Abstract
Background
In the last decade, many studies have reported abnormal connectivity within the default mode network (DMN) in patients with Alzheimer disease. Few studies, however, have investigated other networks and their association with pathophysiological proteins obtained from cerebrospinal fluid (CSF).
Methods
We performed 3 T imaging in patients with mild Alzheimer disease, patients with amnestic mild cognitive impairment (aMCI) and healthy controls, and we collected CSF samples from the patients with aMCI and mild Alzheimer disease. We analyzed 57 regions from 8 networks. Additionally, we performed correlation tests to investigate possible associations between the networks’ functional connectivity and the protein levels obtained from the CSF of patients with aMCI and Alzheimer disease.
Results
Our sample included 41 patients with Alzheimer disease, 35 with aMCI and 48 controls. We found that the main connectivity abnormalities in those with Alzheimer disease occurred between the DMN and task-positive networks: these patients presented not only a decreased anticorrelation between some regions, but also an inversion of the correlation signal (positive correlation instead of anti-correlation). Those with aMCI did not present statistically different connectivity from patients with Alzheimer disease or controls. Abnormal levels of CSF proteins were associated with functional disconnectivity between several regions in both the aMCI and mild Alzheimer disease groups, extending well beyond the DMN or temporal areas.
Limitations
The presented data are cross-sectional in nature, and our findings are dependent on the choice of seed regions used.
Conclusion
We found that the main functional connectivity abnormalities occur between the DMN and task-positive networks and that the pathological levels of CSF biomarkers correlate with functional connectivity disruption in patients with Alzheimer disease.
Introduction
In the last decade, there has been an increasing number of studies that use functional MRI (fMRI) to explore the resting-state networks (RSNs), among which the default mode network (DMN) is the most studied in the field of Alzheimer disease. Fewer studies, however, have investigated the functional abnormalities of task-positive RSNs. Results showing, for example, disrupted connectivity in the salience (SAL),1 executive control (ECN)2 and language networks (LN),3 among others, show us that the deleterious effects of Alzheimer disease extend well beyond the regions included in the DMN.
Because the pathology substrates are thought to be spatially spread across brain networks,4 alterations in one network could be associated with alterations in others, affecting their connectivity. Previous studies have found, for example, disrupted connections between the SAL and ECN5 and between visuospatial (VSN) and frontoparietal networks6 in patients with Alzheimer disease. Even individuals at risk for Alzheimer disease (i.e., those with amnestic mild cognitive impairment [aMCI]) have been shown to have disruptions between the SAL and both the ECN and DMN1 as well as between the dorsal attention (DAN) and sensorimotor networks.7 Moreover, intercorrelations between the DMN and DAN, between the DMN and sensorimotor network and between the ECN and sensorimotor network have been reported to be reduced with increasing Alzheimer disease severity.8
The spatial distribution of the DMN has a striking overlap with the burden of amyloid β (Aβ1–42)-pathology in patients with Alzheimer disease,9 which has been suggested to be a possible explanation for the connectivity disruption involving this network. The link between Aβ burden and disconnectivity of the DMN, for example, has been demonstrated to be true, as detected with Pittsburgh compound B positron emission tomography (PiB-PET) studies in healthy elderly individuals presenting high loads of Aβ pathology.10 Another widely known pathophysiological feature of Alzheimer disease, however, is the presence of neurofibrillary tangles composed of hyperphosphorylated τ protein (phospho-τ), which may also affect the connectivity of RSNs. As far as we know, however, no studies have investigated the association between the pathophysiological factors of Alzheimer disease obtained from cerebrospinal fluid (CSF) and connectivity between RSNs. Thus, in the present study we investigated connectivity alterations within RSNs, connectivity alterations between RSNs, and possible associations between pathophysiological features of Alzheimer disease extracted from CSF and intra- and internetwork functional connectivity in patients with Alzheimer disease and aMCI.
Methods
Participants
We recruited patients with mild Alzheimer disease, patients with aMCI and healthy controls for participation in our study. To be included in the Alzheimer disease group, patients had to fulfil the standards of the National Institute on Aging–Alzheimer’s Association (NIA-AA),11 and have a Clinical Dementia Rating Scale (CDR)12 score of 1. Alzheimer disease was diagnosed with the help of MRI.
