Abstract
This study aimed to evaluate the efficacy of Ahmed glaucoma valve (AGV) implantation in treating neovascular glaucoma (NVG) and to analyze the factors influencing the surgical success rate.
This is a retrospective review of 40 eyes of 40 NVG patients who underwent AGV implantation at Xiangya Hospital of Central South University, China, between January 2014 and December 2016. Pre- and postoperative intraocular pressure (IOP), visual acuity, surgical success rate, medications, and complications were observed. Surgical success criteria were defined as IOP ≤21 and >6 mm Hg with or without additional medications. Kaplan–Meier survival curves and Multivariate cox regression analysis were used to examine success rates and risk factors for surgical outcomes.
The mean follow-up period was 8.88 ± 3.12 months (range: 3–17). IOP declined at each visit postoperatively and it was statistically significant (P < .001). An average of 3.55 ± 0.86 drugs was applied preoperatively, while an average of 0.64 ± 0.90 drugs was used postoperatively, with the difference being of statistical significance (P < .05). The complete surgical success rate of 3, 6, and 12 months after the operation was 85%, 75%, and 65%, respectively. Meanwhile, the qualified success rate of 3, 6, and 12 months after the operation was 85%, 80%, and 77.5%, respectively. The multivariate cox regression analysis showed that age (hazard ratio: 3.717, 7.246; 95% confidence interval: 1.149–12.048, 1.349–38.461; P = .028, .021) was influencing factors for complete success rate and qualified success rate among all NVG patients. Gender, previous operation history, primary disease, and preoperative IOP were found to be not significant.
AGV implantation is an effective and safe surgical method to treat NVG. Age is an important factor influencing the surgical success rate.
Keywords: Ahmed glaucoma valve, influence factor, neovascular glaucoma, surgical success rate
1. Introduction
Glaucoma is the top irreversible disease causing blindness in the world.[1,2] Neovascular glaucoma (NVG) is a severe secondary glaucoma that is characterized by the final manifestation of iris and chamber angle neovascularization. With the increasing morbidities of diabetes and vascular disease, NVG morbidity shows an increasing trend, which has now accounted for over 30% of refractory glaucoma.[3] Selection of treatment for NVG has become difficult for ophthalmologists in clinic. Surgical procedures for NVG, which have included endoscopic cyclophotocoagulation, cyclocryotherapy, and traditional filtering surgery, have been explored for decades. Cyclophotocoagulation and cyclocryotherapy have attained excellent efficacy in reducing intraocular pressure (IOP). However, they can lead to severe complications such as postoperative intraocular hypertension, retinal detachment, loss of vision, atrophy of the eyeball, and bulbi phthisis.[4–6] Simple trabeculectomy is associated with a surgical failure rate as high as 80%.[7,8] Even though antimetabolites such as 5-fluorouracil or mitomycin C (MMC) are used intraoperatively, the scarring of the filtering bleb after surgery also results in a low surgical success rate.[9,10]
Molteno[11] formulated the first aqueous humor drainage device using allergen-free, nontoxic, and biologically inert silica gel in 1968. Since then, some scholars have treated glaucoma drainage valve implantation as the preferred NVG surgical treatment due to its advantages, such as relatively high surgical success rate and fewer postoperative complications.[12–14] Current glaucoma drainage devices can be classified into 2 broad categories: flow-restrictive or nonflow-restrictive implants.[15] The flow-restrictive implants include Ahmed valve, Krupin slit-valve, White pump shunt, Joseph implants, while the nonflow-restrictive, or open tube drainage, implants include the Baerveldt glaucoma implant (BGI), the Molteno implant, and the Schocket implant. The Ahmed valve, which was first applied in the clinic in 1993, has gradually become the mainstream choice of NVG drainage valve implantation. The Ahmed valve is characterized by its 1-way pressure-sensitive control valve, which can restrict the drainage device to be opened only under an IOP of 8 to 14 mm Hg. This contributes to preventing early and late complications after surgery, including excessive aqueous drainage, intraocular hypotension, and shallow anterior chamber, thus enhancing the surgical success rate.[16,17] As mentioned above, the surgical success rate remains a major concern of ophthalmologists in the clinic. Thus, in this retrospective study, we collected the clinical data from NVG patients that underwent Ahmed glaucoma valve (AGV) implantation from January 2014 to December 2016 in this research. Meanwhile, the efficacy of AGV implantation in treating NVG was evaluated and analyzed, and factors affecting surgical success rate were further analyzed.
2. Methods
2.1. Clinical data
2.1.1. Data source
We retrospectively reviewed the medical records of patients with NVG who underwent AGV implantation at Xiangya Hospital of Central South University, China, between January 2014 and December 2016 (model: FP7, New World Medical Inc., Rancho Cucamonga, CA). The study conformed to the Declaration of Helsinki and its subsequent revisions, and the ethics approval was obtained from Central South University Xiangya Hospital Medical Ethics Committee.
2.1.2. Inclusion criteria
-
(1)
Patients who were diagnosed with NVG clinically. NVG diagnostic criteria: typical iris neovascularization and ectropion uveae of pupillary margin, chamber angle trabecular meshwork neovascularization and peripheral anterior synechia, increased IOP, decreased visual acuity, characteristic visual field defect, characteristic glaucomatous cup, and previous primary disease.
-
(2)
IOP maintained higher than 21 mm Hg after applying IOP-lowering medications.
-
(3)
Postoperative follow-up period of >3 months.
-
(4)
No severe systemic disease or mental disease apart from the primary disease.
