Abstract
Common to all fibrotic and metastatic diseases is the uncontrollable remodeling of tissue that leads to the accumulation of fibrous connective tissue components such as collagen and elastin. Build-up of fibrous tissue occurs through the cross-linking of collagen or elastin monomers, which is initiated through the oxidation of lysine residues to form α-aminoadipic-δ-semialdehyde (allysine). To provide a measure of the extent of collagen oxidation in disease models of fibrosis or metastasis, a rapid, sensitive HPLC method was developed to quantify the amount of allysine present in tissue. Allysine was reacted with sodium 2-naphthol-7-sulfonate under conditions typically applied for acid hydrolysis of tissues (6M HCl, 110 °C, 24 h) to prepare a fluorescent bis-naphthol derivative of allysine. High performance liquid chromatography was applied for analysis of allysine content. Under optimal reaction and detection conditions, successful separation of AL-NP was achieved with excellent analytical performance attained. Good linear relationship (R2=0.994) between peak area and concentration for AL-NP was attained for 0.35 – 175 pmol of analyte. A detection limit of 0.02 pmol in the standard sample with a 20 μL injection was achieved for AL-NP, with satisfactory recovery from 88–100% determined. The method was applied in the quantification of allysine in healthy and fibrotic mouse lung tissue, with the fibrotic tissue showing a 2.5 fold increase in the content of allysine.
Keywords: Allysine, Collagen, Fibrogenesis, Fibrosis, lysyl oxidase
1. Introduction
Increased extracellular matrix (ECM) formation and tissue stiffening is a fundamental feature of wound healing, fibrotic diseases, and in the regulation of cancer cell metastasis. Lysyl oxidase (LOX) and its paralogs, LOX-like 1-4 (LOXL1-4) are extracellular, matrix embedded proteins that play a critical role establishing the structural integrity and stability of the ECM through the cross-linking of collagen and elastin fibrils.[1–2] The LOX/LOXL1-4 dependent oxidation of lysine residues on collagen yields α-aminoadipic-δ-semialdehyde (allysine),[3] which undergoes a series of condensation reactions generating the cross-linking structures responsible for collagen and elastin fiber formation.[4–9]
During fibrosis, chronic injury leads to the excess accumulation of ECM scar tissue,[10–11] and the disruption of normal tissue function, which can lead to organ failure and mortality. Elevated levels of the LOX family is a consistent feature across different organs and tissues types, with increased levels of the enzymes reported in patients with enhanced myocardial stiffness,[12] chronic hepatic fibrosis, adriamycin-induced kidney fibrosis[13] and several types of cancer (breast,[14–15] head and neck,[14] and colorectal,[16–17] among others[18]). High levels of LOX expression have similarly been described in mouse models of liver and lung fibrosis.[19] LOX expression has been shown to be an important regulator of tumor progression, with implications for the promotion of cancer cell invasion, metastasis and angiogenesis, as well as in the malignant transformation of solid tumors through remodeling of the tumor microenvironment.[20–23] Secreted LOX has also been shown to be involved in the recruitment of inflammatory cells to distant sites, which contribute to metastasis by initiating the formation of the pre-metastatic niche in breast and liver cancers.[24]
The molecular pathways common to ECM remodeling in fibrosis and solid tumor metastasis across different tissue types suggest that the LOX and LOXL1-4 enzymes may be useful targets for developing anti-tumorigenic and anti-fibrotic therapeutics. LOX and LOXL2, in particular, have been the basis of extensive investigation, with LOX identified as a therapeutic target for patients with cancers,[14] while LOXL2 has received focus in the monitoring and treatment of fibrotic lung disease.[25–26] Over the last few decades several selective inhibitors of LOX, from small molecule inhibitors (e.g. β-aminopropionitrile[27]) to function blocking antibodies, have been developed,[28–29] with Simtuzumab, an antibody inhibitor of LOXL2[25] currently under active investigation.
The formation of allysine is a fundamental component in the LOX and LOXL dependent mechanism of ECM deposition during malignancy. Since allysine is present only transiently in tissue during periods of LOX upregulation, we hypothesize that measurement of the allysine content of tissue would provide a way to quantify the fibrogenic activity. This would allow the initial stages of fibrosis and/or metastasis to be identified and provide a quantitative biochemical measure of the effectiveness of LOX and LOXL1-4 targeted therapies.
Quantification of allysine has proven to be challenging as chemical derivatization is required to avoid loss of aldehyde functionality during tissue hydrolysis steps. Early methods included quantification using conventional amino acid chromatography analyzers following protein reduction using [3H]NaBH4 to give hydroxynorleucine/chloronorleucine, oxidation with performic acid to give α-aminoadipic acid or reaction with [14C]NaCN and NH3 to give α,ε-diaminopimelic acid and ε-hydroxy-α-aminopimelic acid.[30–31] More recently in the context of quantifying protein oxidation several methods have been described in biological samples with GC-MS, HPLC or LC-MS used for quantification.[32–34] Reductive amination with NaCNBH3 in the presence of p-aminobenzoic acid has been performed to produce an acid stable fluorescent allysine derivative that can be detected by HPLC following hydrolysis by either conventional thermal heating or microwave irradiation.[35–36] Reductive amination with fluoresceinamine has also been reported but the fluorescein-allysine conjugate was reported to be unstable during acid hydrolysis of the protein.[37] Requena et. al. have developed methods based on GC-MS analysis with isotopic dilution for determination of allysine following reduction with NaBH4.[38] The equilibrium between allysine and piprideine-6-carboxylate (P6C) has provided an indirect means to estimate allysine concentrations using LC-MS/MS quantification of P6C.[39–40] While these methods provide accurate assessments of the concentrations of allysine present in soluble proteins samples they have not been extended to analyzing whole tissue samples. Methods to quantify the allysine content present in the insoluble collagen matrix include labeling whole tissue samples with phenol and p-cresol.[41–43] Umeda et. al. show that under conditions suitable for tissue digestion (110 °C, 6M HCl, 24h), allysine is derivatized with 2 equivalents of p-cresol to yield a bis–p-cresol condensation derivative, termed ‘APC’, enabling quantification of allysine by HPLC-UV analysis. A limitation of the Umeda’s protocol is its low sensitivity associated with the detection mode.