To be included in the aMCI group, patients had to fulfill the standards of the NIA-AA13 and have a CDR score of 0.5 with an obligatory memory score of 0.5. We included only those with positive neuroimaging and/or CSF biomarkers. Hippocampal volume was one of the selection criteria for patients with aMCI to enter the study: we used FreeSurfer (https://surfer.nmr.mgh.harvard.edu) for cortical surface reconstruction and anatomic segmentation of the brain MRI scans and included only those whose total hippocampal volume was 20% below the mean volume of the control group (either right or left hippocampus) owing to the higher risk of conversion to Alzheimer disease.14
Participants in the control group had to be free of neurologic and psychiatric disorders, have no abnormalities on their structural images and have a CDR score of zero.
Additional exclusion criteria for all participants were history of other neurologic/psychiatric diseases or head injury with loss of consciousness, drug or alcohol addiction, a Hachinski ischemic score15 above 4 and a Fazekas Scale score16 above 1. The Medical Research Ethics Committee of the University of Campinas (UNICAMP) approved our study, and we obtained written informed consent from all participants (or from their responsible guardians if the participants were incapable of consenting) before study initiation.
Cerebrospinal fluid sample
We obtained CSF biomarker samples from 27 of the patients with aMCI and 19 of those with Alzheimer disease. The samples were centrifuged at 800g for 10 minutes to remove cells and stored at −80º C until protein analysis. We measured Aβ1–42, total τ and phospho-τ using the Luminex xMAP platform (Inno-Bia Alzbio3 immunoassay reagents, Innogenetics); cut-off values were chosen based on previous research.17
Neuropsychological assessment
Experienced neuropsychologists (A.F.C.-C. and J.E.V.) who were blinded to the MRI data performed the neuropsychological evaluations.
Data acquisition
All MRI scans were acquired using a 3.0 T MRI Philips Achieva scanner. From each participant we obtained a sagittal high-resolution T1-weighted image with isotropic voxels of 1 mm3, repetition time (TR) 7 ms, echo time (TE) 3.2 ms, field of view (FOV) 240 × 240 mm and 180 slices; and a functional echo planar image acquisition with TR 2000 ms, TE 30 ms, FOV 240 × 240, isotropic voxels set to 3 mm3 and no gap with a total scan time of 6 minutes, resulting in 180 dynamics (full brain volumes) with 40 axial slices each. All participants were instructed to keep their eyes closed, to relax, to move as little as possible, and to avoid falling asleep. The MRI procedure lasted 30 minutes.
Functional connectivity analysis
Functional MRI preprocessing and identification of RSNs
We performed the resting-state functional connectivity preprocessing and analysis using an in-house toolbox (UF2C; www.lni.hc.unicamp.br/app/uf2c)18 that runs within the MATLAB platform (2014b, The MathWorks) with SPM12. The image preprocessing routines were based on functional image realignment using the least squares approach with rigid body transformation according to 6 parameters (3 translational and 3 rotational). Because the images were not resliced at this point to avoid redundant interpolation procedures, we used the estimated parameters in the time series regression (described later in this section). The T1-weighted image was coregistered to the functional mean image (resliced representative functional volume) and segmented into grey matter, white matter and CSF. Subsequently, both the functional and T1-weighted images were normalized to Montreal Neurological Institute (MNI) space (MNI-152). The functional images were smoothed (3-dimensional spatial Gaussian filter with a 6 × 6 × 6 mm3 full-width at half-maximum kernel), filtered (bandpass filter 0.008–0.1 Hz) to avoid physiologic confounders, and regressed for the 6 movement parameters (estimated on the realign step) and for white matter and CSF global signals. Finally, we removed possible remaining linear trends of the time series.
Because it has recently been reported that submillimetre head motion during data scanning can have a substantial impact on some measurements of resting-state fMRI,19 some participants were excluded from the analysis. No participants exceeded 3 mm of absolute rotation or translation or a maximum spin larger than 3°. We evaluated group differences in head motion among the groups to ensure there were no significant differences in maximum head motion and framewise displacement.
