Abstract
Leydig cell testosterone (T) production is reduced with age, resulting in reduced serum T levels (hypogonadism). A number of cellular changes have been identified in the steroidogenic pathway of aged Leydig cells that are associated with reduced T formation, including reductions in luteinizing hormone (LH)-stimulated cAMP production, the cholesterol transport proteins steroidogenic acute regulatory (STAR) protein and translocator protein (TSPO), and downstream steroidogenic enzymes of the mitochondria and smooth endoplasmic reticulum. Many of the changes in steroid formation that characterize aged Leydig cells can be elicited by the experimental alteration of the redox environment of young cells, suggesting that changes in the intracellular redox balance may cause reduced T production. Hypogonadism is estimated to affect about 5 million American men, including both aged and young. This condition has been linked to mood changes, worsening cognition, fatigue, depression, decreased lean body mass, reduced bone mineral density, increased visceral fat, metabolic syndrome, decreased libido, and sexual dysfunction. Exogenous T administration is now used widely to elevate serum T levels in hypogonadal men and thus to treat symptoms of hypogonadism. However, recent evidence suggests that men who take exogenous T may face increased risk of stroke, heart attack, and prostate tumorigenesis. Moreover, it is well established that administered T can have suppressive effects on LH, resulting in lower Leydig cell T production, reduced intratesticular T concentration, and reduced spermatogenesis. This makes exogenous T administration inappropriate for men who wish to father children. There are promising new approaches to increase serum T by directly stimulating Leydig cell T production rather than by exogenous T therapy, thus potentially avoiding some of its negative consequences.
Keywords: aging, testosterone, hypogonadism, TSPO
Leydig Cell Steroidogenic Function
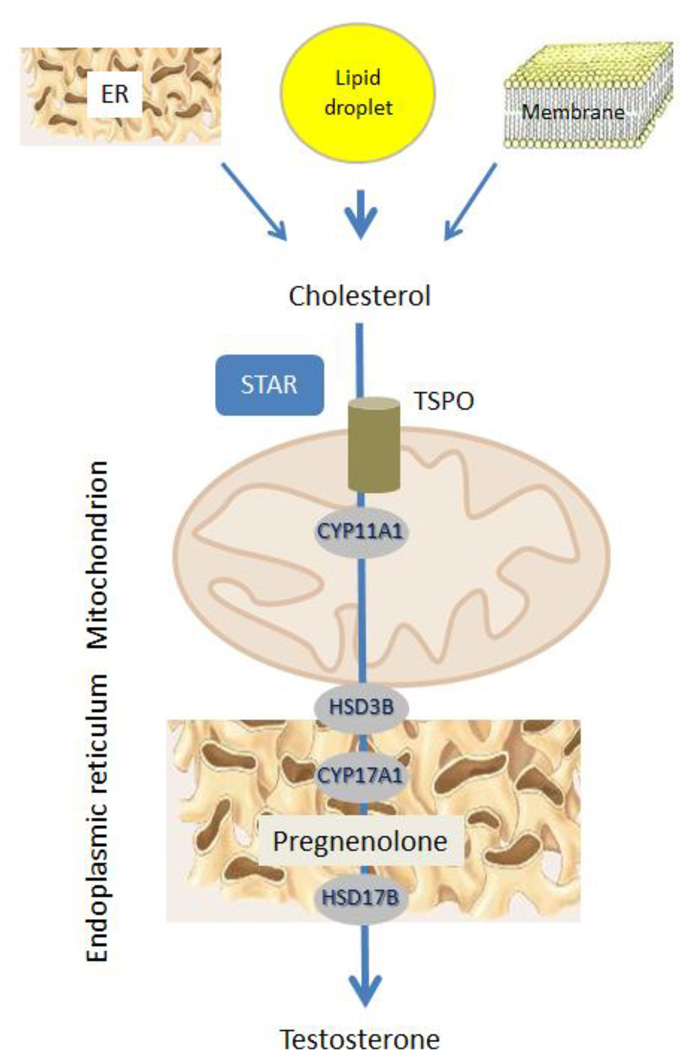
Leydig cells are the testicular cells responsible for testosterone (T) biosynthesis. Adult Leydig cells synthesize T in response to luteinizing hormone (LH). LH binds to and activates G protein-coupled receptors, resulting in the activation of adenylyl cyclase and thus increased cAMP formation. The acute stimulation of Leydig cells by LH results in cholesterol transfer from intracellular stores (mainly lipid droplets but also endoplasmic reticulum (ER) and plasma membrane) into the mitochondria (Fig. 1). This is the rate-limiting step in steroid biosynthesis, and is followed by the conversion of cholesterol to pregnenolone by the C27 cholesterol side-chain cleavage cytochrome P450 enzyme (CYP11A1), located on the matrix side of the inner mitochondrial membrane. Pregnenolone then undergoes enzymatic transformation in the smooth endoplasmic reticulum to produce T (Miller and Bose, 2011; Rone et al, 2009).