In this paper we describe a new procedure that modifies the protocol of Umeda et. al. to use 2-naphthol-7-sulfonate for derivatization of allysine with a fluorescent bis-naphthol. The new allysine derivative, ‘AL-NP’, is stable under the conditions of hydrolysis and allows quantification of allysine in whole tissue with an eight-fold improved sensitivity compared to the p-cresol method. Indeed, we showed that when using 2-naphthol-7-sulfonate it is possible to quantify allysine by HPLC with detection sensitivity as low as pmoles using milligram quantities of whole tissue digest without the prior need to isolate the ECM components.
2. Materials and Methods
2.1. Reagents and materials
All reactants and reagents were of the highest purity. HPLC-grade acetonitrile was obtained from EMD Millipore (Billerica, MA, USA). Water was purified with a Milli-Q system (Millipore, Billerica, MA, USA). L-Allysine ethylene acetal (>98%), potassium hydrogen phthalate (>99%) and fluorescein (99%) were supplied by Sigma (Sigma-Aldrich, Saint Louis, MO, USA). Trifluoroacetic acid (99.5%)was purchased from Alfa Aesar (MA, USA), formic acid (99.0%) from Fisher Scientific (Fisher Scientific, Fair Lawn, NJ, USA) and sodium 2-naphthol-7-sulfonate (>98%) from TCI America (PA, USA). Analytical grade methanol and chloroform were obtained from VWR (PA, USA).
2.2. NMR
NMR spectra were recorded on a Varian 500 NMR system equipped with a 5 mm broadband probe (1H NMR: 499.81 MHz, 13C: 125.68 MHz). 1H resonance assignments were obtained using 1H-1H correlated spectroscopy.
2.3. HPLC
2.3.1
Mobile phases were prepared by adding 1 mL of trifluoroacetic acid to 999 mL water for Solvent A, 1 mL of trifluoroacetic acid to 999 mL HPLC grade acetonitrile for Solvent B, 1 mL of formic acid to water for Solvent C and 1 mL of formic acid to 999 mL HPLC grade acetonitrile for Solvent D. The mobile phases were filtered under a vacuum through a 0.45 mm nylon filters and degassed continuously with an on-line degasser.
2.3.2. Preparative HPLC
Purification of AL-NP was performed using a MetaChem Technologies Inc. LUNA C18(2) column (250×21.2 mm, particle size: 10 μm). UV detection was at 220, and 254 nm. The flow rate was 15 mL/min and a gradient method was employed using Solvents A and B: 0–2 min hold at 5% B, 2–24 min gradient to 95% B, 24–25 min hold at 95% B, 25–28 min gradient to 5% B, 28–30 min re-equilibrate at 5% B.
2.3.3. HPLC-MS
HPLC-MS analysis for purity and identity was carried out using an Agilent 1260 LC system coupled to an Agilent Technologies 6130 Quadrupole MS system. The LC method used a Phenomenex Luna C18(2) column (100×2 mm, particle size: 5 μm). The flow rate was 0.8 mL/min, with UV detection at 220, 254 and 280 nm. A gradient chromatographic method was employed using solvents C and D: 0–1 min hold at 5% D, 1–9 min gradient to 95% D, 9–10 min hold at 95% D, 10–12 min gradient to 5% D, 12–15 min re-equilibrate at 5% D.
2.3.3. Analytical HPLC-FL
HPLC analysis was carried out on an Agilent 1260 system with an Agilent 1260 FLD fluorescence detector (λex = 254 nm and λem = 310 nm for AL-NP; λex = 490 nm and λem = 510 nm for fluorescein used as internal standard). Control of the HPLC system and data collection was by Agilent OpenLAB CDS ChemStation software (version 1.90). A C8 analytical Discovery® column (25 mm × 4 mm, 5 μm particle size) was used. The flow rate was 1 mL/min and the oven temperature was set to 25 °C. The injection volume was set at 20 μL. A gradient chromatographic method was employed using solvents A and B: 0–40 min; 5–30% solvent B, 40–42 min; 30–95% solvent B, 42–45 min; 95% solvent B, 45–47 min; 95–5% solvent B, 47–50 min; 5% solvent B.
2.4. Synthesis of allysine-bis-p-naphthol derivative
L-Allysine ethylene acetal (25 mg, 0.13 mmol) in 6N HCl (10 mL) containing 2-naphthol-7-sulfonate (325 mg, 1.32 mmol) was heated at 110 °C for 24 h. The reaction was cooled to r.t., neutralized with 6 N NaOH, filtered and purified by preparative HPLC to yield a pale brown solid (65.5 mg, 87%).
1H NMR (D2O, 500 MHz, 25 °C): 8.25 (s, 2H, ArH), 7.86 (d, 2H, J = 7.2 Hz, ArH), 7.73 (d, 2H, J = 7.2 Hz, ArH), 7.43 (m, 2H, ArH), 6.67 (m, 2H, ArH), 4.41 (s, 1H, CH(NH2)CO2H), 3.14 (t, 1H, J = 7.4 Hz, CH), 1.49 (m, 2H, CH2), 1.16 (pseudo dt, 1H, CHH′), 1.06 (pseudo dt, 1H, CHH′), 0.56 (m, 2H, CH2).