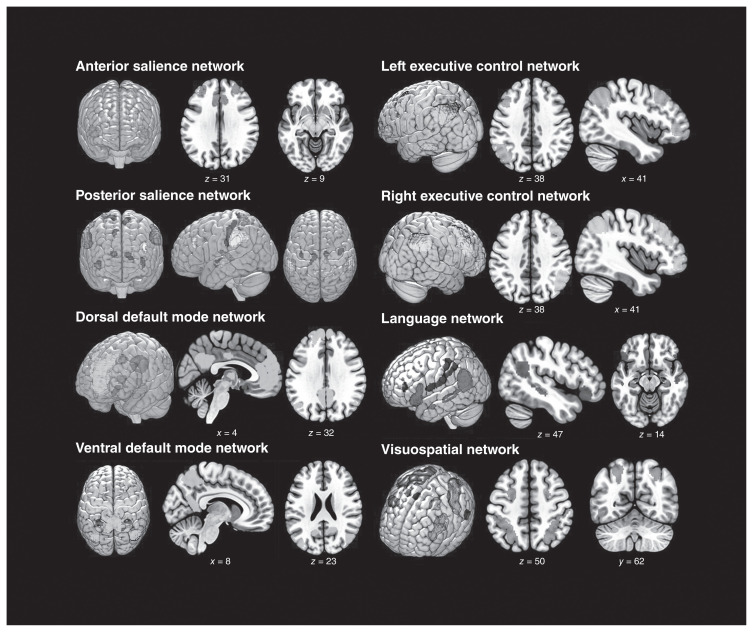
Instead of applying anatomic parcellation as seen in previous studies, we obtained regions of interest (ROIs) from a functional atlas20 (http://findlab.stanford.edu/functional_ROIs.html). We used a total of 57 ROIs in 8 functional networks: the anterior and posterior SAL, the dorsal and ventral DMN, the left and right ECN, the LN, and the VSN/DAN (Fig. 1, Table 1). The seeds time series extraction criteria were protective against nonfunctionally representative signals. The toolbox uses segmented masks and performs an intraseed voxel covariance analysis, excluding possible outlier voxels from the averages of each seed. This procedure enables connectivity analysis unbiased by atrophic or altered tissues.
Fig. 1.
Spatial maps of the 8 resting-state networks analyzed in this study. Functional regions of interest were obtained from http://findlab.stanford.edu/functional_ROIs.html
Table 1.
Anatomic location of the 57 regions of interest used to characterize the 8 resting-state networks*
| Network | ROI | Anatomic location | MNI reference, x, y, z | ROI size (voxels) |
|---|---|---|---|---|
| Anterior salience (n1) | r1n1 | Left middle frontal gyrus | −30, 46, 8 | 651 |
| r2n1 | Left insula | −46, 14, −12 | 305 | |
| r3n1 | Anterior cingulate cortex, medial prefrontal cortex | −8, 32, 16 | 2887 | |
| r4n1 | Right middle frontal gyrus | 28, 46, 16 | 470 | |
| r5n1 | Right insula | 48, 14, −10 | 319 | |
| Posterior salience (n2) | r1n2 | Left middle frontal gyrus | −38, 34, 20 | 93 |
| r2n2 | Left supramarginal gyrus, inferior parietal gyrus | −58, −38, 24 | 1205 | |
| r3n2 | Left precuneus | −4, −50, 56 | 98 | |
| r4n2 | Right middle cingulate cortex | 14, −26, 40 | 56 | |
| r5n2 | Right precuneus, superior parietal gyrus | 12, −58, 62 | 133 | |
| r6n2 | Right supramarginal gyrus, inferior parietal gyrus | 62, −24, 18 | 1002 | |
| r7n2 | Left thalamus | −16, −32, −2 | 142 | |
| r8n2 | Left posterior insula | −36, −10, −14 | 114 | |
| r9n2 | Right thalamus | 14, −22, 4 | 63 | |
| r10n2 | Right posterior insula | 38, −4, −16 | 134 | |
| Dorsal default mode (n3) | r1n3 | Medial prefrontal cortex, anterior cingulate cortex | 2, 44, −28 | 5257 |
| r2n3 | Left angular gyrus | −50, −68, 30 | 97 | |
| r3n3 | Right superior frontal gyrus | 22, 40, 40 | 137 | |
| r4n3 | Posterior cingulate cortex, precuneus | −4, −54, 12 | 1555 | |
| r5n3 | Midcingulate cortex | 4, −16, 32 | 114 | |
| r6n3 | Right angular gyrus | 52, −64, 28 | 38 | |
| r7n3 | Left and right thalamus | −8, −12, −8 | 220 | |
| r8n3 | Left hippocampus | −22, −16, −26 | 393 | |
| r9n3 | Right hippocampus | 26, −16, −26 | 142 | |
| Ventral default mode (n4) | r1n4 | Left retrosplenial cortex, posterior cingulate cortex | −8, −54, 4 | 462 |
| r2n4 | Left middle frontal gyrus | −24, 10, 44 | 405 | |
