Abstract
Background:
Rapid increases in desflurane concentration can transiently increase the heart rate (HR). Esmolol possesses a high β1-adrenoceptor selectivity and a short duration of action. This preliminary study aimed at investigating the effects of esmolol on the HR and autonomic modulation during a desflurane-induced HR increase.
Methods:
American Society of Anesthesiologists physical status I female subjects, aged 20 to 50 years, who were undergoing minor breast surgery were randomly assigned to 2 groups. Rapid increases in desflurane concentration were commenced after induction of anesthesia. Each subject received either i.v. saline (control group) or esmolol 0.5 mg/kg (esmolol group) before desflurane inhalation. Using time-frequency spectral analysis of HR variability, the HR indices were studied at baseline, postinduction, posttreatment, as well as at minimal alveolar concentrations of desflurane reaching 1.0, 1.3, and 1.5. The low frequency (LF) power is influenced by both the sympathetic and parasympathetic activity, whereas the high frequency (HF) power reflects the parasympathetic activity. The LF/HF ratio is thought to reflect either sympathovagal balance or sympathetic modulation.
Results:
Electrocardiograms for data analysis were obtained from 8 subjects in each group. Rapid increases in desflurane concentration after induction caused a HR increase. Both the corresponding LF and HF powers were low and the LF/HF ratio remained unchanged. This indicates that the desflurane-induced HR increase may be attributed to parasympathetic inhibition and may be independent of sympathetic activation. Esmolol pretreatment effectively attenuated desflurane-induced HR increase. Moreover, subjects receiving esmolol pretreatment had increased LF and HF powers, but did not have changes in their LF/HF ratios, as compared to those without esmolol.
Conclusion:
Esmolol pretreatment attenuates HR increase and parasympathetic inhibition during rapid increases in desflurane concentration.
Keywords: β-adrenergic antagonist, autonomic nervous system, heart rate variability, time-frequency spectral analysis
1. Introduction
Desflurane is one of the most widely used modern volatile anesthetics in clinical anesthesia. It has lower blood–gas partition coefficient than other volatile anesthetics, which is conducive to rapid induction and recovery from anesthesia.[1] Despite its clinical popularity, rapid increases in end-tidal desflurane concentration greater than 1 minimum alveolar concentration (MAC) can transiently increase the heart rate (HR).[2–6] Regardless of its cause, the transient HR increase is generally tolerated in patients with robust health, but it may cause myocardial ischemia in those with limited coronary reserve. Prior administration of β1-adrenergic antagonists has been reported to attenuate the hemodynamic perturbation induced by desflurane in a controlled experimental situation.[7]
The mechanisms of desflurane-induced HR increase were investigated after steady-state anesthesia in most studies[4,5,8,9]; however, these studies could not reflect the rapid changes of autonomic activity during induction of anesthesia immediately followed by a rapid wash-in of desflurane in clinical practice. Spectral analysis of heart rate variability (HRV) is a noninvasive tool used to assess cardiovascular neural control.[10] The fluctuations of RR intervals (RRIs) assessed by frequency domain analysis allows for a better understanding of the sympathovagal interaction in the autonomic nervous system.[11,12] Frequency domain analysis is conventionally calculated by either fast Fourier transformation or autoregressive modeling.[13] It provides information on how the power is distributed as a function of frequency over the time period chosen. The prerequisite for using this tool is that the signals should be stationary. Because of the rapid changes in HRV during induction of anesthesia and the ensuing rapid increases in desflurane concentration, it is not possible to obtain stationary data in a clinical situation. To analyze the nonstationary signals, an alternative time-frequency spectral analysis is able to delineate the time-related frequency components of signals.[14,15] Instead of the traditional single spectrum generated in the whole data segment, multiple successive spectra are generated with time in overlapped window lengths. Time-frequency spectral analysis has been properly used to study the rapidly changing HRV data during general anesthesia.[15]
Esmolol holds the pharmacological advantage of high β1-adrenoceptor selectivity and short duration of action (elimination half-life of approximately 9 minutes). It has been widely used in clinical anesthesia and has been shown to be effective in decreasing the hemodynamic response to endotracheal intubation,[16,17] inducing intraoperative controlled hypotension,[18,19] and reducing postoperative pain intensity.[20] Moreover, esmolol has been shown to effectively decrease HR and has the potential to protect against myocardial ischemia in patients undergoing noncardiac surgery.[21] These characteristics suggest that esmolol may be advisable to blunt the transient HR increase induced by desflurane.
Consequently, we hypothesized that autonomic activity may be altered during a desflurane-induced HR increase, which may be modulated by esmolol pretreatment. We conducted a preliminary study by using time-frequency analysis of HRV in control and esmolol-pretreated subjects to evaluate the rapid increases in desflurane concentration after induction of general anesthesia.