2.1.3. Exclusion criteria
-
(1)
Age of <14 years.
-
(2)
Extensive conjunctival scarring.
-
(3)
Poor general condition (such as unsatisfactory blood glucose control in diabetics).
2.2. Surgical method
All surgeries were completed by the same experienced glaucoma specialist.
-
(1)
Three milliliters of 2% lidocaine + 0.75% bupivacaine (mixed at a ratio of 1:1) was applied for peribulbar anesthesia.
-
(2)
A suspension wire was made in the corneal limbus, the bulbar conjunctiva above the temple was cut open along the corneal limbus, the fascia was separated, and hemostasis was accomplished by cautery. A 4 × 4 mm sclera flap with a 50% scleral thickness was made above the temple.
-
(3)
A 0.04% mitomycin cotton patch was placed under the sclera flap near the equator for 3 to 5 min based on patient age, Tenon capsule thickness and preoperative IOP, followed by washing with a large amount of balanced salt solution (>100 mL).
-
(4)
The Ahmed valve was placed between the lateral rectus and superior rectus, and the 6-0 slide wire was fastened at 10 mm behind the corneal limbus.
-
(5)
A lateral corneal incision was made, sodium hyaluronate was injected into the anterior chamber, and an anterior chamber puncture was carried out in the corneal limbus under the sclera flap. The drainage tube was cut off and inserted 1.5 to 2 mm into the anterior chamber from the puncture site.
-
(6)
The sclera flap was sutured with 10-0 line, the drainage tube was fixed and partly ligated for 1 stitch with 8-0 absorbable line, and the conjunctival flap was sutured.
2.3. Evaluation criteria of surgical efficacy
Different from primary glaucoma, NVG patients were frequently comorbid with diseases of the ocular fundus, such as diabetic retinopathy and central retinal vein occlusion (CRVO), which have led to poor visual performance and affected examinations of the cup-disc ratio, field of view, and retinal nerve fiber layer thickness. Therefore, visual acuity, cup-disc ratio, field of view, and retinal nerve fiber layer thickness could not be treated as the criteria of surgical success. In this research, IOP served as the major indicator for evaluating surgical success or failure.
2.3.1. Evaluation of surgical success or failure
-
(1)
Complete success: IOP was 6 to 21 mm Hg without application of antiglaucoma medications postoperatively.
-
(2)
Conditional success: IOP was 6 to 21 mm Hg after local application of antiglaucoma medications postoperatively.
-
(3)
Failure: IOP maintained higher than 21 mm Hg after applying antiglaucoma medications postoperatively; AGV needed to be taken out due to all causes; a second antiglaucoma surgery should be conducted; and severe eye complications occurred, such as retinal detachment and endophthalmitis.[18]
2.3.2. Evaluation of vision change
-
(1)
Improved visual acuity: the best corrected visual acuity in the last follow-up had improved at least 1 line compared with that before surgery.
-
(2)
Stable visual acuity: the best corrected visual acuity in the last follow-up had not changed or had improved within 1 line compared with that before surgery, and the affected eye with no light perception remained with no light perception.
-
(3)
Decreased visual acuity: the best corrected visual acuity in the last follow-up had decreased over 1 line compared with that before surgery or the best corrected visual acuity had a qualitative change (such as finger number changed to hand motion, and hand motion changed to light perception).[19]
2.4. Observational indexes
Slit-lamp microscopic examination, best corrected visual acuity, preoperative and postoperative IOP (Goldmann applanation tonometer), gonioscopy, type and number of local and systemic application of antiglaucoma medications, and postoperative complications.
2.5. Follow-up period
Regular follows-ups were conducted 1 day and 1 week after surgery, as well as 3, 6, and 12 months after surgery.
2.6. Statistical methods
Statistical analysis of all data was conducted using the SPSS 20.0 statistical package, measurement data were analyzed using a t test, ranked data were analyzed by a rank-sum test, Kaplan–Meier survival-curve analysis, and the log-rank test were used to summarize cumulative probability of success and to assess the influencing factors in the univariate analysis. The following variables were assessed as influencing factors for surgical failure: age, gender, previous operation history, primary disease, and preoperative IOP. Multivariate influencing factor analysis was performed with the multivariate cox regression analysis in order to confirm the effects of the influencing factors and identify the hazard ratio (HR) for surgical failure. A P value < .05 was considered statistically significant.
3. Results
3.1. Basic data of patients
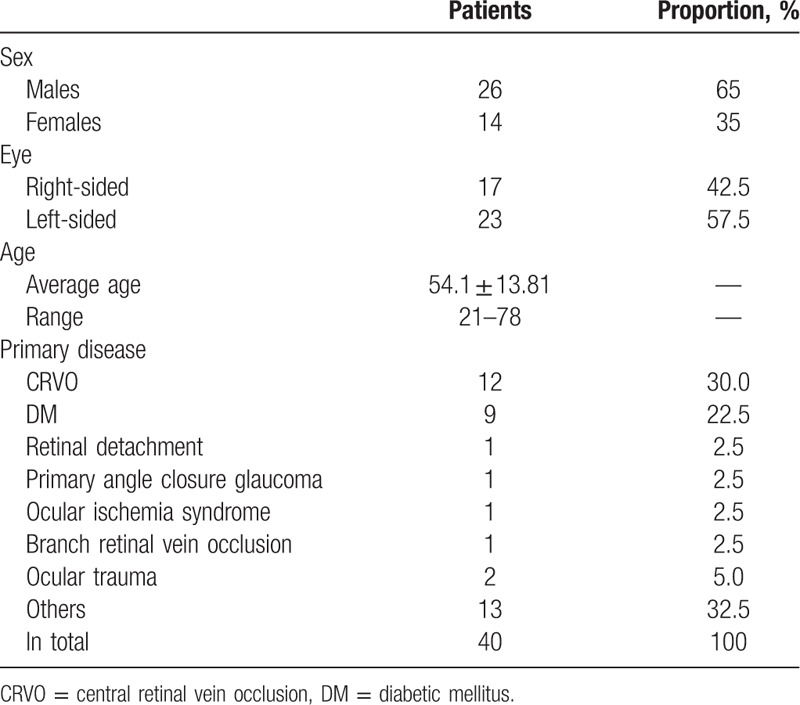
A total of 40 cases (40 eyes) were enrolled in this research, with an average age of 54.1 ± 13.81 years. Twenty-six cases were male while 14 were female, including 17 right eyes and 23 left eyes. The average postoperative follow-up period was 8.88 ± 3.12 months (range: 3–17).