| r3n4 | Left parahipocampal gyrus | −28, −30, −24 | 134 | |
| r4n4 | Left middle occipital gyrus | −40, −82, 22 | 491 | |
| r5n4 | Right retrosplenial cortex, posterior cingulate cortex | 14, −46, 0 | 590 | |
| r6n4 | Precuneus | 4, −60, 40 | 1921 | |
| r7n4 | Right superior frontal gyrus, middle frontal gyrus | 24, 32, 32 | 399 | |
| r8n4 | Right parahipocampal gyrus | 30, −32, 24 | 90 | |
| r9n4 | Right angular gyrus, middle occipital gyrus | 52, −66, 18 | 752 | |
| Language (n5) | r1n5 | Left inferior frontal gyrus | −46, 24, −20 | 652 |
| r2n5 | Left middle temporal gyrus | −52, −4, −24 | 27 | |
| r3n5 | Left middle temporal gyrus, angular gyrus | −50, −26, −14 | 317 | |
| r4n5 | Left middle temporal gyrus, superior temporal gyrus | −56, −54, 4 | 1420 | |
| r5n5 | Right inferior frontal gyrus | 46, 28, −16 | 58 | |
| r6n5 | Right middle temporal gyrus, angular gyrus | 48, −14, −18 | 1106 | |
| Left executive control (n6) | r1n6 | Left middle frontal gyrus, superior frontal gyrus | −48, 18, 28 | 1501 |
| r2n6 | Left inferior frontal gyrus, orbitofrontal gyrus | −38, 42, −12 | 437 | |
| r3n6 | Left superior parietal gyrus, inferior parietal gyrus, angular gyrus | −50, −64, 28 | 2110 | |
| r4n6 | Left inferior temporal gyrus, middle temporal gyrus | −56, −30, −20 | 350 | |
| r5n6 | Left thalamus | −14, −29, 0 | 8 | |
| Right executive control (n7) | r1n7 | Right middle frontal gyrus, superior frontal gyrus | 50, 30, 16 | 2093 |
| r2n7 | Right middle frontal gyrus | 44, 46, −14 | 356 | |
| r3n7 | Right inferior parietal gyrus, supramarginal gyrus, angular gyrus | 48, −60, 32 | 1873 | |
| r4n7 | Right superior frontal gyrus | 6, 34, 38 | 83 | |
| r5n7 | Right caudate | 10, −4, 8 | 188 | |
| Visuospatial/dorsal attention (n8) | r1n8 | Left middle frontal gyrus, superior frontal gyrus, precentral gyrus | −22, −4, 46 | 338 |
| r2n8 | Left inferior parietal gyrus | −54, −30, 30 | 2020 | |
| r3n8 | Left frontal operculum, inferior frontal gyrus | −40, 30, 8 | 1105 | |
| r4n8 | Left inferior temporal gyrus | −48, −64, −12 | 93 | |
| r5n8 | Right middle frontal gyrus | 28, −2, 48 | 97 | |
| r6n8 | Right inferior parietal gyrus | 30, −64, 36 | 1193 | |
| r7n8 | Right frontal operculum, inferior frontal gyrus | 50, 12, 16 | 326 | |
| r8n8 | Right inferior temporal gyrus | 50, −62, −14 | 76 |
MNI = Montreal Neurological Institute; ROI = region of interest.
Regions of interest obtained from http://findlab.stanford.edu/functional_ROIs.html
Statistical analysis
We performed nonimaging statistical data analysis using SPSS software version 22 (SPSS Inc.). We conducted a χ2 test to compare the frequency distribution of sex and analyses of variance (ANOVAs) to compare age and years of education among the groups. Nonimaging results were considered to be statistically significant at p < 0.05, Bonferroni-corrected for multiple comparisons.
