still indispensable for the treatment of cancer.[20–22]
Immunotherapy in the field of innovation has made great efforts. In recent years, it has become an important part of cancer treatment, in addition to the standard therapy. The method of cellular immunotherapy is based on 2 different principles[23–27]: On the one hand, the body's own immune system can be active and specific to stimulate the immune cells by confrontation with autologous or allogeneic tumor antigen in situ. On the other hand, the specific affinity of autologous or allogeneic immune cells to tumor-associated antigens can be activated in vitro, and subsequently directly applied to the human organism as a cellular immunotherapy. Therefore, the use of autologous tumor antigen-specific cellular immunotherapy is particularly interesting, because they promise an effective, low side effects and continuous treatment options based on the use of their own resources. In such a therapeutic approach, CIK cells are currently emerging as an effective treatment option, especially when combined with standard therapies for adjuvant therapy settings.[8] In the literature of the first report and the first phase I trial by Schmidt-Wolf has confirmed that the new higher cytotoxic activity of antitumor effector cells, and emphasized their good safety and tolerability.[28,29] Meanwhile, 25 years after their first description, a large number of clinical trials, showed encouraging results and demonstrated that CIK cells can prevent recurrence, improve progression and overall survival, and improve the quality of life of cancer patients.
In this study, we conducted a meta-analysis to determine the efficacy and safety of DC/CIK plus chemotherapy regimen (Exp) compared with chemotherapy (Con) alone regimen for breast carcinoma. Eleven relevant studies including 941 patients were included for this meta-analysis study. We observed that CR (RR = 1.54, 95% CI: 1.09–2.19), PR (RR = 1.33, 95% CI: 1.11–1.59), and ORR (RR = 1.37, 95% CI: 1.20–1.57) in BC patients treatment with DC/CIK plus chemotherapy regimen was significantly improved than that with chemotherapy alone. There was no significant difference in the incidence of leukopenia, thrombocytopenia, hair loss, nausea/vomiting, hepatic complications, and neurologic complications in BC patient's treatment with DC/CIK plus chemotherapy regimen and with chemotherapy alone. The present study, therefore, provides valuable information to help physicians make treatment decisions for their patients with BC.
There are advantages over the conventional therapy using DC-CIK cellular immunotherapy as follows: First of all, it is better to kill tumor cells. Compared with chemotherapy and radiotherapy to kill all cells, DC-CIK treatment is more like precision guided, can accurately kill tumor cells without killing innocent cells. Therefore, the treatment of patients has relatively small side effects. Second, the possibility of the development of drug resistance is smaller, so it can be used clinically for a long period of time.[30] Finally, but most importantly, it can still play an important role in immune surveillance after killing the tumor cells, and to protect the body's life.
The biggest advantage of the adoptive infusion of CIK cells for treating malignant disorders is safety. A growing number of animal studies published in recent years have indicated that the adoptive CIK cell transfer revealed considerable antitumor effect, and no severe adverse reactions in animals with malignant tumors occurred.[31–33] Based on the findings in animals, it has also been proved by many clinical trials that AEs were rarely observed following CIK cell infusion, and most of them reported were of mild intensity, such as fatigue, low grade fever/chills, and graft-versus-host disease (GVHD).[34,35] All of these events were resolved without treatment or with symptomatic treatments.[36]
Although immunotherapy has a significant clinical advantage, considerable uncertainty remains about its widespread clinical use, mainly due to economic reasons. Reimbursement is a key component of market access for new therapeutics including cancer vaccines and immunotherapeutic drugs for cancer. To our knowledge, there are currently no economic evaluations of DC-CIK or other immunotherapeutics.[37]
Several limitations in this meta-analysis should be addressed. First, evaluation of the data set was considered to be too small for visual or statistical examination of publication bias, and the potential existence of such bias could not be determined. Therefore, we assume that publication bias may exist. Second, eligibility criteria for inclusion in BC patients are different, which may affect the apparent consistency of the effects in these studies, and lead to heterogeneity among studies. Third potential limitation is that country can also introduce a bias. As increasing number of studies dealing with the treatment of patients with BC with CIK cells were published only in Chinese, so the results of this meta-analysis are based on Chinese patients.