13C NMR (D2O, 125 MHz, 25 °C): 173.7, 149.7, 138.1, 131.6, 129.4, 129.1, 126.3, 123.7, 122.9, 118.0, 115.4, 53.9, 34.7, 30.1, 29.6, 19.8.
LC-MS: tR = 6.51 min. LC/MS (ESI-): C26H25NO10S2: m/z: calcd 576.10 [MH+]; found m/z-18: C26H23NO9S2 558.1 (MH+).
2.5. Preparation of standard solution of AL-NP
AL-NP (25 mg) was weighed accurately and dissolved in 500 μL of D2O to create a stock solution of AL-NP. The NMR internal standard, potassium hydrogen phthalate (KHP) (50 mg), was weighed accurately and dissolved in 500 μL of D2O to create a stock solution of KHP. Accurately weighed aliquots of AL-NP solution (200 μL) and KHP solution (200 μL) were then mixed thoroughly together and assayed by 1H-NMR (32 scans, relaxation delay time: 5 s). The AL-NP and KHP peak areas were measured and their ratio in combination with the known KHP stock solution concentration used to determine an accurate concentration of AL-NP in the stock solution. That AL-NP solution was then used to make the HPLC standards.
2.6. Method validation
2.6.1. Recovery analysis
In high-pressure reaction tubes was added increasing amounts (1–20 μL) of an L-allysine ethylene acetal solution (4.15 mg in 20.0 mL water), 100 μL 4 mM fluorescein as an internal standard, 1 mL of 12 M HCl, 40 mg of sodium 2-naphthol-7-sulfonate and water to achieve a constant volume of 2 mL. The reaction vessels were capped with a teflon cap and heated at 110 °C for 24 h. The reaction solutions were cooled, neutralized with 6 M NaOH and analyzed by HPLC-FL.
2.6.2. Standard addition analysis
In high-pressure reaction tubes each containing 250 μL lung homogenate was added 0, 5, 10 or 25 μL L-allysine ethylene acetal solution (4.15 mg in 20.0 mL water), 650, 645, 640 or 625 μL water respectively, 100 μL 4 mM fluorescein as an internal standard, 1 mL of 12 M HCl and 40 mg of sodium 2-naphthol-7-sulfonate. The reaction vessels were capped with a teflon cap and heated at 110 °C for 24 h. The reaction solutions were cooled, neutralized with 6 M NaOH and analyzed by HPLC-FL.
2.6.3. Repeatability analysis
Lung homogenate sample labeled by naphthol method was analyzed by HPLC-FL in triplicate, and again in triplicate at 24 h and 48 h later.
2.7. Aorta labeling
2.7.1. Elastin preparation
Elastin from porcine aorta was purified using the mild method reported by Umeda to avoid degradation of elastin[43]. Briefly, after removing connective tissue and lipids, the aorta was cut into small pieces and suspended in 1 M NaCl for 24 h. The 1 M NaCl was replaced and the washing procedure repeated for a further 2 × 24 h. The aorta segments were washed with water and defatted with chloroform:methanol (2:1) for 48 h. The aorta was then lyophilized.
2.7.2. Aorta derivatization with sodium 2-naphthol-7-sulfonate for HPLC analysis
In a high-pressure reaction tube containing portions (5–50 mg) of porcine aorta, was added 100 μL of 4 mM fluorescein as an internal standard, 900 μL water, 1 mL of 12 M HCl and 40 mg of sodium 2-naphthol-6-sulfonate (TCI America). The reaction vessel was capped with a teflon cap and heated at 110 °C for 24 h. The reaction solution was then cooled, neutralized with 6 M NaOH and analyzed by analytical HPLC-FL.
2.8. Animal protocol
All experiments and procedures were performed in accordance with the National Institutes of Health’s “Guide for the Care and Use of Laboratory Animals” and were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
2.8.1. Lung fibrosis model
C57Bl/6 adult male mice at 6 weeks of age (Charles River Laboratories, Wilmington MA) received a single dose of bleomycin (Fresenius Kabi, Lake Zurich, Il), prepared in sterile PBS. Bleomycin was intratracheally injected at 1.0 units/kg body weight (50 μL total volume). Naïve mice were used as controls. The left lung was harvested after 2 weeks (advance disease state) and homogenized in 1 mL of PBS buffer.
2.8.2. Characterization of allysine levels in mouse lung
In a high-pressure reaction tube containing 250 μL lung homogenate was added 650 μL water, 100 μL 4 mM fluorescein as an internal standard, 1000 μL 12 M HCl and 40 mg of sodium 2-naphthol-7-sulfonate. The reaction vessel was capped with a teflon cap and heated at 110 °C for 24 h. The reaction solution was cooled, neutralized with 6 M NaOH and analyzed by HPLC-FL.
2.9.1. Statistics
Results are expressed as means ± 1 standard deviation. Statistical analyses were performed using GraphPad Prism 5 software (LA Jolla, CA). The comparisons between a bleomycin group and naive group were analyzed by an unpaired t-test. A p value of less than 0.05 was considered significant.
3. Results
3.1. AL-NP standard synthesis
L-Allysine ethylene acetal, used as precursor of allysine, was reacted with 2 equivalents of sodium 2-naphthol-7-sulfonate. Simultaneous deprotection of the acetal protected aldehyde and subsequent labeling of the aldehyde functionality were performed under acidic conditions typically used for tissue hydrolysis (110 °C, 6 M HCl, 24 h). The bis-naphthol structure of AL-NP (Scheme 1) was confirmed by 1H and 13C NMR experiments (SI Figure 1–2). The mass ion associated with the single peak in the UV channel of the LC-MS trace (SI Figure 3) was not the mass of the AL-NP parent ion (m/z: 575.1) but instead corresponded to a mass of 558.1 (m/z -18) suggesting loss of water under the MS condition. The isolated purified AL-NP was used subsequently as a HPLC standard.