Adjacency matrices (ROI × ROI analysis)
The approach used to compare network connectivity among the groups was an ROI-wise analysis. At this step, each ROI of any given network was independently compared with all the other ROIs. We created the cross- correlation matrices by performing Pearson correlation tests (pairwise combination of all 57 ROIs, removing auto [diagonal] and symmetric correlations). These individual correlation matrices were subsequently converted to z scores (Fisher z transformation) and taken to a group comparison analysis to investigate differences between the control and patient groups. In addition to the comparisons between groups, we also tested for associations between the ROI × ROI functional connectivity and CSF values by performing correlation tests in patients with aMCI and Alzheimer disease. Group comparison and correlation tests between functional connectivity and CSF values were performed in our UF2C toolbox.
For the group comparison analysis, the terms “decreased connectivity” and “increased connectivity” were used to classify the alterations among groups and indicate whether the patients had lower or higher absolute connectivity values (using a group score as the reference). In this sense, “decreased” refers to Pearson correlation values farther away from zero when compared with controls, and “increased” refers to Pearson correlation values closer to zero when compared with controls. The statistical tests, however, were performed with the original values, making sure that comparisons among correlations in opposite directions had been considered accordingly in the statistical analysis. The UF2C toolbox not only shows statistical differences among groups, but also distinctly presents comparisons among positive values and negative values and highlights situations in which the correlation signals are opposite among groups, whereas most fMRI toolboxes exclude negative correlations from the analysis or use absolute values. As 2 networks with negative correlation values are said to be anticorrelated, decreased negative value is equivalent to less anticorrelation, whereas increased negative value is equivalent to more anti-correlation. We considered results for imaging data to be significant at p < 0.05, false discovery rate (FDR)–corrected for multiplicity.
Results
Demographic and neuropsychological data
This study included 124 participants: 41 patients with mild Alzheimer disease, 35 with aMCI and 48 healthy controls. Table 2 shows the demographic and neuropsychological data of the sample. Groups did not differ in sex or age; however, they differed in years of education, and this variable was included as a confounding factor in the analysis.
Table 2.
Demographic and neuropsychological characteristics of the study population
| Characteristic | Group; mean ± SD* | p value; comparison | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Control, n = 48 | aMCI, n = 35 | Alzheimer, n = 41 | Control v. aMCI | aMCI v. Alzheimer | Control v. Alzheimer | |
| Female sex, no. (%) | 33 (69) | 23 (65) | 26 (63) | — | — | — |
| Age, yr | 68.12 ± 8.78 | 68.32 ± 6.4 | 72.21 ± 12.94 | > 0.99 | 0.25 | 0.16 |
| Education, yr | 11.86 ± 5.6 | 9.05 ± 5.45 | 7.33 ± 5.3 | 0.047 | 0.33 | 0.001 |
| CDR score | 0 | 0.5 | 1 | — | — | — |
| MMSE | 28.61 ± 1.68 | 22.94 ± 2.6 | 19.53 ± 3.82 | < 0.001 | 0.048 | < 0.001 |
| Episodic memory tests | ||||||
| RAVLT encoding | 45.02 ± 9.19 | 28.94 ± 8.44 | 20.12 ± 6.76 | < 0.001 | 0.003 | < 0.001 |
| RAVLT A7 | 8.64 ± 2.74 | 3.21 ± 2.09 | 1 ± 1.37 | < 0.001 | < 0.001 | < 0.001 |