2. Materials and methods
2.1.1. Patient selection
This prospective randomized preliminary study was approved by the Hospital Research and Ethics Committee of Chang Gung Memorial Hospital (No. 94-060). After written informed consent was obtained, we recruited American Society of Anesthesiologists physical status I female subjects, aged 20 to 50 years, undergoing minor breast surgery including lumpectomies, quadrantectomies, and radiograph wire-localized breast biopsies. All subjects were free of known systemic diseases, were not receiving medication, had no improper alcohol or drug use, and had a body mass index between 18.5 and 24.9 kg/m2.
2.2. Anesthesia and experimental procedure
Each subject arrived in the operating room after an 8- to 10-hour overnight fast without premedication. A 20-gauge catheter was inserted into a forearm vein and 5 mL/kg of lactated Ringer solution was administered over 10 minutes, afterward Lactated Ringer solution was infused at a rate of 2 mL/kg/h throughout the study. Perioperative monitoring of electrocardiogram (ECG), peripheral oxygen saturation, noninvasive blood pressure, end-tidal concentration of CO2, and desflurane level were registered by means of a Datex-Ohmeda S/5 Monitor (Datex-Ohmeda, Helsinki, Finland) that was connected to the subject. In order to obtain data for HRV analysis, the analogue signals of QRS complex were sampled by a Biopac MP 35 system (Biopac Systems, Inc., Goleta, CA) and its companion Biopac Student Lab software. The sampling rate for this was 1000 Hz.
Before the experimental procedure, the subjects were divided into either control or esmolol groups using a sealed envelope technique. Preceded by a 10-minute stabilization period, continuous ECG recording was started at least 5 minutes before the induction of anesthesia. After routine administration of oxygen, anesthesia was induced with i.v. thiamylal sodium 5 mg/kg. After a neuromuscular block had been achieved with i.v. rocuronium 1 mg/kg, a ProSeal laryngeal mask airway (PLMA; Laryngeal Mask Co. Limited, Mahe, Seychelles) size 3 or 4 was inserted by a single experienced anesthesiologist using an introducer tool. The cuff was inflated to a pressure of 60 cm H2O as measured by a calibrated aneroid manometer. The presence of an effective airway was initially judged by a capnograph trace with no audible leaks with peak airway pressures no less than 12 cm H2O during manual ventilation. Mechanical ventilation was initiated at a rate of 10/minute and a tidal volume of 10 mL/kg. Oropharyngeal and drainage tube leaks were confirmed by listening over the mouth and placing lubricant over the proximal end of the drainage tube, respectively. Subjects requiring reposition of PLMA, with air leaks, or with ineffective ventilation were excluded from the study. Tidal volume was set to maintain the end-tidal CO2 at 35 to 40 mm Hg. After reaching a stable hemodynamic status (transient fluctuations in HR within 5 beats/minute and blood pressure within 10 mm Hg/minute), each subject received either i.v. saline 0.05 mL/kg (control group) or esmolol 0.5 mg/kg (esmolol group). One minute later, desflurane anesthesia was abruptly started at an inspired concentration of 11% with oxygen at a flow rate of 3 L/minute. The study's endpoint was reached when the end-tidal concentration of desflurane reached 1.5 MAC and was held constant for at least 2 minutes. Surgical preparation was not commenced until the study's endpoint was reached. The effects of esmolol pretreatment and relevant sympathovagal changes on rapid increases of desflurane concentration were evaluated.
2.3. Data management
The ECG data was used for off-line analysis. All programs used for data analysis were self-developed in the MATLAB (The MathWorks, Inc., Natick, MA). Automatic detection of RRIs was performed by using threshold and slope detection combined with rejection of candidate peaks during estimated refractory periods. Artifacts and premature beats were identified by visual inspection and were replaced by linear interpolation. The RRIs were resampled at a frequency of 4 Hz to obtain an even sampling sequence and avoid signal aliasing.
2.4. Time-frequency analysis of HRV
HRV was analyzed with a time-frequency domain method of analysis. The time-frequency spectra were computed based on the smoothed pseudo Wigner–Ville transformation, which has been demonstrated to be a good estimation method for short nonstationary time series.[14,22,23] This detailed method was described previously.[15,24] In brief, the spectrum was obtained every 4 seconds with a window length of 64 seconds. The spectral power of each spectrum in ms2 was calculated at low frequency (LF, 0.04–0.15 Hz) and at high frequency (HF, 0.15–0.40 Hz). The ratio of LF/HF was also calculated. The sympathetic and parasympathetic components of HRV are active over different frequency ranges. The LF power is influenced by both the sympathetic and parasympathetic activity,[12] whereas the HF power reflects the parasympathetic activity.[13] The LF/HF ratio is thought to either mirror sympathovagal balance or reflect sympathetic modulation.[13,25] To abate the between-subject variability, logarithmic transformations were performed on these autonomic-related HR indices. Spectral powers were averaged in each selected 2-minute interval during the following 6 periods: Baseline: before anesthetic induction; Postinduction or preinsertion: between postinduction and PLMA insertion; Posttreatment: 1 minute after esmolol or saline treatment; 1.0 MAC: after end-tidal concentration of desflurane reaching 1.0 MAC; 1.3 MAC: after end-tidal concentration of desflurane reaching 1.3 MAC; 1.5 MAC: after end-tidal concentration of desflurane reaching 1.5 MAC. The data analysis was accomplished by an observer who was blinded regarding the study.