In conclusion, compared with chemotherapy alone, DC/CIK plus chemotherapy treatment significantly increased CR, PR, and ORR. However, there was no difference between the safety of DC/CIK plus chemotherapy and chemotherapy alone. DC/CIK plus chemotherapy treatment is a valuable new option for the treatment of breast carcinoma in women. However, further studies are needed to verify the results of this study, due to the presence of unstable factors.
Acknowledgments
We acknowledge the support and input from Beijing Luhe Hospital, Capital Medical University.
Footnotes
Abbreviations: AEs = adverse events, BC = breast cancer, CI = confidence interval, CIK = cytokine-induced killer, CNKI = China National Knowledge Infrastructure, Con = chemotherapy, CR = complete response, DCs = dendritic cells, ORR = overall response rate, PR = partial response, RCTs = randomized controlled trials, RR = relative risk.
The authors report no funding and conflicts of interest.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279–89. [DOI] [PubMed] [Google Scholar]
- [3].Demicheli R. Tumour dormancy: findings and hypotheses from clinical research on breast cancer. Semin Cancer Biol 2001;11:297–306. [DOI] [PubMed] [Google Scholar]
- [4].Stefanovic S, Schuetz F, Sohn C, et al. Adoptive immunotherapy of metastatic breast cancer: present and future. Cancer Metastasis Rev 2014;33:309–20. [DOI] [PubMed] [Google Scholar]
- [5].Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther 2012;12:1597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Criscitiello C, Curigliano G. Immunotherapy of Breast Cancer. Prog Tumor Res 2015;42:30–43. [DOI] [PubMed] [Google Scholar]
- [7].Hao MZ, Lin HL, Chen Q, et al. Efficacy of transcatheter arterial chemoembolization combined with cytokine-induced killer cell therapy on hepatocellular carcinoma: a comparative study. Chin J Cancer 2010;29:172–7. [DOI] [PubMed] [Google Scholar]
- [8].Hontscha C, Borck Y, Zhou H, et al. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC). J Cancer Res Clin Oncol 2011;137:305–10. [DOI] [PubMed] [Google Scholar]
- [9].Ren J, Di L, Song G, et al. Selections of appropriate regimen of high-dose chemotherapy combined with adoptive cellular therapy with dendritic and cytokine-induced killer cells improved progression-free and overall survival in patients with metastatic breast cancer: reargument of such contentious therapeutic preferences. Clin Transl Oncol 2013;15:780–8. [DOI] [PubMed] [Google Scholar]
- [10].Zhang WJ, Liu LL, Wang X. The efficacy of chemotherapy alone with chemotherapy combined DC-CIK cell immunotherapy of metastatic breast cancer. Chin J Gerontol 2012;32:247–50. [Google Scholar]
- [11].Zhi-Qiang N, Fang YQ, Liu D. Effect of DC-CIK combined with chemotherapy on immune function, progression-free survival and quality of life of patients with breast cancer. Maternal Child Health Care China 2013;28:5134–7. [Google Scholar]
- [12].Geng Q, Li XP, Zhang P. Clinical observation of DC-CIK cells in the treatment of advanced breast cancer. Chin J Trauma Disabil Med 2014;22:69–70. [Google Scholar]
- [13].Shen YL. The clinical value and feasibility study of CIK combined with DC cells in the treatment of metastatic breast cancer. Maternal Child Health Care China 2014;29:5180–2. [Google Scholar]
- [14].Jiao L, Chen F, Jiang QQ, et al. Dendritic cell-cytokine induced killer cells combined with neoadjuvant chemotherapy and targeted therapy in treatment of advanced breast cancer patients: the clinical effectiveness. China J Mod Med 2015;25:21–5. [Google Scholar]
- [15].Luan JW. Analysis of the effect of chemotherapy combined with CIK-DC cells in the treatment of metastatic breast cancer. China Foreign Med Treatment 2015;1:35–6. [Google Scholar]
- [16].Shi L, Bai YJ, Ma H, et al. Clinical efficacy of DC-CIK immunotherapy combined with chemotherapy in the treatment of advanced breast cancer. Yiayao Qianyan 2015;5:201–2. [Google Scholar]