Figure 1. Leydig cell steroid biosynthesis.
In response to LH, cAMP stimulates the transport of cholesterol into mitochondria. Cholesterol is delivered by STAR and TSPO to the inner mitochondrial membrane where it is cleaved into pregnenolone, used for the biosynthesis of testosterone.
Leydig Cell Aging
In the aging male, serum T levels decline as a consequence of the reduced ability of Leydig cells to produce T (Chen et al., 2002). In studies conducted using the aging Brown Norway rat, reduced T production without loss of Leydig cells was found to be associated with age-related reductions in serum T levels (Wang et al, 1993; Chen et al, 1994, 1996). Multiple defects have been identified in the steroidogenic pathway of aged Leydig cells, including reductions in each of LH-stimulated cAMP production, the cholesterol transport inducing protein steroidogenesis acute regulatory protein (STAR) and the outer mitochondrial membrane cholesterol-binding translocator protein (18-kDa; TSPO), and downstream steroidogenic enzymes of the mitochondria (CYP11A1, HSD3B) and smooth endoplasmic reticulum (HSD3B, CYP17A1, HSD17B) (Luo et al, 1996, 2001, 2005; Culty et al, 2002). Other agerelated changes have been suggested to indirectly impact steroidogenesis, including decreased Leydig cell cholesterol synthesis and mobilization (Liao et al, 1993), decreased autophagic activity of the cells (Li et al, 2011), increased nitric oxide (NO) and cGMP signaling (Sokanovic et al, 2013), and increased cellular lipofuscin accumulation (Wang et al, 2012).
Although the mechanism by which these age-related defects occur remains uncertain, there is evidence that changes in the redox balance within the Leydig cells are involved. For example, numerous studies have suggested that imbalance of prooxidants and antioxidants within cells can lead to DNA, protein and/or lipid damage, and thus to functional changes (Finkel and Holbrook, 2000; Drew and Leeuwenburgh, 2002). Cells produce reactive oxygen species (ROS) during normal metabolism. In steroidogenic cells, ROS production would be expected to be particularly high because in addition to the mitochondrial electron transport chain, steroid hydroxylations by the cytochrome P450 enzymes produce ROS (Hornsby, 1989; Peltola et al, 1996). This may have significance for Leydig cell function because of the detrimental effects that ROS can have on critical components of the steroidogenic pathway (Quinn and Payne, 1984, 1985; Georgiou et al, 1987; Diemer et al, 2003). As Leydig cells age, the antioxidant defense molecules superoxide dismutase-1 and -2, glutathione peroxidase, and glutathione (GSH) are significantly reduced (Cao et al, 2004; Luo et al, 2006). Additionally, the superoxide content of aging Leydig cells is significantly increased compared to young Leydig cells (Chen et al, 2001). Lipid peroxidation also increases, perhaps as a consequence of changes in the redox environment (Cao et al, 2004). Increased lipid peroxidation also occurs in aged adrenal cells (Azhar et al, 1995). These results, though correlative, suggest that alteration in the redox environment of aged Leydig cells may be involved in the reduced T formation that characterizes these cells.
Does alteration of the redox environment cause age-related reductions in Leydig cell steroidogenesis? This has been addressed in part by manipulating the intracellular antioxidant environment in young Leydig cells so as to create the intracellular environment that is characteristic of old cells, and then determining whether, acutely or over time, doing so results in decreased T production (Chen et al., 2008). Reduced glutathione, the most abundant intracellular small molecule thiol present in mammalian cells, serves as a potent intracellular antioxidant (Fang et al, 2002) and is particularly abundant in Leydig cells. The intracellular biosynthesis of GSH is mediated by two ATP-dependent enzymes, γ-glutamylcysteine synthetase (the rate-limiting enzyme) and glutathione synthetase (Anderson, 1998; Griffith, 1999). GSH decreases significantly as Leydig cells age (Cao et al, 2004; Luo et al, 2006;). The Leydig cell is not novel in this regard; age-related reductions in GSH occur in a number of systems, including human serum (Jones et al, 2002), rat liver, and rat brain (Liu, 2002; Sandhu and Kaur, 2002; Liu et al, 2004;).