All primary diseases in this research included CRVO, diabetic mellitus (DM), retinal detachment, primary angle-closure glaucoma, ocular ischemia syndrome, branch retinal vein occlusion (BRVO), and others (Table 1). Chamber angles in 35 patients were all-direction N4; 1 had bitemporal N3 while inferior N4, and N2 in remaining directions; 1 had inferior and nasal wide-angle, inferior N4, and bitemporal N3; 1 had inferior N4 while N2 in the remaining directions; and 2 had wide chamber angles.
Table 1.
Basic data of the patients enrolled.
3.2. Visual acuity of patients
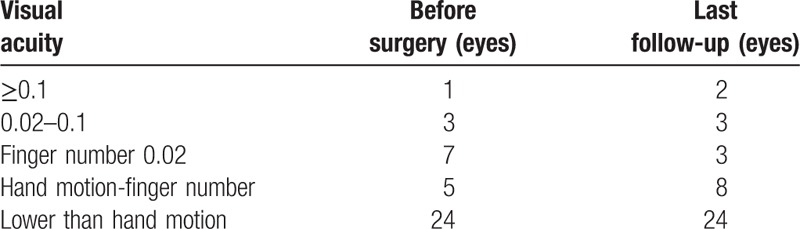
The numbers for preoperative visual acuity of ≥0.1, 0.02 to 0.1, finger number 0.02, hand motion-finger number, lower than hand motion were 1, 3, 7, 5, and 24 eyes, respectively. Upon the last follow-up, 37 eyes had improved or stable visual acuity with no light perception (92.5%), while 3 had decreased visual acuity (7.5%) (Table 2).
Table 2.
Comparison of visual acuity before and after surgery.
3.3. IOP
Preoperative IOP (baseline) of 40 patients ranged from 21 to 73 mm Hg, with an average preoperative IOP of 45.82 ± 12.90 mm Hg. IOP at each follow-up visit is listed in Table 3. IOP at each visit postoperatively had notably decreased compared with that before surgery. The differences were statistically significant (P < .001, paired t test). IOP in some patients displayed an increasing trend, which could be controlled within the normal range after applying antiglaucoma medications.
Table 3.
Intraocular pressure at baseline and follow-up.

3.4. Application of antiglaucoma medications
An average of 3.55 ± 0.86 antiglaucoma medications was applied in all patients preoperatively, while an average of 0.64 ± 0.90 medications was applied postoperatively at the last follow-up. The number of antiglaucoma medications applied before and after surgery was compared using a rank-sum test, with the difference being of statistical significance (Z = 5.417, P < .05).
3.5. Surgical success rate and influencing factor
The complete surgical success rate of 3, 6, and 12 months after the operation was 85%, 75%, and 65%, respectively. Meanwhile, the qualified success rate of 3, 6, and 12 months after the operation was 85%, 80%, and 77.5%, respectively (Fig. 1). The potential factors influencing survival time are listed in Table 4. A multivariate cox regression analysis ascertained the relative predictive ability of these factors (Tables 5 and 6).
Figure 1.
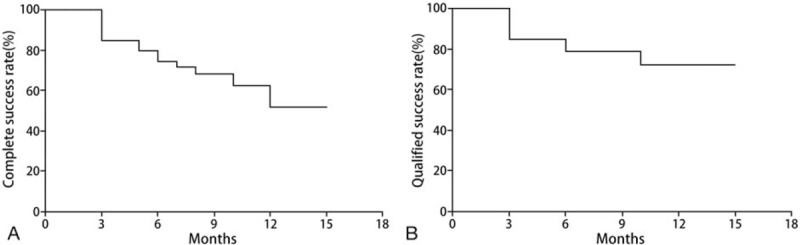
Kaplan–Meier survival curve of surgical outcomes in all 40 patients with neovascular Glaucoma who underwent Ahmed glaucoma valve implantation. (A) The complete success rate. (B) The qualified success rate.
Table 4.
Influencing factors of all 40 patients with neovascular glaucoma who underwent Ahmed glaucoma valve implantation.