2.5. Statistics
Before the study, our experience has been that rapid increases in desflurane concentration raised the HR approximately 15 beats/minute. Assuming a standard deviation (SD) of HR to be 10 and esmolol decreased desflurane-induced HR increase by 50%, 8 subjects in each group would be adequate, with a power of 0.8 and a risk α = 0.05, to detect a difference of HR.
All data sets were expressed as mean ± SD. Shapiro–Wilk tests for normality were conducted to confirm the approximate normal distribution of the variables. Because the P-values of normality tests for demographic, HR and HRV variables were all greater than 0.05, we used parametric tests to test for differences in means. The data sets from subject demographics were compared using the independent-sample Student t test. The data sets from the changes of HR and HRV parameters over time were analyzed using 2-way analysis of variance (ANOVA). Tukey post hoc tests were used for multiple comparisons in both cases. P < .05 was considered to be statistically significant. All data sets were analyzed using SigmaPlot for windows (SPSS Scientific, Chicago, IL).
3. Results
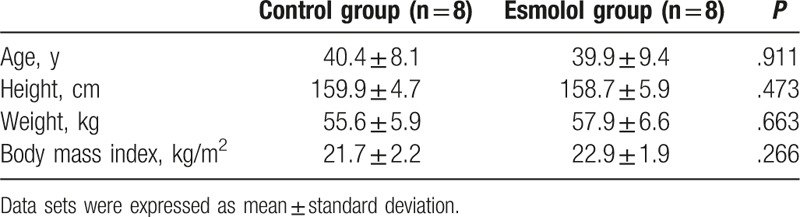
Twenty subjects were randomly assigned to the 2 study groups. One subject in the control group as well as 2 subjects in the esmolol group did not receive allocated intervention because of an unsatisfactory PLMA placement. Moreover, 1 subject in the control group was excluded from analysis due to an unsatisfactory ECG recording. Complete data sets were obtained and analyzed from 8 subjects in each group. Among these subjects, 4 subjects in both groups underwent lumpectomies. Four subjects in the control group as well as three subjects in the esmolol group underwent wire-localized breast biopsies. Moreover, 1 subject in the esmolol group underwent a quadrantectomy. There were no significant differences between the 2 groups with respect to their characteristics (Table 1). No subject exhibited bradycardia (defined as HR < 50 bpm) or severe hypotension (defined as a drop from 30% of baseline systolic blood pressure) throughout the study. In addition, none of the subjects had anesthesia-related perioperative adverse events.
Table 1.
Demographic characteristics.
3.1. HR changes
The mean HR values of the control and esmolol groups are shown in Table 2 and Fig. 1. Two-way ANOVA revealed significant main effects of group (F = 54.9, P < .001) and time (F = 19.5, P < .001) without a significant group × time interaction (F = 1.82, P = .116). The HR values at the baseline and postinduction periods were not significantly different within both the control and esmolol groups (P = .502 and .602, respectively). The HR values at the baseline and postinduction periods were also comparable between the 2 groups (P = .215 and .164, respectively). The HR values of the control group at the posttreatment period were not significantly different from those at the postinduction period. However, the HR values of the control group at the 1 MAC, 1.3 MAC, and 1.5 MAC periods of desflurane anesthesia were significantly higher than those of the postinduction period (P = .006, <.001, and =.028, respectively). In contrast, the HR values of the esmolol group at the posttreatment, 1 MAC, 1.3 MAC, and 1.5 MAC periods were all not significantly higher or lower than those of the postinduction period (P = .997, .794, .541, and 1.000, respectively). It should be noted that the HR values of the esmolol group at the posttreatment, 1 MAC, 1.3 MAC, and 1.5 MAC periods were all significantly lower than those of the control groups (all P < .001).
Table 2.
Values for mean heart rate (beats/minute).
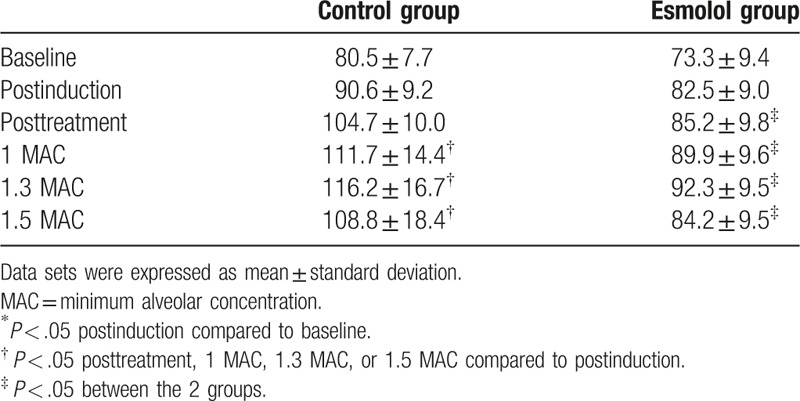
Figure 1.