Given the abundance of GSH in Leydig cells, we hypothesized that the experimental depletion of GSH would result in reduced steroidogenesis. Buthionine sulfoximine (BSO), a specific γ-glutamylcysteine synthetase inhibitor, can block the rate-limiting step of GSH biosynthesis, and by doing so deplete the intracellular GSH pool in both cultured cells and whole animals (Griffith et al, 1979; Anderson, 1998). Experimental depletion of GSH in isolated Leydig cells was found to reduce T production to levels reminiscent of T production by Leydig cells of aged rats (Chen et al, 2008). The antioxidants vitamin E, N-tert-butyl-α-phenylnitrone and Trolox countered BSO’s effect on steroidogenesis. In vivo studies also were conducted. Young (4 month-old) rats were injected with BSO twice a day for 7 days, after which Leydig cells were isolated and analyzed in vitro (Chen et al, 2008). As with cells treated in vitro, BSO treatment in vivo resulted in significant reductions in Leydig cell GSH content and the ability of the Leydig cells to produce T. Reminiscent of aging, decreases were seen in LH-stimulated cAMP production, STAR, CYP11A1, HSD3B and CYP17A1. The results of these studies support the conclusion that alteration in the oxidant/antioxidant environment plays a causative role in the reduced ability of aging Leydig cells to produce T. Although the molecular mechanisms by which an altered redox environment acts to reduce Leydig cell function remain unclear, there is evidence to suggest that changes in membrane fluidity in response to oxidative damage of membrane lipids may be involved (Kolena et al, 1986; Wu et al, 1993; Kodaman et al, 1994; Vega et al, 1995).
The regulatory mechanisms involved in the generation of superoxide and other ROS in Leydig cells are unclear. T is produced in response to stimulation by LH. However, the long-term in vivo suppression of LH, from middle age through old age, was found to prevent the reductions in T formation that characterize aged rat Leydig cells (Chen and Zirkin, 1999), suggesting that LH stimulation of steroid formation, over long periods of time, may play a role in the diminished steroid production that characterizes aging. These observations led us to ask whether and how LH might exert negative effects on Leydig cell function. Using microarray analysis, significant increases were seen in the expression of genes related to stress response in aged cells, including genes whose protein products are involved in the induction or activation of Nrf2, a master regulator of the cellular antioxidant response (Beattie et al, 2013). Additionally, incubation of Leydig cells with LH was found to result in significantly increased generation of ROS and also in increased DNA damage, and these effects were suppressed by incubating the cells with an antioxidant (Beattie et al., 2013).
T replacement therapy for the hypogonadal male
Reduced serum T, or hypogonadism, is estimated to affect about 5 million American men, including both aging and young (Araujo et al., 2007). Hypogonadism is common in aging men, with 20–50% of men over age 60 reported to have serum T levels significantly below those of young men (Harman et al, 2001; Mohr et al, 2005; Araujo et al, 2007; Bhasin and Basaria, 2011; Surampudi et al, 2012). Primary hypogonadism also occurs in many infertile younger men. With regard to the latter, approximately 15% of couples seek infertility-related medical appointments, with male factor effects contributing to 40%–50% of these cases (Kim and Schlegel, 2008; Hwang et al, 2011). About 30% of infertile men are diagnosed as idiopathic, among whom about 50% have primary hypogonadism (Schlegel, 2009; Belchetz et al, 2010). Whether in aging or in young men, reduced serum T is associated with a number of metabolic and quality-of-life changes, including decreased lean body mass, bone mineral density, muscle mass and strength, adiposity, cardiovascular disorders, decreased libido and sexual function, altered mood and fatigue (Matsumoto, 2002; Araujo et al, 2007; Surampudi et al, 2012; Wu et al, 2010).
Although age-related changes in the amplitude of LH pulses have been reported, decline in serum T levels in aging men typically is not a consequence of changes in LH, the levels of which remain unchanged or increased in most men, but rather to decreased responsiveness of the Leydig cells to LH (Bonavera et al, 1997; 1998). Consequently, attempts to increase Leydig cell T production, and thus serum T levels, through LH stimulation typically are not effective in aging men unless the men have clearly identifiable deficiencies in central stimulation, such as is the case of men with hypogonadotropic hypogonadism (Bobjer et al, 2012) or Kallman’s syndrome (Hamada et al, 2012). Similarly, although in some aging men T production can be affected through inhibiting negative feedback effects on the hypothalamic-pituitary-gonadal (HPG) axis by the administration of selective estrogen receptor modulators (SERMS) such as clomiphene or tamoxifen, or aromatase inhibitors (Page, 2011), these approach typically do not increase T production in hypogonadal men with normal or high LH levels. Thus, methods to increase T production through LH stimulation often are not effective clinically in the aging population.
Increasingly, T is prescribed to men diagnosed with low circulating T levels. The primary objective of T replacement therapy is to raise serum T levels into the eugonadal range so as to reduce symptoms of hypogonadism and improve quality of life (Sih et al, 1997; Gruenewald and Matsumoto, 2003). The T preparations in use are injections, scrotal and nonscrotal transdermal patches, and oral, buccal and gel preparations (Dobs et al, 1999; Gooren and Bunck, 2003; Bhasin and Basaria, 2011; Wang et al, 2011; Surampudi et al, 2012; Abadilla and Dobs, 2012). The availability of these methods has made T replacement increasingly accessible and palatable to men. However, exogenous T replacement has drawbacks. With injections, serum T levels initially are supraphysiologic and then reduced (Bhasin and Basaria, 2011), requiring T levels to be measured and sometimes adjusted between injections. T administered by gels and other transdermal methods are easier to use and produce more constant T concentrations, but have the potential for T transfer via skin contact (Bhasin and Basaria, 2011; Surampudi et al, 2012; Abadilla and Dobs, 2012). More seriously, there are recent studies indicating increased risk of cardiovascular disease in men using T replacement (Vigen et al, 2013; Finkle et al, 2014; Xu et al, 2013). Indeed, the FDA recently (September, 2014) expressed concern that men who take exogenous T may face increased risk of stroke and heart attack. There also are reports in rats suggesting that exogenous T treatment might increase the risk of prostate cancer (Bosland, 2014).