Scheme 1.
Synthesis of AL-NP from L-allysine ethylene acetal and 2-naphthol-7-sulfonate
3.2. AL-NP standard solution preparation
The exact concentration of a standard solution of AL-NP was determined by 1H NMR spectroscopy, using potassium hydrogen phthalate (KHP) as an internal NMR standard. A D2O solution of KHP of known exact concentration was mixed with an accurately weighed aliquot of AL-NP standard. 1H NMR of the KHP/AL-NP solution was measured and the peak areas of the KHP protons and the AL-NP analyte protons were measured. The integrated peak intensity of a single KHP or AL-NP analyte nuclei resonance is directly proportional to its molar concentration (and to the number of nuclei that give rise to that resonance). Comparison of the integrals of the AL-NP analyte resonances to those of the KHP standard of exact known concentration allowed determination of the AL-NP concentration in the standard.
3.3. Allysine quantification in tissue
With the AL-NP standard prepared it was next necessary to develop and optimize a set of reaction and HPLC conditions for the labelling of allysine in tissue
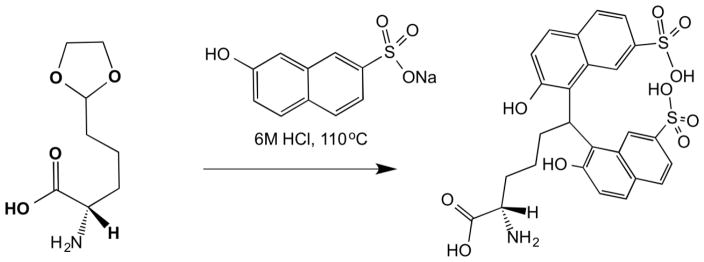
Firstly porcine aorta (25 mg), which is rich in allysine, was labeled with sodium 2-naphthol-7-sulfonate (110 °C, 6 M HCl, 24 h), to form the AL-NP product. Fluorescein (100 μM) was added to the samples at the beginning of the labeling to act as an internal standard. Fluorescein was stable under the labeling conditions, displaying a fluorescence excitation/emission and elution time distinct from the naphthol derivatives. After labeling the sample was neutralized and subject to HPLC analysis. Analytical HPLC-FL was performed on a reverse-phase C8 Discovery® column with fluorescence detection at λex = 254 nm and λem = 310 nm. Reproducible peak separation was found using a gradient method with water/0.1% TFA and acetonitrile/0.1% TFA as mobile phases (Figure 1a). As a control, aorta (25 mg) was digested in the absence of 2-naphthol-7-sulfonate. Its HPLC trace is overlaid with the naphthol labeled aorta trace showing that there is no basal interference with the AL-NP peak (Figure 1a) from the aorta tissue. HPLC gradient, time and solvent conditions were optimized so as to allow identification of the allysine labeled species in porcine aorta as a single isolable peak. Allysine was identified with a retention time of Rt = 29.5 min, by comparison with an injection of the AL-NP standard run under identical HPLC conditions (Figure 1b).
Fig. 1.
a) HPLC-FL trace for aorta labeled with 2-naphthol-7-sulfonate overlaid with HPLC-FL control trace for basal aorta in absence of 2-naphthol-7-sulfonate; ‘A’: Analyte, ‘IS’: Internal standard, b) Expanded view of the HPLC trace of aorta labeled with 2-naphthol-7-sulfonate (grey), overlaid with HPLC trace (offset) of AL-NP HPLC standard (black).
To further confirm the formation of AL-NP in the digested tissue, the aorta labeled sample was subject to HPLC-MS analysis using liquid chromatography conditions identical to the conditions of the HPLC-FL analytical method. First the AL-NP standard (100 pmol) was injected and a UV (254 nm) peak observed at 29.4 min in line with the analytical HPLC-FL analysis. Extracting the mass ion for AL-NP from the total ion count identified the expected mass-ion peak of AL-NP at the same retention time as the peak observed in the UV trace (SI Figure S4A). Then the naphthol labeled aorta sample was injected giving a UV (254 nm) peak at 29.65 min with a mass ion peak corresponding to the AL-NP standard at the same retention time (SI Figure S4B), confirming the presence of an allysine labeled peak in the aorta dige
3.4. Method optimization
Reaction conditions for allysine derivatization in tissue were optimized to deliver complete sample labeling. Portions of aorta (25 mg dry weight) were subjected to increasing concentrations of 2-naphthol-7-sulfonate. With 40 mg of 2-naphthol-7-sulfonate the peak area as measured by HPLC was found to be maximal (SI Figure S5). No increase in peak area was found if more 2-naphthol-7-sulfonate was added. Portions of aorta (25 mg dry weight) were also labeled with 40 mg 2-naphthol-7-sulfonate for increasing lengths of reaction times. No increase in peak area was found if the reaction time was extended beyond 24 h (SI Figure S6).
3.5. Method performance and validation
Following optimization of the labeling and HPLC conditions the analytical method was evaluated in terms of calibration range, linearity, sensitivity, recovery/accuracy and precision.
3.5.1. Linearity
Calibration curves were prepared for AL-NP at eight different concentration levels by plotting the peak area versus the analyte concentration. The calibration curve showed good linearity (equation for regression line: y = (4.082±0.023)x + (4.486±1.867), where y = peak area and x = amount of AL-NP injected (pmol) (n=3)) over a wide range of concentrations, typically 1–160 pmol to cover the expected concentration range of allysine found in murine lung tissue samples. The coefficient of correlation was equal to 0.999 confirming linearity of the method. (SI Figure S7, Table 1)
Table 1.