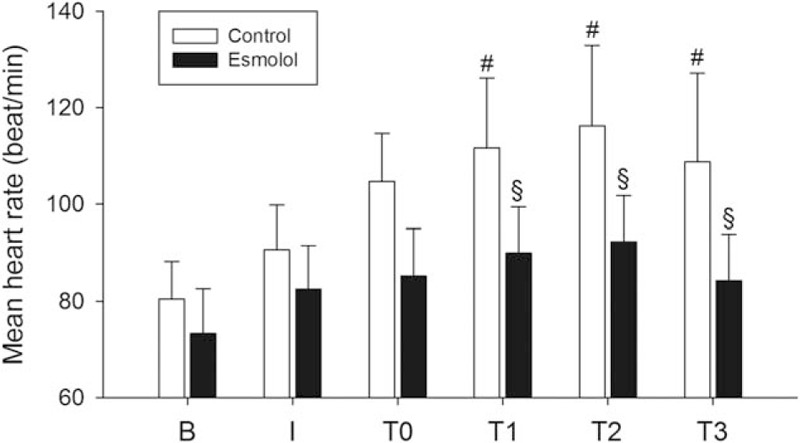
Time course of the mean heart rate at baseline (B), postinduction (I), posttreatment (T0), 1 MAC (T1), 1.3 MAC (T2), and 1.5 MAC (T3). Data sets were expressed as mean ± standard deviation. MAC = minimum alveolar concentration. ∗P < .05 postinduction compared to baseline; #P < .05 T0–T3 compared to postinduction; §P < .05 between the 2 groups.
3.2. LF power
The LF powers of the control and esmolol groups are shown in Table 3 and Fig. 2A. Two-way ANOVA revealed significant main effects of group (F = 21.5, P < .001) and time (F = 44.6, P < .001) without a significant group × time interaction (F = 1.97, P = .092). The LF powers at the baseline and postinduction periods were not significantly different within both the control and esmolol groups (P = .425 and .234, respectively). The LF powers at the baseline and postinduction periods were also comparable between the 2 groups (P = .212 and .371, respectively). The LF powers of the control group at the posttreatment, 1 MAC, 1.3 MAC, and 1.5 MAC periods were all significantly lower than those at the postinduction period (all P < .001). Similarly, the LF powers of the esmolol group at the posttreatment, 1 MAC, 1.3 MAC, and 1.5 MAC periods were also significantly lower than those at the postinduction period (P < .001, <.001, =.002, and .007, respectively). Though the LF powers of the 2 groups were comparable at the posttreatment and 1.0 MAC periods (P = .388 and .324, respectively), the LF powers of the esmolol group were significantly higher than those of the control group at the 1.3 MAC and 1.5 MAC periods (P = .002 and <.001).
Table 3.
Values for spectral powers of heart rate variability.
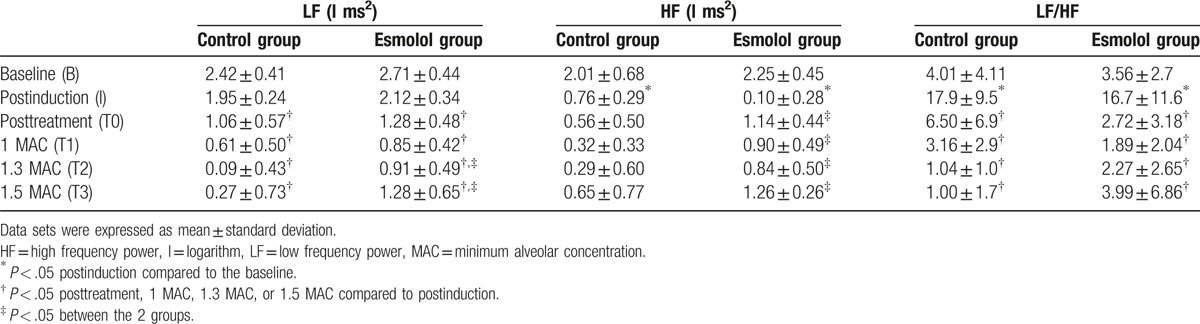
Figure 2.