| RAVLT CR-FP | 11.34 ± 3.35 | 5.42 ± 6.06 | −2.6 ± 5.1 | < 0.001 | < 0.001 | < 0.001 |
| Working memory/attention tests | ||||||
| FDS | 5.48 ± 1.45 | 4.53 ± 0.94 | 3.76 ± 1.4 | 0.008 | 0.80 | < 0.001 |
| BDS | 4.34 ± 1.14 | 3.36 ± 1.06 | 2.2 ± 1.5 | 0.005 | 0.002 | < 0.001 |
| Language tests | ||||||
| SVF | 17.66 ± 4.6 | 12.65 ± 3.95 | 9.72 ± 4.79 | < 0.001 | 0.003 | < 0.001 |
| FAS | 35.5 ± 11.65 | 27.82 ± 9.9 | 20.2 ± 12.03 | 0.015 | 0.026 | < 0.001 |
| BNT | 63.36 ± 71.05 | 49 ± 9.37 | 38.52 ± 12.5 | 0.56 | 0.90 | 0.039 |
| Executive function tests | ||||||
| Stroop C,s | 50.05 ± 16.14 | 47.94 ± 13.25 | 70.4 ± 35.32 | > 0.99 | < 0.001 | < 0.001 |
| Stroop C, errors | 0.09 ± 0.03 | 0.03 ± 0.02 | 0.52 ± 1.05 | > 0.99 | 0.002 | 0.001 |
| Stroop I, s | 104.02 ± 28.08 | 130.47 ± 60.11 | 168.88 ± 90.98 | 0.27 | 0.042 | 0.001 |
| Stroop I, errors | 3.3 ± 4.71 | 6.94 ± 8.9 | 19.83 ± 18.55 | 0.85 | < 0.001 | < 0.001 |
| TMT-A, s | 61.18 ± 18.02 | 91.96 ± 50.45 | 161.52 ± 114.33 | 0.18 | < 0.001 | < 0.001 |
| TMT-B, s | 112.24 ± 74.07 | 141.59 ± 90.2 | 198.24 ± 133.52 | 0.60 | 0.06 | 0.001 |
| Visuospatial skill tests | ||||||
| Rey figure copy | 41.75 ± 3.15 | 27.12 ± 10.23 | 19.16 ± 13.19 | 0.13 | 0.91 | 0.005 |
| Clock drawing (0–10) | 9.09 ± 1.61 | 8.76 ± 1.85 | 5.48 ± 2.64 | 0.07 | < 0.001 | < 0.001 |
| LNI | 17.96 ± 1.38 | 17.94 ± 4.85 | 14.32 ± 4.31 | > 0.99 | 0.005 | < 0.001 |
aMCI = amnestic mild cognitive impairment; BDS = backward digit span; BNT = Boston naming test; C = congruent; CDR = Clinical Dementia Rating; FAS = phonological fluency for letters; FDS = forward digit span; I = incongruent; LNI = Luria’s neuropsychological investigation; MMSE = Mini-Mental Status Examination; RAVLT = Rey Auditory Verbal Learning Test; RAVLT A7 = delayed recall of Rey auditory verbal learning test; RAVLT RC-FP = Rey auditory verbal learning test true recognition (correct recognition – false positives); Rey = Rey-Osterrieth Complex Figure Test; SD = standard deviation; SVF = semantic verbal fluency; TMT = Trail Making Test.
Unless indicated otherwise.
Cerebrospinal fluid values
The mean CSF biomarker protein values of the aMCI sample were as follows: total τ 97.1 ± 72.58, phospho-τ 43.57 ± 32.86, Aβ1–42 386.56 ± 173.71 and Aβ1–42/phospho-τ 8.09 ± 7.6. The mean values for the Alzheimer disease group were as follows: total τ 170.84 ± 73.95, phospho-τ 54.87 ± 30.69, Aβ1–42 360.86 ± 100.88 and Aβ1–42/phospho-τ 7.18 ± 4.91. There were no signicant differences in total τ (p = 0.09), phospho-τ (p = 0.47), Aβ1–42 (p = 0.66), or Aβ1–42/phospho-τ (p = 0.98) between the 2 patient groups.
Functional connectivity analysis
Owing to head motion during scanning, 2 controls, 3 patients with aMCI and 5 with mild Alzheimer disease were excluded from the functional connectivity analysis.
Controls versus patients with aMCI
The ROI × ROI analysis showed that patients with aMCI did not present FDR-corrected statistically different results from controls. Uncorrected (p < 0.001) results for multiplicity, adjusted for years of education, show that compared with controls patients with aMCI had decreased internetwork functional connectivity between some ROIs of the ventral and dorsal DMN, the posterior SAL and the VSN/DAN. We observed inversion of the correlation signal in ROIs belonging to the ventral and dorsal DMN and the LN. From a network perspective, regions of the DMN were mainly affected, specifically parietal and posterior cingulate regions (Appendix 1, Fig. S1, available at jpn.ca/160190-a1).