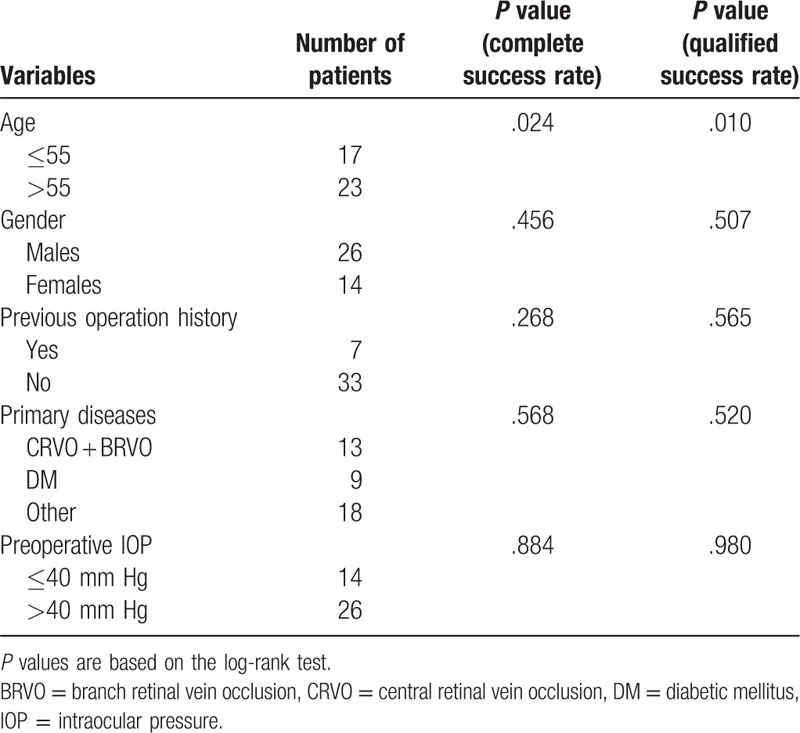
Table 5.
Multivariate cox regression analysis determining likelihood of complete success rates.
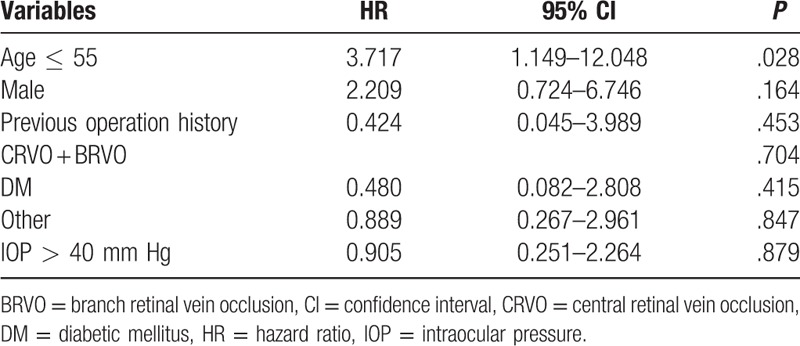
Table 6.
Multivariate cox regression analysis determining likelihood of qualified success rates.

3.5.1. Age
Patients age ≤55 years had a significant prognostic factor for surgical failure in the univariate analysis (Table 4). The Kaplan–Meier survival curves by age group are shown in Fig. 2. When using the multivariate cox regression analysis, the results showed that younger age (≤55 years) was significant risk factor for surgical failure (complete success rate: HR, 3.717; 95% confidence interval [CI]: 1.349–38.461; P = .028; qualified success rate: HR, 7.246; 95% CI: 1.349–38.461; P = .021) (Tables 5 and 6).
Figure 2.
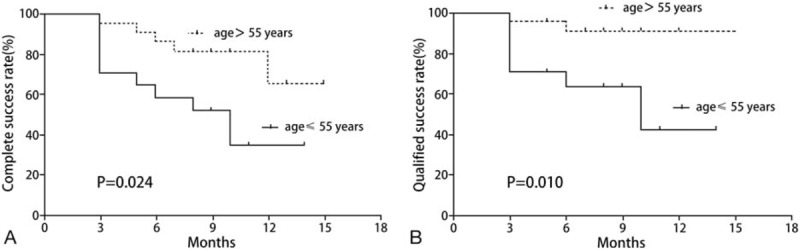
Kaplan–Meier survival curves of surgical outcomes in patients age ≤55 years (17 patients, solid line) versus >55 years (23 patients, dotted line) who underwent Ahmed glaucoma valve implantation for neovascular glaucoma. (A) The complete success rate. (B) The qualified success rate.
3.5.2. Gender
We compared the outcomes of all patients according to their genders. The complete success rate of the patients of different gender was not statistically significant (P = .456, Table 4). As to the qualified success rate, there was also no statistically significant difference in terms of gender (P = .507, Table 4).
3.5.3. Previous operation history
Among the 40 patients, 2 had a previous operation history of trabeculectomy, 2 of phacoemulsification cataract surgery, 1 of phacotrabeculectomy, 1 of secondary intraocular lens implantation, and 1 of external approach retinal detachment reduction over 10 years ago. The complete success rates between patients with and without previous operation history were compared, and the difference was not statistically significant (P = .268, Table 4). The qualified success rates were not statistically significant, either (P = .565, Table 4).
3.5.4. Primary diseases
We compare the differences of complete success rates and qualified success rates by dividing patients into 3 groups: CRVO + BRVO group; DM group; and other diseases group, the differences showed no statistical significance (P = .568 and .520, Table 4).
3.5.5. Preoperative IOP
The complete success rates and qualified success rates between patients with a preoperative IOP of ≤40 mm Hg and those of >40 mm Hg were compared in this research, and the difference was not statistically significant (P = .884 and .980, Table 4).
3.6. Postoperative complications and treatment
Table 7 lists the postoperative complications. The most common postoperative complication was cataract progression, which could be seen in 12 eyes (30.0%). One eye developed drainage tube obstruction (2.5%), which had improved after obstruction removal by Nd:YAG laser. Two had fiber-wrapped tissues in the periphery (5.0%). One had vitreous hemorrhage and received vitrectomy (2.5%). Complications such as drainage tube displacement, drainage tube exposure, drainage tube erosion, retinal detachment, and corneal endothelial decompensation were not seen for a long time after surgery.