- [17].Zhu HH, Dong-Chu MA, Ding ZY, et al. Clinical effect of DC-CIK in treatment of three negative breast cancer after adjuvant chemotherapy. Med Pharma J Chin Peoples Liberation Army 2015;27:48–51. [Google Scholar]
- [18].Dong N, Wu HW, Shen YJ, et al. Efficacy and safety of cytokine induced killer cells combined with chemotherapy in treatment of patients with metastatic breast cancer. Med Recap 2016;22:979–82. [Google Scholar]
- [19].Gao HY, Zhu YL, Sun L. Investigation of the curative effect of chemotherapy combined with DC-CIK cell immunotherapy in metastatic breast carcinoma. J Int Oncol 2016;43:326–9. [Google Scholar]
- [20].Husain SR, Han J, Au P, et al. Gene therapy for cancer: regulatory considerations for approval. Cancer Gene Ther 2015;22:554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Enriquez-Navas PM, Wojtkowiak JW, Gatenby RA. Application of evolutionary principles to cancer therapy. Cancer Res 2015;75:4675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].La-Beck NM, Jean GW, Huynh C, et al. Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacotherapy 2015;35:963–76. [DOI] [PubMed] [Google Scholar]
- [23].Disis ML. Immune regulation of cancer. J Clin Oncol 2010;28:4531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- [25].Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Ann Rev Immunol 2004;22:329–60. [DOI] [PubMed] [Google Scholar]
- [26].Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 2006;6:715–27. [DOI] [PubMed] [Google Scholar]
- [27].Disis ML, Bernhard H, Jaffee EM. Use of tumour-responsive T cells as cancer treatment. Lancet 2009;373:673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schmidt-Wolf IGH, Negrin RS, Kiem HP, et al. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med 1991;174:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schmidt-Wolf IGH, Finke S, Trojaneck B, et al. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-1 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer 1999;81:1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thanendrarajan S, Kim Y, Schmidt-Wolf I. New adoptive immunotherapy strategies for solid tumours with CIK cells. Expert Opin Biol Ther 2012;12:565–72. [DOI] [PubMed] [Google Scholar]
- [31].Nishimura R, Baker J, Beilhack A, et al. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood 2008;112:2563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang Y, Dai H, Li H, et al. Growth of human colorectal cancer SW1116 cells is inhibited by cytokine-induced killer cells. Clin Dev Immunol 2011;2011:621414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Verneris MR, Ito M, Baker J, et al. Engineering hematopoietic grafts: purified allogeneic hematopoietic stem cells plus expanded CD8+ NK-T cells in the treatment of lymphoma. Biol Blood Marrow Transplant 2001;7:532–42. [DOI] [PubMed] [Google Scholar]
- [34].Schmeel FC, Schmeel LC, Gast SM, et al. Adoptive immunotherapy strategies with cytokine-induced killer (CIK) cells in the treatment of hematological malignancies. Int J Mol Sci 2014;15:14632–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chung MJ, Park JY, Bang S, et al. Phase II clinical trial of ex vivo-expanded cytokine-induced killer cells therapy in advanced pancreatic cancer. Cancer Immunol Immunother 2014;63:939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang Y, Wang J, Wang Y, et al. Autologous CIK cell immunotherapy in patients with renal cell carcinoma after radical nephrectomy. Clin Dev Immunol 2013;2013:195691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jönsson B, Wilking N. Cancer vaccines and immunotherapeutics: challenges for pricing, reimbursement and market access. Hum Vaccin Immunother 2012;8:1360–3. [DOI] [PMC free article] [PubMed] [Google Scholar]