Although the administration of exogenous T will elevate serum T levels into the normal range, exogenous T in most men will suppress LH, resulting in reduced Leydig cell T formation and reduced intratesticular T concentrations. Consequently, the administration of T can result in the suppression of spermatogenesis, making this an inadvisable approach to ameliorate hypogonadism in men wishing to father children (Pavlovich et al, 2001; Kim and Schlegel, 2008; Hwang et al, 2011; Ramasamy et al, 2011).
TSPO-mediated induction of endogenous T production
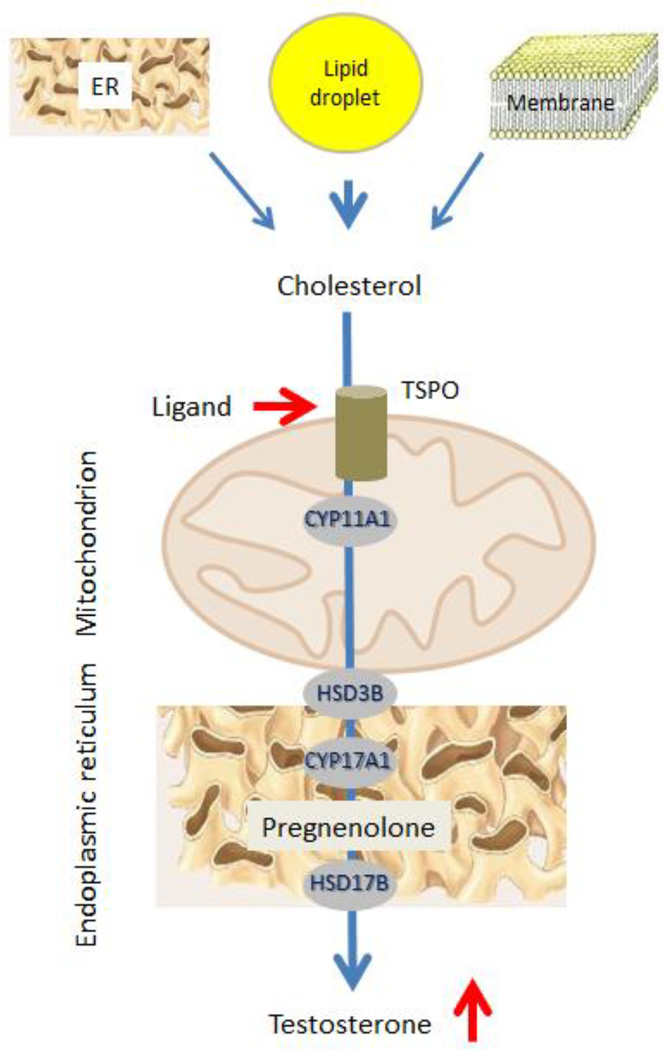
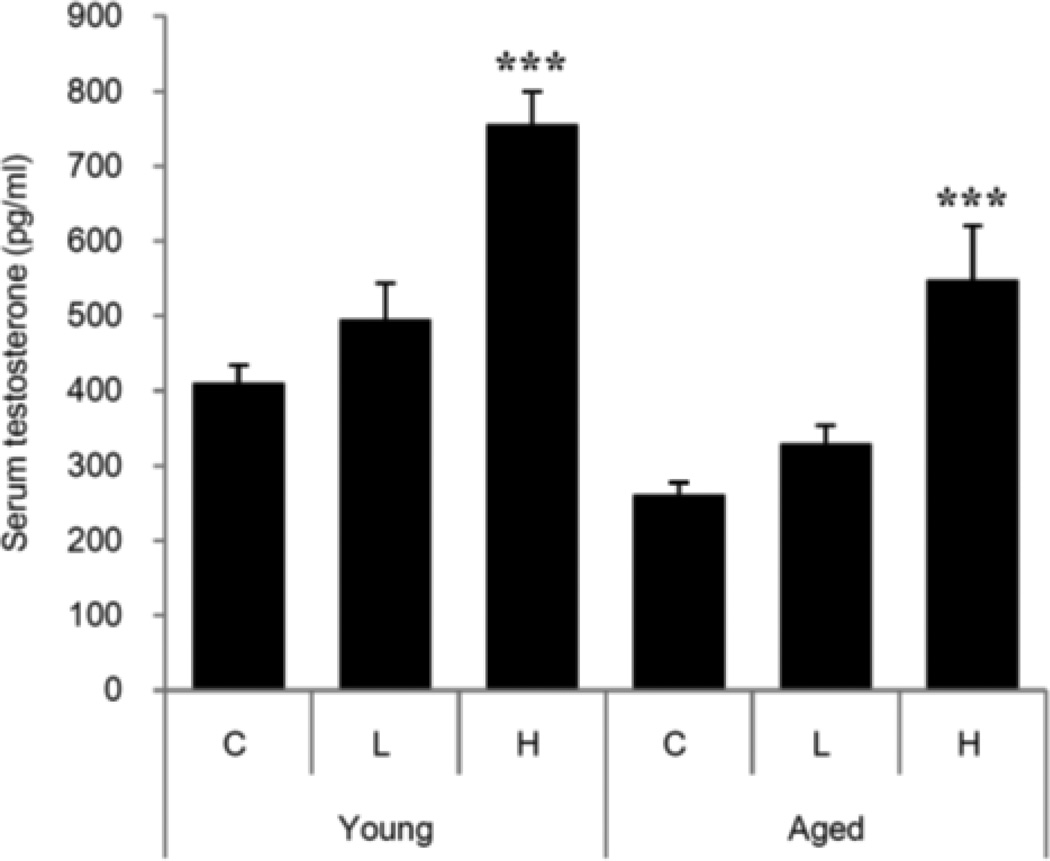
Knowledge of the steps in testosterone formation, and the mechanisms involved, have made it possible to apply pharmacological means to increase serum (and intratesticular) T by stimulating the Leydig cells themselves. Culture of young and aged Leydig cells with dbcAMP was found to increase steroid formation significantly (Chen et al, 2004), indicating that the LH signaling step in steroid formation can be bypassed. Analyses of steroid formation in aged as compared to young cells revealed downstream deficiency in TSPO (Culty et al, 2002; Chung et al, 2013), a protein that is integrally involved in cholesterol translocation from the cytosol to the inner mitochondrial membrane. As cholesterol translocation represents the rate-determining step in steroid formation, we tested the hypothesis that the direct pharmacological activation of TSPO would increase T production by aged cells (Fig. 2). To this end, we examined the effects of the high-affinity TSPO drug ligand FGIN-1-27 (N,N-dihexyl-2-(4-fluorophenyl)indole-3-acetamide) on T formation by Leydig cells isolated from aged (21 month-old) Brown Norway rats, and of administering FGIN-1-27 to these rats in vivo (Chung et al, 2013). LH-stimulated T formation was reduced in aged as compared to young cells. However, when aged cells were incubated with LH plus FGIN-1-27, T production was equivalent to that of LH-stimulated young cells. The stimulatory effect of FGIN-1-27 was abolished by the TSPO cholesterol recognition/interaction amino acid consensus (CRAC) domain inhibitor 3,17,19-androsten-5-triol (19-Atriol). In vivo, administering FGIN-1-27 to aged rats elevated serum T to the level of young rats (Fig. 3). Although the long-term safety of TSPO drug ligands remains unknown, such ligands have been used in clinical studies to increase neurosteroids in men with neurological and psychiatric disease symptoms (Taliani et al, 2009; Rupprecht et al, 2009; Nothdurfter et al, 2012).