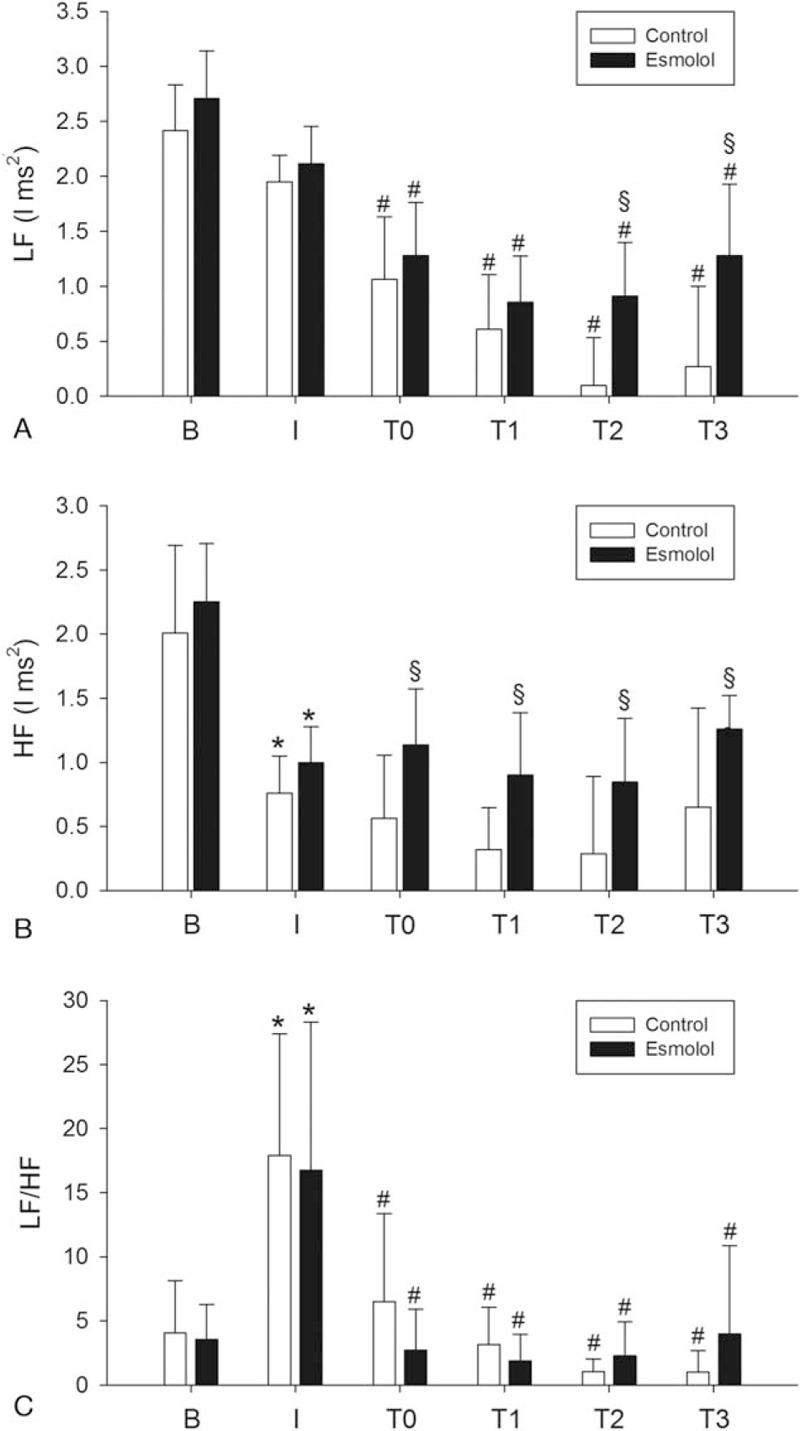
Time course of the heart rate variability indices at baseline (B), postinduction (I), posttreatment (T0), 1 MAC (T1), 1.3 MAC (T2), and 1.5 MAC (T3). Data sets were expressed as mean ± standard deviation. HF = high frequency power, l = logarithm, LF = low frequency power, MAC = minimum alveolar concentration. ∗P < .05 postinduction compared to baseline; #P < .05 T0–T3 compared to postinduction; §P < .05 between the 2 groups.
3.3. HF power
The HF powers of the control and esmolol groups are shown in Table 3 and Fig. 2B. Two-way ANOVA revealed significant main effects of group (F = 21.8, P < .001) and time (F = 22.0, P < .001) without a significant group × time interaction (F = 0.508, P = .770). The HF powers of the control and esmolol groups were significantly decreased at the postinduction periods as compared to those at the baseline periods (both P < .001). The HF powers at the baseline and postinduction periods were comparable between the 2 groups (P = .321 and .330, respectively). The HF powers of the control group at the posttreatment, 1 MAC, 1.3 MAC, and 1.5 MAC periods were not significantly higher or lower than those at the postinduction period (P = .976, .478, .396, and .998, respectively). Similarly, the HF powers of the esmolol group at the posttreatment, 1 MAC, 1.3 MAC, and 1.5 MAC periods were also not significantly higher or lower those that at the postinduction period (P = .791, .999, .988, and .895, respectively). Nonetheless, the HF powers of the esmolol group were significantly higher than those of the control group at the posttreatment, 1.0 MAC, 1.3 MAC, and 1.5 MAC periods (P = .022, .021, .026, and .015, respectively).