Table 7.
Postoperative complications.

4. Discussion
Research on pathogenesis, treatment, and prognosis for NVG has greatly developed in recent years.[20] Over 40 diseases have been reported to induce NVG,[21] among which diabetes, retinal vein obstruction (central or branch, ischemic), central retinal artery obstruction, and ocular ischemia syndrome are the most common. In this research, 13 and 9 patients have primary diseases of retinal vein obstruction and diabetes, respectively, accounting for 32.5% and 22.5% of the total proportion, respectively. This is consistent with previous literature. In this research, 35 patients have the chamber angles of all-direction N4 and only 2 have all-direction wide chamber angles. Most patients have entered stage III, namely, the angle-closure glaucoma stage, based on the current NVG stage.
Research has indicated that the AGV has fewer postoperative complications than other drainage devices, which can better protect visual performance.[22,23] MMC is an antibiotic produced by fermentation of Streptomyces caespitosus, which can inhibit fibroblast proliferation and prevent scar hypertrophy through interfering with DNA at the proliferative phase, thus maintaining the smoothness of the filtering channel.[24] Chen et al[25,26] were the first to report applying MMC in trabeculectomy in 1981. Since then, antimetabolites have been extensively applied in surgery. MMC is also used intraoperatively in this research.
In NVG, as the trabecular meshwork is damaged permanently, the effect of medical treatment is poor. Nevertheless, medical treatment can play a role in protecting the optic nerve from damage, decreasing associated pain, and possibly improving vision secondary to IOP-dependent corneal edema before more definitive treatment can take effect. Recently, studies by Luo and Lai have shown the promising effect of gelatin-g-poly(N-isopropylacrylamide) carriers in antiglaucoma drug delivery,[27,28] giving ophthalmologists more options in treating NVG before surgery. Surgical intervention is indicated when medical therapy is inadequate to control IOP, particularly if synechial angle closure from neovascularization of the angle has occurred. The most important goal of antiglaucoma surgery is to construct target IOP and prevent intraocular hypertension from further damaging visual function, thus leading to irreversible visual impairment. In this research, 2 eyes had an IOP of >21 mm Hg 1 day after surgery. One of them was related to contact of the drainage tube with the corneal endothelium, and the IOP decreased after adjusting the position of the drainage tube. The other one may be related to long-term application of IOP-lowering medications, continuous intraocular hypertension, severe preoperative anterior chamber inflammatory response, and slow postoperative recovery of the anterior chamber. The IOP returned to a normal level 1 week after surgery after active symptomatic treatment. IOP 1 week after surgery and in the last follow-up was remarkably lower than that before surgery, with an average postoperative IOP of <21 mm Hg. The difference in IOP before and after surgery is statistically significant. Meanwhile, 2 to 5 types of drugs were used in the enrolled patients before surgery, with an average of 3.55 types. In the last follow-up after surgery, 2 patients had poorly controlled IOP after applying 3 antiglaucoma medications, which were well under control after cyclocryotherapy. A total of 2 and 5 patients had an IOP of <21 mm Hg after applying 2 and 1 antiglaucoma medications, respectively. Clearly, AGV implantation can safely and effectively decrease IOP. Moreover, most patients have well-controlled IOP without applying any medication.
In previous literature reports, Yalvac et al[29] reported that the 1-year surgical success rate of the 38 NVG eyes receiving Ahmed valve implantation was 63.3%. Bai et al[19] reported that the complete surgical success rate of the 36 patients undergoing Ahmed valve implantation was 80.6% after 18 months of follow-up, while the conditional surgical success rate was 91.7%. Netland[15] carried out research with a sample size of 38, and they reported a 1-year surgical success rate of 73.1% and a 3-year surgical success rate of 20.6%. In the research by Shen et al,[18] the surgical success rate 3 months after Ahmed valve implantation was 100%, which was then 90%, 85%, and 70% after 6, 9, and 12 months, respectively. Meanwhile, the 15-, 18-, and 24-month surgical success rates were 70%, 60%, and 60%, respectively. The conditional surgical success rate in this research is 77.5% upon the last follow-up. Thus, our research results are demonstrably consistent with these literatures. These rates are related to the operation of the surgeon; in addition, it may also be related to intraoperative combination with MMC to inhibit fibroblast proliferation, which conforms to the results from Perkins et al[30] and Alvarado et al.[31]
As is indicated by our results, the surgical success rate in patients aged below 55 years is lower than that in those aged over 55 years. Mermoud et al[32] discovered in their research that NVG patients aged over 55 years had a higher Molteno surgical success rate than those aged below 55 years. Sidoti et al[33] performed Baerveldt drainage valve implantation in 36 NVG patients and discovered that being young was a risk factor of surgical failure. Tsai et al[34] and Takihara et al[35] carried out filtering surgery on NVG patients and came to a similar conclusion. Therefore, we speculate that age is an important factor influencing surgical success rate. This may be linked with the stronger wound healing response in younger patients, making them more likely to develop fiber-wrapped in the periphery of the drainage disc, as well as more aggressive illness when the younger patients develop NVG.