Figure 2. Effect of TSPO ligands on Leydig cell steroid biosynthesis.
In response to TSPO ligands, cholesterol is transported into mitochondria even in the absence of LH, where it is cleaved into pregnenolone, used for the biosynthesis of testosterone.
Figure 3. TSPO drug ligand FGIN-1-27 induction of testosterone formation in hypogonadal aging Brown Norway rats.
Young and aged rats were treated with either low (L; 0.1 mg/kg/day) or high (H, 1 mg/kg/day) doses of FGIN-1-27 by ip for 10 days. Serum testosterone was measured by RIA. From: Chung et al, 2013 Endocrinology 154: 2156-2154.
Administering TSPO drug ligands to increase Leydig cell T production is independent of LH and thus of STAR induction. This approach could result in the regulation of LH release by the T formed, and therefore T production should be pulsatile as under normal physiological circumstances. The fact that TSPO drug ligands act in an LH-independent manner suggest that they can be also used to treat secondary hypogonadism, where there is a defect in LH release from the pituitary, or mixed primary and secondary hypogonadism. Fertility should be preserved, not suppressed, with this approach because the Leydig cells produce relatively high levels of T. Indeed, the local stimulation of Leydig cell T production might actually enhance spermatogenesis because intratesticular T levels should increase, not decrease. Whether or not this approach will affect the heart and prostate differently than exogenous T administration is uncertain at present.
HIGHLIGHTS.
Reviews changes in the steroidogenic enzymes of aging Leydig cells
Discusses effects of the redox environment on Leydig cell testosterone production
Discusses ways to increase serum testosterone by stimulating the Leydig cells
Acknowledgments
This work was supported over the years by grants from the National Institutes of Health (R37 AG021092, R03 AG026721, Canadian Institutes of Health Research, and a Canada Research Chair in Biochemical Pharmacology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest.