3.4. LF/HF ratio
The LF/HF ratios of the control and esmolol groups are shown in Table 3 and Fig. 2C. Two-way ANOVA revealed a significant main effect of time (F = 18.1, P < .001), but there were no significant effects of group (F = 0.133, P = .716) and group × time interaction (F = 0.69, P = .633). The LF/HF ratios of the control and esmolol groups were significantly increased at the postinduction periods as compared to those at the baseline periods (both P < .001). The LF/HF ratios of the control group at the posttreatment, 1 MAC, 1.3 MAC, and 1.5 MAC periods were all significantly lower than those at the postinduction period (all P < .001). Similarly, the LF/HF ratios of the esmolol group at the posttreatment, 1 MAC, 1.3 MAC, and 1.5 MAC periods were also significantly lower than those at the postinduction period (all P < .001). Moreover, the LF/HF ratios were not significantly different between the 2 groups at the baseline, postinduction, posttreatment, 1.0 MAC, 1.3 MAC, and 1.5 MAC periods (P = .864, .673, .180, .650, .662, and .288, respectively).
4. Discussion
This is the first study to measure the rapidly changing HRV during desflurane anesthesia using time-frequency analysis. As expected, our data confirmed that rapid increases in desflurane concentration after induction cause a HR increase. Our data further revealed that both the LF and HF powers of the control and esmolol groups were low during rapid increases in desflurane concentration. Prior administration of esmolol immediately before commencement of desflurane inhalation effectively attenuated the desflurane-induced HR increase. Moreover, prior administration of esmolol resulted in higher LF and HF powers of HRV as compared to those without. Data from this study supports the potential benefits of esmolol to attenuate HR increase and improve HRV during rapid increases in desflurane concentration in clinical practice.
HR fluctuations around a mean HR are attributed to the continuous changes in the sympathovagal balance. Frequency domain analysis of HRV, which unravels and quantifies fluctuations around a stationary period of sinus rhythm within defined frequency ranges, can mirror the sympathetic and parasympathetic activities.[10,13] Reduced HRV, a manifestation of autonomic dysfunction, has been shown to be associated with increased risks of cardiovascular events,[26–28] as well as poor prognosis of cardiovascular diseases.[29] Because the HR characteristics during induction of anesthesia along with rapid wash-in of volatile anesthetics are time-varying and lack data stationarity, the time-related HRV would be better delineated by the quadratic time-frequency analysis.[14,15,30] Based on this technique in the present study, the spectral components of HRV were consistently suppressed after induction of anesthesia, which is consistent with previous reports.[15,31] Therefore, the data sets from the LF and HF powers during rapid increases in desflurane concentration were compared with those of the postinduction period within the control or esmolol groups. Moreover, the data were compared between the 2 groups in order to investigate the relevant changes in HR indices.
HR increase induced by rapid increases in desflurane concentration has been well documented in clinical situations,[2–5] though debate on the mechanisms responsible for this phenomenon still exists. Sympathetic hyperactivity measured from the peripheral nerve and the activation of renin–angiotensin system was reported to support the sympathoexcitation caused by desflurane.[4,5] However, desflurane-induced renal sympathetic nerve hyperactivity has been reported to be vagally mediated, because the rabbits receiving bilateral vagotomy abolished the sympathoexcitation induced by desflurane.[32] The authors concluded that the sympathetic nerve hyperactivity induced by desflurane might be attributed to the vagally mediated reflex sympathoexcitation. Nonetheless, those studies lacked direct measurements of the alternation in cardiac autonomic function. Spectral analysis of HRV provides measurements of the instantaneous RRIs in response to cardiovascular neural control.[13] Previous reports assessing the autonomic system in dogs revealed that desflurane elicited a HR increase and was accompanied by corresponding decreases in the HF and LF powers.[8,9] Their data showed that the HF power correlated inversely with the HR, indicating that desflurane elicits a HR increase primarily resulting from parasympathetic inhibition and is independent of sympathetic activation. In accordance with this notion, the present study demonstrated that both the HF and LF powers were suppressed during rapid increases in desflurane concentration in human subjects. While LF power is influenced by both the sympathetic and parasympathetic activity, the decreased HF power in the present study indicates that desflurane-induced HR increase may be largely attributed to the inhibited parasympathetic activity. Moreover, except for a transient increase of the LF/HF ratio after induction, the LF/HF ratios during desflurane anesthesia remained unchanged as compared to those of the baseline period, indicating that desflurane-induced HR increase is very likely to be independent of sympathetic activation.
A previous study in healthy volunteers demonstrated that esmolol attenuated the HR response to abrupt increases in desflurane concentration after a steady state of anesthesia.[7] The present study further replicated the clinical situation in which rapid increases in desflurane concentration usually follow induction of anesthesia. The unchanged HR in the esmolol-pretreated subjects revealed the potential of esmolol to prevent HR increase attributed to desflurane inhalation. Moreover, the effects of esmolol on HR spectra were also investigated. The β-adrenergic antagonists were reported to augment vagally mediated HF power in normal adults,[33,34] as well as restore HRV petameters in patients with cardiovascular disease.[35,36] Our data revealed that esmolol increased the HF power, an index of the parasympathetic activity, as compared to that of the control group during rapid increases in desflurane concentration. Similarly, the LF power, an index contributed jointly by both the sympathetic and parasympathetic activities, was also increased gradually after esmolol administration. Nonetheless, the LF/HF ratios, an index of the sympathetic activity, exhibited no discernible differences between the 2 groups. We therefore concluded that esmolol primarily improves the parasympathetic component of HRV, which is supposed to be largely suppressed during rapid increases in desflurane concentration.