Our results showed that the surgical success rates between male and female groups was not statistically significant, which is in agreement with a previous study.[29,35,36]
Seven patients in this research have had previous ophthalmologic operation surgery, but the results suggest that the difference in the surgical success rate between patients with and without operation history is not statistically significant. Ohnishi et al[37] and Blankenship[38] discovered in their research that some diabetics developed NVG after cataract surgery. The human lens has a barrier function to prevent angiogenic factors from dispersing into the anterior chamber. An increased number of angiogenic factors disperse to the anterior chamber after cataract surgery, which leads to rapid genesis and development of NVG and may reduce the surgical effects on NVG. However, 2 cases in this research who had once received cataract surgery have a good prognosis after AGV implantation. This finding is contrary to the previous conclusion, which may be related to the rapidly changing cataract surgery technology. It is generally considered that conjunctival scarring after filtering surgery may interfere with the formation of a functional filtering bleb. Honjo et al[39] considered that intraoperative combination with MMC could improve surgical efficacy. In addition, 3 patients in this research have a history of antiglaucoma surgery; 2 of them had good IOP after surgery, while 1 had an IOP of higher than 21 mm Hg after applying antiglaucoma medications, which may be related to the conjunctival scarring after the previous operation.
Different opinions regarding whether primary disease in patients will affect surgical effects are noted in the present study. Every et al[40] discovered in their research that the surgical success rate between CRVO patients receiving Molteno drainage valve implantation and those without CRVO was comparable. Mermoud et al[32] suggested that surgical effects in diabetics were superior to those in CRVO patients. Hayreh[3] proposed similar opinions, and he considered that patients with CRVO as the primary disease generally had more severe illness than diabetics and NVG patients, thus affecting the surgical prognosis. In this research, the difference in the surgical success rates among CRVO patients, diabetics and patients with other diseases is not statistically significant.
We find in the present study that the difference in surgical success rates between patients with a preoperative IOP of ≤40 mm Hg and those of >40 mm Hg is not statistically significant. In the research by Yalvac et al,[29] patients with a preoperative IOP of higher than 35 mm Hg had comparable surgical prognosis to those with a preoperative IOP of lower than 35 mm Hg. Takihara et al[35] pointed out 40 mm Hg as the standard for grouping, and the results also showed no statistically significant difference. These results were consistent with ours.
In our clinical follow-up, AGV implantation is associated with certain complications, with cataract progression being common. Fiber-wrapped in the periphery of the drainage disc is the most common complication after surgery, which is also an important reason leading to surgical failure. Sarkisan[16] discovered in their research that nonrestrictive glaucoma drainage valves, such as Molteno, resulted in greatly fluctuating IOP after surgery, which resulted in a large amount of aqueous humor flowing to the periphery of the drainage disc within a short term. In this research, only 2 cases developed fiber-wrapped in the periphery of the drainage disc, with the occurrence rate of 5%, which is lower than the 15% reported in literature.[41,42]
Our study has several limitations that should be taken into account. First, our study is a nonrandomized retrospective study; second, the scale of sample size in our study was small; third, the average follow-up period was relatively short, despite efforts to contact patients directly or through their referring physicians; what's more, a lack of a control group was also a limitation in our study. As we mentioned above, NVG is blinding, intractable disease, which is difficult to manage. To date, plenty of researches have reported that as the follow-up time went on, the cumulative probability of failure increased. Similarly, NVG has a poor response to other Glaucoma drainage implant surgery except AGV, with variable success rates of 22% to 78%.[17] Thus, in our study, although the success rate can maintain at a relatively high level, due to the short follow-up period, we cannot come to a conclusion that the AGV implantation can achieve good effect in the long term.
At present, the AGV and BGI are the 2 most widely used aqueous shunts in the world.[43] So far, few literatures explore the clinical effect of these 2 aqueous shunts in NVG, thus, it would be of interest to compare the clinical outcomes between AGV and BGI in treating NVG. Future large-volume well-designed Randomized Controlled Trial (RCT)s with extensive follow-up are awaited to confirm and update the findings of this analysis.
Studies have shown that vascular endothelial growth factor (VEGF) is important in ocular abnormalities characterized by neovascularization, including NVG. Thus, anti-VEGF treatment has led to a new era in the management of NVG. Recently, numerous studies have compared clinical outcomes of AGV implantation with intravitreal bevacizumab injection (IVB) with AGV implantation without IVB in the management of NVG.[44–48] The success rates have been variable. Some researchers found that the pretreating with IVB can produce better results, whereas other studies found that the success rates of the 2 procedures were comparable. However, a meta-analysis article has shown that AGV implantation with the IVB pretreatment procedure has a lower hyphema complication incidence compared with the AGV implantation without the IVB procedure for NVG.[49] Therefore, in our future research, we will continue clinical data of NVG patients, according to different primary diseases, stage of the disease, and treatments, to conduct prospective, randomized, larger sample size, and extensive follow-up clinical trials in order to find out a better procedure for NVG.
5. Conclusion
AGV implantation is associated with a simple surgical procedure, little intraocular operation, a high surgical success rate, and a low complication rate. Therefore, it is worthy of being promoted in the clinic. Age is an important factor influencing the surgical success rate.
Footnotes
Abbreviations: AGV = Ahmed glaucoma valve, BGI = Baerveldt glaucoma implant, BRVO = branch retinal vein occlusion, CI = confidence interval, CRVO = central retinal vein occlusion, DM = diabetic mellitus, HR = hazard ratio, IOP = intraocular pressure, IVB = intravitreal bevacizumab injection, MMC = mitomycin C, NVG = neovascular glaucoma, VEGF = vascular endothelial growth factor.
YH and YT have contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (No 81670858) and the Clinical Research Fund of Central South University, Changsha, Hunan, China (No 2013L07).