References
- Abadilla KA, Dobs AS. Topical testosterone supplementation for the treatment of male hypogonadism. Drugs. 2012;72:1591–1603. doi: 10.2165/11635620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact. 1998;111–112:1–14. doi: 10.1016/s0009-2797(97)00146-4. [DOI] [PubMed] [Google Scholar]
- Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- Azhar S, Cao L, Reaven E. Alteration of the adrenal antioxidant defense system during aging in rats. J Clin Invest. 1995;96:1414–1424. doi: 10.1172/JCI118177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie MC, Chen H, Fan J, Papadopoulos V, Miller P, Zirkin BR. Aging and luteinizing hormone effects on reactive oxygen species production and DNA damage in rat Leydig cells. Biol Reprod. 2013;88:1–7. doi: 10.1095/biolreprod.112.107052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belchetz PE, Barth JH, Kaufman JM. Biochemical endocrinology of the hypogonadal male. Ann Clin Biochem. 2010;47:503–515. doi: 10.1258/acb.2010.010150. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Basaria S. Diagnosis and treatment of hypogonadism in men. Best Prac Res Clin Endocrinol Metab. 2011;25:251–270. doi: 10.1016/j.beem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Bobjer J, Naumovska M, Giwercman YL, Giwercman A. High prevalence of androgen deficiency and abnormal lipid profile in infertile men with non-obstructive azoospermia. Int J Androl. 2012;35:688–694. doi: 10.1111/j.1365-2605.2012.01277.x. [DOI] [PubMed] [Google Scholar]
- Bonavera JJ, Swerdloff RS, Leung A, Lue YH, Baravarian S, Superiano L, Sinha-Hikim AP, Wang C. In the male brown-Norway (BN) male rat, reproductive aging is associated with decreased LH-pulse amplitude and area. J Androl. 1997;18:359–365. [PubMed] [Google Scholar]
- Bonavera JJ, Swerdloff RS, Sinha-Hakim AP, Lue YH, Wang C. Aging results in attenuated gonadotropin releasing hormone-luteinizing hormone axis responsiveness to glutamate receptor agonist N-methyl-D-aspartate. J Neuroendocrinol. 1998;10:93–99. doi: 10.1046/j.1365-2826.1998.00177.x. [DOI] [PubMed] [Google Scholar]
- Bosland MC. Testosterone treatment is a potent tumor promoter for the rat prostate. Endocrinology. 2014;155:1–5. doi: 10.1210/en.2014-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004;88:61–67. doi: 10.1016/j.jsbmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, Zirkin BR. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol. 2001;36:1361–1373. doi: 10.1016/s0531-5565(01)00118-8. [DOI] [PubMed] [Google Scholar]
- Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the Brown Norway rat. J. Androl. 1994;15:551–557. [PubMed] [Google Scholar]
- Chen H, Hardy MP, Zirkin BR. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology. 2002;143:1637–1642. doi: 10.1210/endo.143.5.8802. [DOI] [PubMed] [Google Scholar]
- Chen H, Huhtaniemi I, Zirkin BR. Depletion and repopulation of Leydig cells in the testes of aging brown Norway rats. Endocrinology. 1996;137:3447–3452. doi: 10.1210/endo.137.8.8754773. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Luo L, Zirkin BR. Dibutyryl cyclic adenosine monophosphate restores the ability of aged Leydig cells to produce testosterone at the high levels characteristic of young cells. Endocrinology. 2004;145:4441–4446. doi: 10.1210/en.2004-0639. [DOI] [PubMed] [Google Scholar]
- Chen H, Pechenino AA, Liu J, Beattie MC, Brown TR, Zirkin BR. Effect of glutathione depletion on Leydig cell steroidogenesis in young and old brown Norway rats. Endocrinology. 2008;149:2612–2619. doi: 10.1210/en.2007-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zirkin BR. 1999 Long-term suppression of Leydig cell steroidogenesis prevents Leydig cell aging. Proc Nat Acad Sci. 1999;96:14877–14881. doi: 10.1073/pnas.96.26.14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Chen H, Midzak A, Burnett AL, Papadopoulos V, Zirkin BR. Drug ligand-induced activation of translocator protein (TSPO) stimulates steroid production by aged brown Norway rat Leydig cells. Endocrinology. 2013;154:2156–2165. doi: 10.1210/en.2012-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culty M, Luo L, Yao ZX, Chen H, Papadopoulos V, Zirkin BR. Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J Androl. 2002;23:439–447. [PubMed] [Google Scholar]
- Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–2891. doi: 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]
- Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J. Clin Endocrinol Metab. 1999;84:3469–3478. doi: 10.1210/jcem.84.10.6078. [DOI] [PubMed] [Google Scholar]