Linear range of the naphthol analytical method for allysine quantification
| AL-NP injected (pmol) | Mean Peak Area | Peak area RSD (%) |
|---|---|---|
| 3.62 | 13.9 | 1.29 |
| 7.83 | 32.4 | 1.54 |
| 19.76 | 86.1 | 1.40 |
| 39.58 | 169.2 | 0.78 |
| 59.34 | 250.8 | 0.86 |
| 79.79 | 326.8 | 1.35 |
| 119.48 | 501.5 | 0.78 |
| 159.76 | 649.3 | 0.83 |
Correlation coefficient R2 = 0.994; Equation for regression line y = (4.082±0.023)x + (4.486±1.867) (n=3)
3.5.2. Accuracy/Recovery
The conversion of allysine to AL-NP under standard assay conditions was quantified. A stock solution of L-allysine ethylene acetal was prepared and increasing concentrations were labeled with 2-naphthol-7-sulfonate. HPLC analysis of the reaction solutions was performed and the amount of AL-NP product formed was quantified using the AL-NP calibration curve, results are provided in Table 2. Recovery of allysine converted into AL-NP was studied using 6 different concentrations of the allysine standard solution. The recoveries were expressed as average values of two separate determinations. High recoveries of AL-NP in range of 89–100% were found over an allysine range of 1–22.5 nmol (SI Figure S8).
Table 2.
Recovery of the naphthol analytical method for allysine quantification
| Allysine reagent (nmol/reaction) | AL-NP Recovery, mean ±SD (%) |
|---|---|
| 1.10 | 91 ± 2.9 |
| 2.25 | 100 ± 6.07 |
| 5.51 | 98 ± 5.43 |
| 11.15 | 92 ± 8.6 |
| 16.82 | 96 ± 3.81 |
| 22.50 | 89 ± 11.1 |
3.5.3. Standard addition
A standard addition reaction was performed to assess the affect the presence of tissue would have on the allysine labeling efficiency. Homogenized mouse lung was added to reaction tubes containing increasing amounts of L-allysine ethylene acetal (3 different concentrations) of an allysine standard solution), and the standard naphthol labeling protocol performed. After labeling, the amount of AL-NP product formed was quantified by analytical-HPLC, with correction for the basal levels of allysine present in the mouse lung, results are provided in Table 3. The recoveries were expressed as average values of two separate determinations. High recoveries of AL-NP in range of 89–96% were found over an allysine range of 0–25 nmol (SI Figure S9).
Table 3.
Recovery of AL-NP with correction for basal concentrations in lung tissue homogenate
| Allysine reagent added (nmol) | Injected allysine concentration (μg/mL) | AL-NP formed (nmol) | AL-NP concentration (μg/mL) | Corrected Recovery, mean ±SD (%) |
|---|---|---|---|---|
| - | 0 | 1.44 | 0.046 | - |
| 4.76 | 0.05 | 5.66 | 0.181 | 89 ± 6.73 |
| 11.43 | 0.12 | 12.40 | 0.396 | 96 ± 1.28 |
| 23.81 | 0.25 | 23.10 | 0.738 | 91 ± 2.64 |
3.5.4. Specificity
Lung tissue homogenate was treated under the assay conditions in the absence of the 2-naphthol-7-sulfonate. Figure 1a shows the HPLC trace of the assay in absence of naphthol labeling. No interfering peaks were observed that co-eluted at the same retention time as the AL-NP, demonstrating the method to be specific for AL-NP
3.5.5. Repeatability
Precision of the method was studied by analyzing three replicates of a sample of digested lung tissue homogenate labeled with naphthol. The RSDs for the AL-NP peak obtained under repeatability (intra-day precision) conditions were 0.19% for migration time and 4.02% for peak area. The RSDs obtained under repeatability (inter-day precision) conditions were 1.59% for migration time and 6.11% for peak area.
3.5.6. Limit of Detection
The limit of detection calculated as the analyte concentration for which the peak height was three times the background noise (3S/N) was attained as 0.02 pmol for AL-NP. The limit of quantification calculated as the analyte concentration for which the peak height was ten times the background noise (10S/N) was attained as 0.078 pmol for AL-NP.
3.6. Comparison of current napthol derivatization method with cresol derivatization
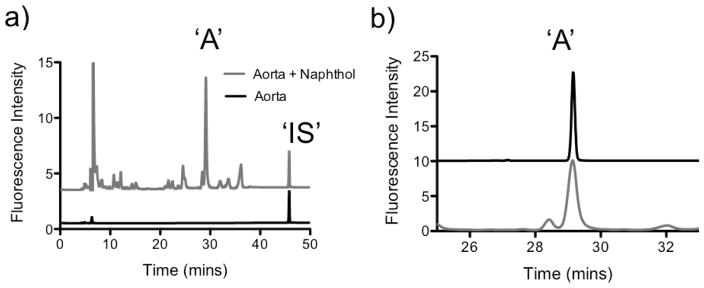
Increasing amounts of aorta (5 – 50 mg) were labeled with 2-naphthol-7-sulfonate giving rise to a linear correlation between HPLC peak area and the dry weight of aorta (correlation coefficient R2 = 0.996, y = (11.24±0.11)x + (3.57±2.67), where y = peak area and x = aorta mass (mg)) (Figure 2, Table 4). The amount of AL-NP formed was quantified giving an allysine concentration in porcine aorta of 7.48 nmol/mg dry aorta.
Fig. 2.
Comparison of peak area for aorta derivatized with 2-naphthol-7-sulfonate versus tissue derivatized with p-cresol as a function of aorta mass digested.
Table 4.