Certain limitations of this preliminary study need to be acknowledged. Firstly, to abate the gender and aging effects on HRV,[25] the subjects enrolled were restricted to healthy young females underwent similar procedures. The safety and efficacy profiles of esmolol pretreatment in patients with different genders and ages as well as in higher-risk patients have yet to be defined. Secondly, our data revealed that esmolol improved HRV parameters which were supposed to be suppressed by desflurane. Due to the fact that reduced HRV may imply autonomic dysfunction that carries increased risks of cardiovascular morbidity and mortality,[26–28] the association between improved HRV and perioperative cardiac events especially in higher-risk patients remains unstudied. Thirdly, this small-sample study focused on the cardiac autonomic control mechanisms underlying the effects of esmolol on desflurane-induced HR increase. The preliminary results encourage further studies before it can be implemented on a daily basis in clinical practice.
In conclusion, rapid increases in desflurane concentration after induction of anesthesia elicits a HR increase. Time-frequency analysis of HRV revealed that this phenomenon may be largely attributed to the parasympathetic inhibition by desflurane. Esmolol pretreatment not only attenuates desflurane-induced HR increase, but also enhances the recovery of parasympathetic inhibition. These preliminary findings provide clinical implications that warrant further exploration.
Footnotes
Abbreviations: ANOVA = analysis of variance, ECG = electrocardiogram, HF = high frequency, HR = heart rate, HRV = heart rate variability, LF = low frequency, MAC = minimum alveolar concentration, PLMA = ProSeal laryngeal mask airway, RRIs = RR intervals, SD = standard deviation.
Funding: This work was supported by grants from Chang Gung Memorial Hospital, Taiwan (CMRPG340391) and the Ministry of Science and Technology, Taiwan (MOST 105-2221-E-182-035).
The authors have no conflicts of interest to disclose.
References
- [1].Uhlig C, Bluth T, Schwarz K, et al. Effects of volatile anesthetics on mortality and postoperative pulmonary and other complications in patients undergoing surgery: a systematic review and meta-analysis. Anesthesiology 2016;124:1230–45. [DOI] [PubMed] [Google Scholar]
- [2].Weiskopf RB, Cahalan MK, Eger EI, II, et al. Cardiovascular actions of desflurane in normocarbic volunteers. Anesth Analg 1991;73:143–56. [PubMed] [Google Scholar]
- [3].Weiskopf RB, Eger EI, II, Daniel M, et al. Cardiovascular stimulation induced by rapid increases in desflurane concentration in humans results from activation of tracheopulmonary and systemic receptors. Anesthesiology 1995;83:1173–8. [DOI] [PubMed] [Google Scholar]
- [4].Ebert TJ, Muzi M. Sympathetic hyperactivity during desflurane anesthesia in healthy volunteers. A comparison with isoflurane. Anesthesiology 1993;79:444–53. [DOI] [PubMed] [Google Scholar]
- [5].Weiskopf RB, Moore MA, Eger EI, II, et al. Rapid increase in desflurane concentration is associated with greater transient cardiovascular stimulation than with rapid increase in isoflurane concentration in humans. Anesthesiology 1994;80:1035–45. [DOI] [PubMed] [Google Scholar]
- [6].Do HS, Kim SY, Heo SJ, et al. The effect of intravenous labetalol administration on hemodynamic responses during desflurane inhalation. Korean J Anesthesiol 2012;62:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weiskopf RB, Eger EI, II, Noorani M, et al. Fentanyl, esmolol, and clonidine blunt the transient cardiovascular stimulation induced by desflurane in humans. Anesthesiology 1994;81:1350–5. [DOI] [PubMed] [Google Scholar]
- [8].Picker O, Scheeren TW, Arndt JO. Inhalation anaesthetics increase heart rate by decreasing cardiac vagal activity in dogs. Br J Anaesth 2001;87:748–54. [DOI] [PubMed] [Google Scholar]
- [9].Picker O, Schwarte LA, Schindler AW, et al. Desflurane increases heart rate independent of sympathetic activity in dogs. Eur J Anaesthesiol 2003;20:945–51. [DOI] [PubMed] [Google Scholar]
- [10].Akselrod S, Gordon D, Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science 1981;213:220–2. [DOI] [PubMed] [Google Scholar]
- [11].Pagani M, Lombardi F, Guzzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 1986;59:178–93. [DOI] [PubMed] [Google Scholar]
- [12].Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol 1985;248:H151–3. [DOI] [PubMed] [Google Scholar]
- [13].Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–65. [PubMed] [Google Scholar]
- [14].Novak P, Novak V. Time/frequency mapping of the heart rate, blood pressure and respiratory signals. Med Biol Eng Comput 1993;31:103–10. [DOI] [PubMed] [Google Scholar]