- Drew B, Leeuwenburgh C. Aging and the role of reactive nitrogen species. Ann NY Acad Sci. 2002;959:66–81. doi: 10.1111/j.1749-6632.2002.tb02084.x. [DOI] [PubMed] [Google Scholar]
- Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF, Jr, Hoover RN. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou M, Perkins LM, Payne AH. Steroid synthesis-dependent, oxygen-mediated damage of mitochondrial and microsomal cytochrome P-450 enzymes in rat Leydig cell cultures. Endocrinology. 1987;121:1390–1399. doi: 10.1210/endo-121-4-1390. [DOI] [PubMed] [Google Scholar]
- Gooren LJ, Bunck MCM. Transdermal testosterone delivery: testosterone patch and gel. World J Urol. 2003;21:316–319. doi: 10.1007/s00345-003-0368-6. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J Biol Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- Gruenewald DA, Matsumoto AM. Testosterone supplementation therapy for older men: potential benefits and risks. J Am Geriatr Soc. 2003;51:101–115. doi: 10.1034/j.1601-5215.2002.51018.x. [DOI] [PubMed] [Google Scholar]
- Hamada AJ, Montgomery B, Agarwal A. Male infertility:a critical review of pharmacologic management. Expert Opin Pharmacother. 2012;13:2511–2531. doi: 10.1517/14656566.2012.740011. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter J, Tobin JD, et al. Longitudinal effects of aging on serum total free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hornsby PJ. Steroid and xenobiotic effects on the adrenal cortex: mediation by oxidative and other mechanisms. Free Radic Biol Med. 1989;6:103–115. doi: 10.1016/0891-5849(89)90163-9. [DOI] [PubMed] [Google Scholar]
- Hwang K, Walters RC, Lipshultz LI. Contemporary concepts in the evaluation and management of male infertility. Nat Rev Urol. 2011;8:86–94. doi: 10.1038/nrurol.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- Kim HH, Schlegel PN. Endocrine manipulation in male infertility. Urol Clin N Am. 2008;35:303–318. doi: 10.1016/j.ucl.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Kodaman PH, Aten RF, Behrman HR. Lipid hydroperoxides evoke antigonadotropic and antisteroidogenic activity in rat luteal cells. Endocrinology. 1994;135:2723–2730. doi: 10.1210/endo.135.6.7988463. [DOI] [PubMed] [Google Scholar]
- Kolena J, Blazícek P, Horkovics-Kováts S, Ondrias K, Seböková E. Modulation of rat testicular LH/hCG receptors by membrane lipid fluidity. Mol. Cell. Endocrinology. 1986;44:69–76. doi: 10.1016/0303-7207(86)90107-3. [DOI] [PubMed] [Google Scholar]
- Li WR1, Chen L, Chang ZJ, Xin H, Liu T, Zhang YQ, Li GY, Zhou F, Gong YQ, Gao ZZ, Xin ZC. Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells. Asian J Androl. 2011;13:881–888. doi: 10.1038/aja.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C1, Reaven E, Azhar S. Age-related decline in the steroidogenic capacity of isolated rat Leydig cells: a defect in cholesterol mobilization and processing. J Steroid Biochem Mol Biol. 1993;46:39–47. doi: 10.1016/0960-0760(93)90207-d. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Shenvi S, Hagen TM, Liu RM. Glutathione metabolism during aging and in Alzheimer disease. Ann NY Acad Sci. 2004;1019:346–349. doi: 10.1196/annals.1297.059. [DOI] [PubMed] [Google Scholar]
- Liu RM. Down-regulation of γ-glutamylcysteine synthetase regulatory subunit gene expression in rat brain tissue during aging. J Neurosci Res. 2002;68:344–351. doi: 10.1002/jnr.10217. [DOI] [PubMed] [Google Scholar]
- Luo L, Chen H, Trush MA, Show MD, Anway MD, Zirkin BR. Aging and the brown Norway rat Leydig cell antioxidant defense system. J Androl. 2006;27:240–247. doi: 10.2164/jandrol.05075. [DOI] [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Leydig cell aging: steroidogenic acute regulatory protein (StAR) and cholesterol side-chain cleavage enzyme. J Androl. 2001;22:149–156. [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Temporal relationships among testosterone production, steroidogenic acute regulatory protein (StAR), and P450 side-chain cleavage enzyme (P450scc) during Leydig cell aging. J Androl. 2005;26:25–31. [PubMed] [Google Scholar]
- Luo L, Chen H, Zirkin BR. Are Leydig cell steroidogenic enzymes differentially regulated with aging? J Androl. 1996;17:509–515. [PubMed] [Google Scholar]
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57:M76–M99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011;52:2111–2135. doi: 10.1194/jlr.R016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr BA, Guay AT, O’Donnell AB, McKinlay JB. Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts Male Ageing Study. Clin Endocrinol. 2005;62:64–73. doi: 10.1111/j.1365-2265.2004.02174.x. [DOI] [PubMed] [Google Scholar]