Precision of the naphthol analytical method for allysine quantification in aorta
| Mass of Aorta (mg) | Mean Peak Area (n=3) | RSD (%) | Allysine nmol/mg |
|---|---|---|---|
| 5.0 | 55.9 | 1.79 | 7.32 |
| 11.2 | 127.1 | 5.59 | 7.54 |
| 15.1 | 177.8 | 5.46 | 7.75 |
| 22.3 | 249.7 | 6.33 | 7.42 |
| 28.3 | 319.8 | 4.88 | 7.46 |
| 34.1 | 385.1 | 3.58 | 7.41 |
| 38.7 | 440.0 | 4.09 | 7.38 |
Allysine was also labeled with p-cresol using the conditions of Umeda et. al. to prepare APC. HPLC analysis of APC was carried out using the Agilent 1260 HPLC module with C8 Discovery column as per the naphthol analysis, but using the solvent and gradient conditions as described by Umeda (0.05 M sodium dihydrogen phosphate (pH 2.2)-acetonitrile (3:1), flow rate: 1 mL/min). A limit of quantification for APC of 0.63 pmol/injection was measured, which suggest the AL-NP method to be ~8 times more sensitive than the cresol method.
A side-by-side comparison of the labeling of aorta by the naphthol and cresol methods was made. As for the naphthol method, the cresol protocol demonstrated a good linear correlation between HPLC peak area and the dry weight of aorta (correlation coefficient R2 = 0.975, y = (2.59±0.38)x + (1.28±4.28), where y = peak area and x = aorta mass (mg)) (Figure 2). For equivalent masses of aorta the naphthol method showed a ~4.5 fold increase in peak area measured compared to the cresol method, an increase in detection sensitivity that is in line with the increased limit of quantification measured for the naphthol method.
3.7. Allysine quantification in lung tissue
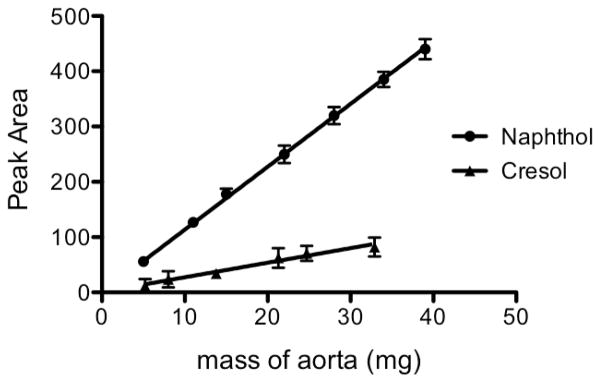
We next assessed the ability of the assay to detect allysine levels in milligram quantities of mouse lung tissue. The lungs from 14-day bleomycin-treated and sham treated C57Bl/6 mice were homogenized in PBS and labeled with sodium 2-naphthol-7-sulfonate under the conditions used for aorta labeling. Samples were then analyzed by analytical HPLC-FL (Figure 3a). By comparison with the AL-NP standard it was possible to identify an allysine labeled peak in mouse lung tissue with retention time of 29.4 mins (Figure 3b). The fluorescein:allysine peak area ratio normalized to total lung mass shows a three-fold increase for animals treated with bleomycin compared to PBS-treated control mice. In healthy C57Bl/6 mice the average total allysine content in the left lung was found to be 6 nmol/lung, equivalent to a concentration of allysine of 80±6 nmol/g in lung tissue. In mice treated with bleomycin, the allysine content was found to increase to 14 nmol/lung after 2 weeks post bleomycin insult, equivalent to a concentration of allysine of 150±16 nmol/g (Figure 3c). A recent report indicated increasing allysine present at 1, 1.5 and 2 weeks post bleomycin insult compared to a sham group. However at 4 weeks post bleomycin insult allysine was significantly reduced, as injury became stable fibroelastic scar.[44]
Fig. 3.
a) HPLC-FL trace for mouse lung labeled with 2-naphthol-7-sulfonate, b) Expansion of the HPLC-FL trace in the region where AL-NP elutes. Mouse lung labeled with 2-naphthol-7-sulfonate (grey), overlaid with HPLC trace (offset) of AL-NP HPLC standard (black), c) comparison of the concentration of allysine measured per gram of tissue in the left lung of sham-treated (n = 8) and bleomycin-treated mice (n = 14)
4. Discussion
Allysine is a fundamental component in the cross-linking of collagen and fiber formation in the extracellular matrix during metastasis and fibrotic disease. Methods to quantify allysine concentration may provide insight into disease progression and the response to therapeutics. Previously reported methods for quantification of allysine using radiolabelled agents were found to be time consuming, complex and often not reproducible.[4, 30–31] More recently GC-MS, HPLC and LC-MS/MS methods have been reported for the quantification of allysine. Sadilkova et. al.[40] and Pena et. al.[39] have both quantified the free allysine content in blood and plasma samples using ‘P6C’ as a marker, with reported LOD’s of 0.1 μmol/L, (~2 pmol/20 μL injection) and 0.0235 pmol respectively which is identical to what we have demonstrated with the current naphthol derivative. Reductive amination studies by Guo et. al.[35] and Akagawa et. al.[36] have isolated allysine labeled with p-aminobenzoic acid from BSA protein samples with Akagawa et. al. reporting a LOD of 0.01 pmol which is comparable with the present naphthol study. No LOD was provided with the Guo study.