- [15].Huang HH, Chan HL, Lin PL, et al. Time-frequency spectral analysis of heart rate variability during induction of general anaesthesia. Br J Anaesth 1997;79:754–8. [DOI] [PubMed] [Google Scholar]
- [16].Fernandez-Galinski S, Bermejo S, Mansilla R, et al. Comparative assessment of the effects of alfentanil, esmolol or clonidine when used as adjuvants during induction of general anaesthesia. Eur J Anaesthesiol 2004;21:476–82. [DOI] [PubMed] [Google Scholar]
- [17].Menigaux C, Guignard B, Adam F, et al. Esmolol prevents movement and attenuates the BIS response to orotracheal intubation. Br J Anaesth 2002;89:857–62. [DOI] [PubMed] [Google Scholar]
- [18].Blau WS, Kafer ER, Anderson JA. Esmolol is more effective than sodium nitroprusside in reducing blood loss during orthognathic surgery. Anesth Analg 1992;75:172–8. [DOI] [PubMed] [Google Scholar]
- [19].Lim YJ, Kim CS, Bahk JH, et al. Clinical trial of esmolol-induced controlled hypotension with or without acute normovolemic hemodilution in spinal surgery. Acta Anaesthesiol Scand 2003;47:74–8. [DOI] [PubMed] [Google Scholar]
- [20].Watts R, Thiruvenkatarajan V, Calvert M, et al. The effect of perioperative esmolol on early postoperative pain: a systematic review and meta-analysis. J Anaesthesiol Clin Pharmacol 2017;33:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yu SK, Tait G, Karkouti K, et al. The safety of perioperative esmolol: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg 2011;112:267–81. [DOI] [PubMed] [Google Scholar]
- [22].Baillard C, Goncalves P, Mangin L, et al. Use of time frequency analysis to follow transitory modulation of the cardiac autonomic system in clinical studies. Auton Neurosci 2001;90:24–8. [DOI] [PubMed] [Google Scholar]
- [23].Pereira de Souza Neto E, Custaud MA, Frutoso J, et al. Smoothed pseudo Wigner-Ville distribution as an alternative to Fourier transform in rats. Auton Neurosci 2001;87:258–67. [DOI] [PubMed] [Google Scholar]
- [24].Chan HL, Lin MA, Chao PK, et al. Correlates of the shift in heart rate variability with postures and walking by time-frequency analysis. Comput Methods Programs Biomed 2007;86:124–30. [DOI] [PubMed] [Google Scholar]
- [25].Kuo TB, Lin T, Yang CC, et al. Effect of aging on gender differences in neural control of heart rate. Am J Physiol 1999;277:H2233–9. [DOI] [PubMed] [Google Scholar]
- [26].Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996;94:2850–5. [DOI] [PubMed] [Google Scholar]
- [27].Tsuji H, Venditti FJ, Jr, Manders ES, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation 1994;90:878–83. [DOI] [PubMed] [Google Scholar]
- [28].Maida KD, Gastaldi AC, de Paula Facioli T, et al. Physical training associated with Enalapril but not to Losartan, results in better cardiovascular autonomic effects. Auton Neurosci 2017;203:33–40. [DOI] [PubMed] [Google Scholar]
- [29].Stein PK, Domitrovich PP, Huikuri HV, et al. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electrophysiol 2005;16:13–20. [DOI] [PubMed] [Google Scholar]
- [30].Pola S, Macerata A, Emdin M, et al. Estimation of the power spectral density in nonstationary cardiovascular time series: assessing the role of the time-frequency representations (TFR). IEEE Trans Biomed Eng 1996;43:46–59. [DOI] [PubMed] [Google Scholar]
- [31].Howell SJ, Wanigasekera V, Young JD, et al. Effects of propofol and thiopentone, and benzodiazepine premedication on heart rate variability measured by spectral analysis. Br J Anaesth 1995;74:168–73. [DOI] [PubMed] [Google Scholar]
- [32].Pac-Soo CK, Wang C, Ma D, et al. Vagally mediated sympathoexcitation and central depression by desflurane in rabbits. Br J Anaesth 2000;84:777–82. [DOI] [PubMed] [Google Scholar]
- [33].Cook JR, Bigger JT, Jr, Kleiger RE, et al. Effect of atenolol and diltiazem on heart period variability in normal persons. J Am Coll Cardiol 1991;17:480–4. [DOI] [PubMed] [Google Scholar]
- [34].Lai HY, Yang CC, Cheng CF, et al. Effect of esmolol on positive-pressure ventilation-induced variations of arterial pressure in anaesthetized humans. Clin Sci (Lond) 2004;107:303–8. [DOI] [PubMed] [Google Scholar]
- [35].Floras JS, Ponikowski P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur Heart J 2015;36:1974–82b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mortara A, La Rovere MT, Pinna GD, et al. Nonselective beta-adrenergic blocking agent, carvedilol, improves arterial baroflex gain and heart rate variability in patients with stable chronic heart failure. J Am Coll Cardiol 2000;36:1612–8. [DOI] [PubMed] [Google Scholar]