- Nothdurfter C, Rammes G, Baghai TC, Schüle C, Schumacher M, Papadopoulos V, Rupprecht R. Translocator protein (18 kDa) as a target for novel anxiolytics with a favourable side-effect profile. Neuroendocrinol. 2012;24:82–92. doi: 10.1111/j.1365-2826.2011.02166.x. [DOI] [PubMed] [Google Scholar]
- Page ST. Physiologic role and regulation of intratesticular sex steroids. Curr Opin Endocrinol Diabetes Obes. 2011;18:217–223. doi: 10.1097/MED.0b013e328345d50e. [DOI] [PubMed] [Google Scholar]
- Pavlovich CP, King P, Goldstein M, Schlegel PN. Evidence of a treatable endocrinopathy in infertile men. J Urol. 2001;165:827–841. [PubMed] [Google Scholar]
- Peltola V, Huhtaniemi I, Metsa-Ketela T, Ahotupa M. Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinology. 1996;137:105–112. doi: 10.1210/endo.137.1.8536600. [DOI] [PubMed] [Google Scholar]
- Quinn PG, Payne AH. Oxygen-mediated damage of microsomal cytochrome P-450 enzymes in cultured Leydig cells. Role in steroidogenic desensitization. J Biol Chem. 1984;259:4130–4135. [PubMed] [Google Scholar]
- Quinn PG, Payne AH. Steroid product-induced, oxygen-mediated damage of microsomal cytochrome P-450 enzymes in Leydig cell cultures. Relationship to desensitization. J Biol Chem. 1985;260:2092–2099. [PubMed] [Google Scholar]
- Ramasamy R, Stahl PJ, Schlegel PN. Medical therapy for spermatogenic failure. Asian J Androl. 2011;14:57–60. doi: 10.1038/aja.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta. 2009;1791:646–658. doi: 10.1016/j.bbalip.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schule C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, Uzunov V, McAllister KH, Bertaina-Anglade V, La Rochelle CD, Tuerck D, Floesser A, Kiese B, Schumacher M, Landgraf R, Holsboer F, Kucher K. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Sandhu SK, Kaur G. Alterations in oxidative stress scavenger system in aging rat brain and lymphocytes. Biogerontology. 2002;3:161–173. doi: 10.1023/a:1015643107449. [DOI] [PubMed] [Google Scholar]
- Schlegel PN. Evaluation of male infertility. Minerva Ginecol. 2009;61:261–283. [PubMed] [Google Scholar]
- Sih R, Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–1667. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- Sokanovic SJ, Baburski AZ, Janjic MM, Stojkov NJ, Bjelic MM, Lalosevic D, Andric SA, Stojilkovic SS, Kostic TS. The opposing roles of nitric oxide and cGMP in the age-associated decline in rat testicular steroidogenesis. Endocrinology. 2013;154:3914–3924. doi: 10.1210/en.2013-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surampudi PN, Wang C, Swerdloff R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int J Endocrinol. 2012;2012:1–20. doi: 10.1155/2012/625434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliani S, Da Settimo F, Da Pozzo E, Chelli B, Martini C. Translocator protein ligands as promising therapeutic tools for anxiety disorders. Curr Med Chem. 2009;16:3359–3380. doi: 10.2174/092986709789057653. [DOI] [PubMed] [Google Scholar]
- Vega M, Carrasco I, Castillo T, Troncoso JL, Videla LA, Devoto L. Functional luteolysis in response to hydrogen peroxide in human luteal cells. J Endocrinol. 1995;147:177–182. doi: 10.1677/joe.0.1470177. [DOI] [PubMed] [Google Scholar]
- Vigen R, O'Donnell CI, Barón AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- Wang C, Ilani N, Arvert S, McLachlan RI, Soulis T, Watkinson A. Efficacy and safety of the 2% formulation of testosterone topical solution applied to the axillae in androgen-deficient men. Clin Endocrinol. 2011;75:836–843. doi: 10.1111/j.1365-2265.2011.04152.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Leung A, Sinha-Hikim AP. Reproductive aging in the male Brown-Norway rat: a model for human. Endocrinology. 1993;133:2773–2781. doi: 10.1210/endo.133.6.8243304. [DOI] [PubMed] [Google Scholar]
- Wang FF, Wang Q, Chen Y, Lin Q, Gao HB, Zhang P. Chronic stress induces ageing-associated degeneration in rat Leydig cells. Asian J Androl. 2012;14:643–648. doi: 10.1038/aja.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva FF, Forti G, Giwercam A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Boonen S, Vanderschueren D, Labrie F, Huhtaniemi IT. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- Wu X, Yao K, Carlson JC. Plasma membrane changes in the rat corpus luteum induced by oxygen radical generation. Endocrinology. 1993;133:491–495. doi: 10.1210/endo.133.2.8344194. [DOI] [PubMed] [Google Scholar]
- Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108. doi: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]