Umeda et. al. developed a p-cresol method with good recovery and reproducibility for time-efficient quantification of allysine from tissue.[43] Given the small quantities of murine lung tissue available for ex-vivo analysis, in this paper we looked to increase the sensitivity of detection of the labeled allysine product by replacing the p-cresol with fluorescent sodium 2-naphthol-7-sulfonate. AL-NP is produced through acid hydrolysis similar to the p-cresol ‘APC’ product and is stable in acid at 110 °C over a 48 h period. AL-NP can be quantitatively determined by reverse phase HPLC using fluorescence detection, with strong linearity seen between peak area and AL-NP concentration over the concentration range of allysine found in murine lung tissue samples. Optimized HPLC-FL analysis of the tissue digest samples delivers peak separation, allowing allysine identification and quantification. Reaction conditions of 24 h tissue digest and 40 mg of 2-naphthol-7-sulfonate (~1000 fold excess) ensure complete allysine conversion to AL-NP as determined by recovery experiments where known concentrations of allysine starting material were labeled under the conditions for tissue digest. The presence of lung tissue material had limited effect on the accuracy of allysine labeling with AL-NP recovery largely unaffected by background matrix effects. Labeling with 2-naphthol-7-sulfonate showed an eight-fold increase in detection sensitivity compared to the p-cresol labeling. Labelling of porcine aorta and murine lung gave results on the same order of magnitude as p-cresol results with bovine tissue. Quantification of allysine levels in milligram amounts of mouse lung tissue was possible and allowed changes in allysine content in disease tissue compared to healthy tissue to be detected. Quantification of allysine required only a quarter of total lung homogenate leaving the remainder available for other assays commonly used in pulmonary biology, including hydroxyproline assay and western blot analysis. This analytical technique provides a method to follow the changes in allysine concentration in tissue with increasing disease severity and provide a means to quantify the extent of ECM remodeling in fibrotic and metastatic diseases.
Our results have shown that 2-naphthol-7-sulfonate labeling of allysine under conditions of hydrolysis can be applied to study the total tissue concentrations of allysine. The naphthol-allysine derivative, AL-NP, is stable under the conditions of acid hydrolysis and allows quantification of allysine directly from whole tissue samples without the need for time-consuming tissue processing to isolate collagen proteins. We have demonstrated increased sensitivity compared to the previous p-cresol method, while providing comparable sensitivity to current LC-MS and GC-MS methods used with soluble protein samples. We have demonstrated quantification of allysine in artery and lung tissue, and it may be an effective technique in other tissues as well.
Supplementary Material
Highlights.
Improved method to quantify the allysine concentration in tissue samples using HPLC
Assay sensitive to 0.02 pmol of allysine from milligram quantities of tissue
Method provides a means to assess the effects of novel anti-fibrotic therapeutics
Acknowledgments
This work was supported by the following National Institutes of Health grants: EB009062, DK104302, HL116315, HL131907 (P.C.)
Footnotes
Conflict of interest statement:
P.C. has equity in and is a consultant to Collagen Medical LLC, has equity in Reveal Pharmaceuticals Inc, and has research support from Biogen, Pfizer, and Agilent. This work was not supported by any of these companies. The other authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:206–210. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- 2.Smith-Mungo LI, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16:387–398. doi: 10.1016/s0945-053x(98)90012-9. [DOI] [PubMed] [Google Scholar]
- 3.Pinnell SR, Martin GR. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc Nat Acad Sci. 1968;61:708–716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lent RW, Smith B, Salcedo LL, Faris B, Franzblau C. Reduction of elastin. II. Evidence for the presence of α-aminoadipic acid. delta.-semialdehyde and its aldol condensation product. Biochemistry. 1969;8:2837–2845. doi: 10.1021/bi00835a022. [DOI] [PubMed] [Google Scholar]
- 5.Starcher B, Partridge S, Elsden D. Isolation and Partial Characterization of a New Amino Acid from Reduced Elastin*. Biochemistry. 1967;6:2425–2432. doi: 10.1021/bi00860a019. [DOI] [PubMed] [Google Scholar]
- 6.Franzblau C, Sinex FM, Faris B, Lampidis R. Identification of a new crosslinking amino acid in elastin. Biochem Biophys Res Commun. 1965;21:575–581. doi: 10.1016/0006-291x(65)90524-3. [DOI] [PubMed] [Google Scholar]
- 7.Lent R, Franzblau C. Studies on the reduction of bovine elastin: evidence for the presence of Δ6, 7-dehydrolysinonorleucine. Biochem Biophys Res Commun. 1967;26:43–50. doi: 10.1016/0006-291x(67)90250-1. [DOI] [PubMed] [Google Scholar]
- 8.Partridge S, Elsden D, Thomas J. Constitution of the cross-linkages in elastin. Nature. 1963;197:1297–1298. doi: 10.1038/1971297a0. [DOI] [PubMed] [Google Scholar]
- 9.Thomas J, Elsden D, Partridge S. Degradation products from elastin: partial structure of two major degradation products from the cross-linkages in elastin. Nature. 1963;200:651–652. doi: 10.1038/200651a0. [DOI] [PubMed] [Google Scholar]
- 10.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Inter Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 12.López B, González A, Hermida N, Valencia F, de Teresa E, Díez J. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am J Physiol-Heart C. 2010;299:H1–H9. doi: 10.1152/ajpheart.00335.2010. [DOI] [PubMed] [Google Scholar]
- 13.Mäki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol. 2009;24:651–660. doi: 10.14670/HH-24.651. [DOI] [PubMed] [Google Scholar]