The authors report no conflicts of interest.
References
- [1].Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jonas JB, Yang D, Wang N. Effect of intraocular pressure on glaucomatous damage to the optic nerve. Ophthalmologe 2014;111:181–8. [DOI] [PubMed] [Google Scholar]
- [3].Hayreh SS. Neovascular glaucoma. Prog Retin Eye Res 2007;26:470–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yap-Veloso MI, Simmons RB, Echelman DA, et al. Intraocular pressure control after contact transscleral diode cyclophotocoagulation in eyes with intractable glaucoma. J Glaucoma 1998;7:319–28. [PubMed] [Google Scholar]
- [5].Shen SY, Lai JS, Lam DS. Necrotizing scleritis following diode laser transscleral cyclophotocoagulation. Ophthalmic Surg Lasers Imaging 2004;35:251–3. [PubMed] [Google Scholar]
- [6].Delgado MF, Dickens CJ, Iwach AG, et al. Long-term results of noncontact neodymium:yttrium-aluminum-garnet cyclophotocoagulation in neovascular glaucoma. Ophthalmology 2003;110:895–9. [DOI] [PubMed] [Google Scholar]
- [7].Allen RC, Bellows AR, Hutchinson BT, et al. Filtration surgery in the treatment of neovascular glaucoma. Ophthalmology 1982;89:1181–7. [DOI] [PubMed] [Google Scholar]
- [8].Mietz H, Raschka B, Krieglstein GK. Risk factors for failures of trabeculectomies performed without antimetabolites. Br J Ophthalmol 1999;83:814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Katz GJ, Higginbotham EJ, Lichter PR, et al. Mitomycin C versus 5-fluorouracil in high-risk glaucoma filtering surgery. Extended follow-up. Ophthalmology 1995;102:1263–9. [DOI] [PubMed] [Google Scholar]
- [10].Hyung SM, Kim SK. Mid-term effects of trabeculectomy with mitomycin C in neovascular glaucoma patients. Korean J Ophthalmol 2001;15:98–106. [DOI] [PubMed] [Google Scholar]
- [11].Molteno AC. New implanted for drainage in glaucoma: clinical trials. Br J Ophthalmol 1969;53:606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ortiz JM. Molteno implants. Arch Ophthalmol 1991;109:1417–20. [DOI] [PubMed] [Google Scholar]
- [13].Minckler DS, Vedula SS, Li TJ, et al. Aqueous shunts for glaucoma. Cochrane Database Syst Rev 2006;19:CD004918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bernardino CR, Chang EL, Hatton MP, et al. Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol 2005;50:48–60. [DOI] [PubMed] [Google Scholar]
- [15].Netland PA. The Ahmed glaucoma valve in neovascular glaucoma (an AOS thesis). Trans Am Ophthalmol Soc 2009;107:325–42. [PMC free article] [PubMed] [Google Scholar]
- [16].Sarkisan SR., Jr Tube shunt complications and their prevention. Curr Opin Ophthalmol 2009;20:126–30. [DOI] [PubMed] [Google Scholar]
- [17].Schwartz KS, Lee RK, Gedde SJ. Glaucoma drainage implants: a critical comparison of types. Curr Opin Ophthalmol 2006;17:181–9. [DOI] [PubMed] [Google Scholar]
- [18].Shen CC, Salim S, Du H, et al. Trabeculectomy versus Ahmed glaucoma valve implantation in neovascular glaucoma. Clin Ophthalmol 2011;5:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bai YJ, Wang M, Li YQ, et al. Clinical efficacy and safety of FP-7 Ahmed glaucoma valve implantation in neurovascular glaucoma patients. Zhonghua Yan Ke Za Zhi 2011;47:893–7. [PubMed] [Google Scholar]
- [20].Sivak-Callcott JA, O’Day DM, Gass DM, et al. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology 2001;108:1767–76. [DOI] [PubMed] [Google Scholar]
- [21].Zhang XL. Current approaches in neovascular glaucoma. Zhonghua Yan Ke Za Zhi 2012;48:488–91. [PubMed] [Google Scholar]
- [22].Hong CH, Arosemena A, Zurakowski D, et al. Comparison of the Ahmed glaucoma valve, the Krupin eye valve with disk, and the double-plate molten implant. J Glaucoma 2002;11:347–53. [DOI] [PubMed] [Google Scholar]
- [23].Tsai JC, Johndon CC, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma: a single-surgeon comparison of outcome. Ophthalmology 2003;110:1814–21. [DOI] [PubMed] [Google Scholar]
- [24].Shields MB, Scroggs MW, Sloop CM, et al. Clinical and histopathologic observations concerning hypotony after trabeculactomy with adjunctive mitomycin C. Am J Ophthalmol 1993;116:673–83. [DOI] [PubMed] [Google Scholar]
- [25].Chen CW, Huang HT, Sheu MM. Enhancement of IOP control effect of trabeculectomy by local application of anticancer drug. Acta Ophthalmol Scand 1986;25:1487–91. [Google Scholar]
- [26].Chen CW, Huang HT, Bair JS, et al. Trabeculectomy with stimultaneous topical application of mitomycin C in refractory glaucoma. J Ocul Pharmacol 1990;6:175–82. [DOI] [PubMed] [Google Scholar]