- 14.Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le QT, Chi JTA, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 15.Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K, Hendrix MJ. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–4483. [PubMed] [Google Scholar]
- 16.Baker A, Bird D, Lang G, Cox TR, Erler J. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene. 2013;32:1863–1868. doi: 10.1038/onc.2012.202. [DOI] [PubMed] [Google Scholar]
- 17.Baker AM, Cox TR, Bird D, Lang G, Murray GI, Sun XF, Southall SM, Wilson JR, Erler JT. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J Natl Cancer Inst. 2011;103:407–424. doi: 10.1093/jnci/djq569. [DOI] [PubMed] [Google Scholar]
- 18.Lapointe J, Li C, Higgins JP, Van De Rijn M, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Nat Acad Sci. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, Erler JT. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 2013;73:1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Discher DE, Janmey P, Wang Y-l. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 22.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskel. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 24.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nature Med. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 26.Chien JW, Richards TJ, Gibson KF, Zhang Y, Lindell KO, Shao L, Lyman SK, Adamkewicz JI, Smith V, Kaminski N. Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. European Resp J. 2014;43:1430–1438. doi: 10.1183/09031936.00141013. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson M, Adamo H, Bergh A, Bergström SH. Inhibition of Lysyl Oxidase and Lysyl Oxidase-Like Enzymes Has Tumour-Promoting and Tumour-Suppressing Roles in Experimental Prostate Cancer. Sci Rep. 2016;6 doi: 10.1038/srep19608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schohe-Loop R, Burchardt E, Faeste C, Hirth-Dietrich C, Keldenich J, Knorr A, Lampe T, Naab P, Schmidt D, Schmidt G. 1999 EP1998005622. [Google Scholar]
- 29.Kagan HM, Gacheru SN. 4997854 A. US. 1991
- 30.Diedrich DL, Schnaitman CA. Lysyl-derived aldehydes in outer membrane proteins of Escherichia coli. Proc Nat Acad Sci. 1978;75:3708–3712. doi: 10.1073/pnas.75.8.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paz MA, Keith DA, Traverso HP, Gallop PM. Isolation, purification, and cross-linking profiles of elastin from lung and aorta. Biochemistry. 1976;15:4912–4918. doi: 10.1021/bi00667a025. [DOI] [PubMed] [Google Scholar]
- 32.Sell DR, Kleinman NR, Monnier VM. Longitudinal determination of skin collagen glycation and glycoxidation rates predicts early death in C57BL/6NNIA mice. FASEB J. 2000;14:145–156. doi: 10.1096/fasebj.14.1.145. [DOI] [PubMed] [Google Scholar]
- 33.Sell DR, Strauch CM, Shen W, Monnier VM. 2-aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: effects of diabetes, renal failure and sepsis. Biochemical J. 2007;404:269–277. doi: 10.1042/BJ20061645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills PB, Struys E, Jakobs C, Plecko B, Baxter P, Baumgartner M, Willemsen MA, Omran H, Tacke U, Uhlenberg B. Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nature Med. 2006;12:307. doi: 10.1038/nm1366. [DOI] [PubMed] [Google Scholar]
- 35.Afiuni-Zadeh S, Guo X, Azimi G, Lankmayr E. Optimization and application of microwave-assisted acid hydrolysis for rapid quantification of protein oxidation markers using LC–MS. Talanta. 2011;85:1835–1841. doi: 10.1016/j.talanta.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 36.Akagawa M, Sasaki D, Ishii Y, Kurota Y, Yotsu-Yamashita M, Uchida K, Suyama K. New method for the quantitative determination of major protein carbonyls, α-aminoadipic and γ-glutamic semialdehydes: investigation of the formation mechanism and chemical nature in vitro and in vivo. Chem Res Toxicol. 2006;19:1059–1065. doi: 10.1021/tx060026p. [DOI] [PubMed] [Google Scholar]
- 37.Daneshvar B, Frandsen H, Autrupand H, Dragsted L. γ-Glutamyl semialdehyde and 2-amino-adipic semialdehyde: biomarkers of oxidative damage to proteins. Biomarkers. 1997;2:117–123. doi: 10.1080/135475097231841. [DOI] [PubMed] [Google Scholar]
- 38.Requena JR, Chao CC, Levine RL, Stadtman ER. Glutamic and aminoadipic semialdehydes are the main carbonyl products of metal-catalyzed oxidation of proteins. Proc Nat Acad Sci. 2001;98:69–74. doi: 10.1073/pnas.011526698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pena IA, Marques LA, Laranjeira AB, Yunes JA, Eberlin MN, Arruda P. Simultaneous detection of lysine metabolites by a single LC–MS/MS method: monitoring lysine degradation in mouse plasma. SpringerPlus. 2016;5:172. doi: 10.1186/s40064-016-1809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadilkova K, Gospe SM, Hahn SH. Simultaneous determination of alpha-aminoadipic semialdehyde, piperideine-6-carboxylate and pipecolic acid by LC–MS/MS for pyridoxine-dependent seizures and folinic acid-responsive seizures. J Neurosci Meth. 2009;184:136–141. doi: 10.1016/j.jneumeth.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Akagawa M, Sasaki T, Suyama K. Oxidative deamination of lysine residue in plasma protein of diabetic rats. FEBS J. 2002;269:5451–5458. doi: 10.1046/j.1432-1033.2002.03243.x. [DOI] [PubMed] [Google Scholar]
- 42.Jahanmard E, Suyama K. Bisphenol derivative of allysine for high-performance liquid chromatographic analysis of allysine residue of proteins. J Chromatogr B. 2000;739:273–280. doi: 10.1016/s0378-4347(99)00556-3. [DOI] [PubMed] [Google Scholar]
- 43.Umeda H, Kawamorita K, Suyama K. High-performance liquid chromatographic quantification of allysine as bis-p-cresol derivative in elastin. Amino acids. 2001;20:187–199. doi: 10.1007/s007260170059. [DOI] [PubMed] [Google Scholar]
- 44.Chen HH, Waghorn PA, Wei L, Tapias LF, Schühle DT, Rotile NJ, Jones CM, Looby RJ, Zhao G, Elliott JM. Molecular imaging of oxidized collagen quantifies pulmonary and hepatic fibrogenesis. JCI insight. 2017;2 doi: 10.1172/jci.insight.91506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.