- [27].Luo LJ, Lai JY. The role of alkyl chain length of monothiol-terminated alkyl carboxylic acid in the synthesis, characterization, and application of gelatin-g-poly(N-isopropylacrylamide) carriers for antiglaucoma drug delivery. Acta Biomater 2017;49:344–57. [DOI] [PubMed] [Google Scholar]
- [28].Lai JY, Luo LJ. Chitosan-g-poly(N-isopropylacrylamide) copolymers as delivery carriers for intracameral pilocarpine administration. Eur J Pharm Biopharm 2017;113:140–8. [DOI] [PubMed] [Google Scholar]
- [29].Yalvac IS, Eksioglu U, Satana B, et al. Long-term results of Ahmed glaucoma valve and Molteno implant in neovascular glaucoma. Eye 2007;21:65–70. [DOI] [PubMed] [Google Scholar]
- [30].Perkins TW, Cardakli UF, Eisele JR, et al. Adjunctive mitomycin C in Molteno implant surgery. Ophthalmology 1995;102:91–7. [DOI] [PubMed] [Google Scholar]
- [31].Alvarado JA, Hollander DA, Juster RP, et al. Ahmed valve implantation with adjunctive mitomycin C and 5-fluorouracil: long-term outcomes. Am J Ophthalmol 2008;146:276–84. [DOI] [PubMed] [Google Scholar]
- [32].Mermoud A, Salmon JF, Alexander P, et al. Molteno tube implantation for neovascular glaucoma. Long-term results and factors influencing the outcome. Ophthalmology 1993;100:897–902. [PubMed] [Google Scholar]
- [33].Sidoti PA, Dunphy TR, Baerveldt G, et al. Experience with the Baerveldt glaucoma implant in treating neovascular glaucoma. Ophthalmology 1995;102:1107–18. [DOI] [PubMed] [Google Scholar]
- [34].Tsai JC, Feuer WJ, Parrish RJ, et al. 5-Fluorouracil filtering surgery and neovascular glaucoma. Long-term follow-up of the original pilot study. Ophthalmology 1995;102:887–93. [DOI] [PubMed] [Google Scholar]
- [35].Takihara Y, Inatani M, Fukushima M, et al. Trabeculectomy with mitomycin C for neovascular glaucoma: prognostic factors for surgical failure. Am J Ophthalmol 2009;147:912–8. [DOI] [PubMed] [Google Scholar]
- [36].Higashide T, Ohkubo S, Sugiyama K. Long-term outcomes and prognostic factors of trabeculectomy following intraocular bevacizumab injection for neovascular glaucoma. PLoS ONE 2015;10:e0135766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ohnishi Y, Ishibashi T, Sagawa T, et al. Fluorescein gonioangiography in diabetic neovascularization. Graefes Arch Clin Exp Ophthalmol 1994;232:199–204. [DOI] [PubMed] [Google Scholar]
- [38].Blankenship GW. The lens influence on diabetic vitrectomy results. Report of a prospective randomized study. Arch Ophthalmol 1980;98:2196–8. [DOI] [PubMed] [Google Scholar]
- [39].Honjo M, Tanihara H, Inatani M, et al. Mitomycin C trabeculectomy in eyes with cicatricial conjunctiva. Am J Ophthalmol 1998;126:823–4. [DOI] [PubMed] [Google Scholar]
- [40].Every SG, Molteno ACB, Bevin TH, et al. Long-term results of Molteno implant insertion in cases of neovascular glaucoma. Arch Ophthalmol 2006;124:355–60. [DOI] [PubMed] [Google Scholar]
- [41].Moras Y, Donaldson CE, Kim YM, et al. The Ahmed drainage implant in the treatment of pediatric glaucoma. Am J Ophthalmol 2003;135:821–9. [DOI] [PubMed] [Google Scholar]
- [42].Huang MC, Netland PA, Coleman AL, et al. Intermediate-term clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol 1999;127:27–33. [DOI] [PubMed] [Google Scholar]
- [43].Budenz DL, Barton K, Feuer WJ, et al. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology 2011;118:443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sevim MS, Buttanri IB, Kugu S, et al. Effect of intravitreal bevacizumab injection before Ahmed glaucoma valve implantation in neovascular glaucoma. Ophthalmologica 2013;229:94–100. [DOI] [PubMed] [Google Scholar]
- [45].Mahdy RA, Nada WM, Fawzy KM, et al. Efficacy of intravitreal bevacizumab with panretinal photocoagulation followed by Ahmed valve implantation in neovascular glaucoma. J Glaucoma 2013;22:768–72. [DOI] [PubMed] [Google Scholar]
- [46].Kang JY, Nam KY, Lee SJ, et al. The effect of intravitreal bevacizumab injection before Ahmed valve implantation in patients with neovascular glaucoma. Int Ophthalmol 2014;34:793–9. [DOI] [PubMed] [Google Scholar]
- [47].Ma KT, Yang JY, Kim JH, et al. Surgical results of Ahmed valve implantation with intraoperative bevacizumab injection in patients with neovascular glaucoma. J Glaucoma 2012;21:331–6. [DOI] [PubMed] [Google Scholar]
- [48].Arcieri ES, Paula JS, Jorge R, et al. Efficacy and safety of intravitreal bevacizumab in eyes with neovascular glaucoma undergoing Ahmed glaucoma valve implantation: 2-year follow-up. Acta Ophthalmol 2015;93:e1–6. [DOI] [PubMed] [Google Scholar]
- [49].Zhou M, Xu X, Zhang X, et al. Clinical outcomes of Ahmed glaucoma valve implantation with or without intravitreal bevacizumab pretreatment for neovascular glaucoma: a systematic review and meta-analysis. J Glaucoma 2016;25:551–7. [DOI] [PubMed] [Google Scholar]