Controls versus patients with mild Alzheimer disease
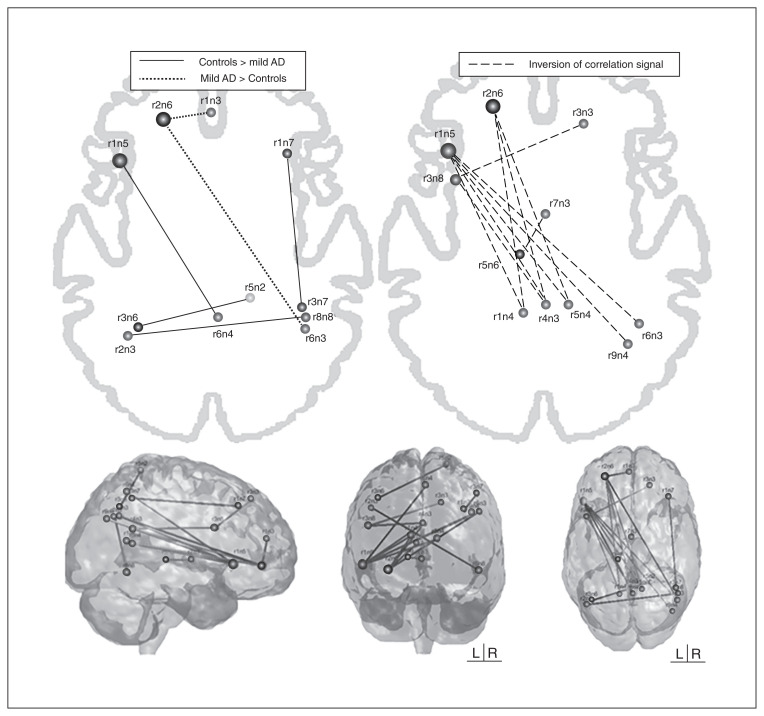
Compared with controls, patients with mild Alzheimer disease had 7 networks with alterations (only the anterior SAL showed no functional connectivity disruption) in the pairwise ROI analysis, adjusted for years of education, that survived to FDR-correction. In total, 19 ROIs linked by 17 connections showed disrupted functional connectivity in this group relative to the control sample. Patients with mild Alzheimer disease showed an intranetwork positive connectivity decrease (closer to zero) between 2 ROIs of the right ECN (r1n7–r3n7), whereas a decreased internetwork anticorrelation (closer to zero) was found between an ROI belonging to the LN (r1n5) and a region of the ventral DMN (r6n4), between the left ECN (r3n6) and posterior SAL (r5n2), and between the dorsal DMN (r2n3) and the VSN/DAN (r8n8; Fig. 2). We observed increased internetwork positive connectivity (farther away from zero) between the left ECN (r2n6) and 2 regions of the dorsal DMN (r1n3 and r6n3; Fig. 2). An inversion of the correlation signal was found mainly in 3 regions: the first belonging to the VSN/DAN, the second belonging to the left ECN, and the third belonging to the LN (Table 3, Fig. 2), and in all of these connections the correlation signal was negative in controls and positive in patients with Alzheimer disease.
Fig. 2.
Region of interest functional connectivity analysis between controls and patients with mild Alzheimer disease (AD). Solid lines represent higher values of functional connectivity in controls, dotted lines represent higher values in patients with Alzheimer disease (absolute differences in functional connectivity strength between regions of interest), and dashed lines represent an inversion of correlation signal). See Table 1 for anatomic regions.
Table 3.
Connections between regions of interest that presented an inversion of the correlation signal in patients with mild Alzheimer disease relative to controls (negative correlation in controls and positive correlation signal in patients with mild Alzheimer disease)
| ROI/region | ROI name | Network | Anatomic region |
|---|---|---|---|
| Visuospatial/dorsal attention network (r3n8 — left frontal operculum/inferior frontal gyrus) |
r3n3 | Dorsal default mode | Right superior frontal gyrus |
| r4n3 | Dorsal default mode | Posterior cingulate cortex/precuneus | |
| Left executive control network (r2n6 — left inferior frontal gyrus) |
r5n4 | Ventral default mode | Right retrosplenial cortex/posterior cingulate cortex |
| r1n4 | Ventral default mode | Left retrosplenial cortex/posterior cingulate cortex | |
| r4n3 | Dorsal default mode | Posterior cingulate cortex/precuneus | |
| Language network (r1n5 — left inferior frontal gyrus) |
r1n4 | Ventral default mode | Left retrosplenial cortex/posterior cingulate cortex |
| r5n4 | Ventral default mode | Right retrosplenial cortex/posterior cingulate cortex | |
| r9n4 | Ventral default mode | Right angular gyrus/middle occipital gyrus | |
| r6n3 | Dorsal default mode | Right angular gyrus | |
| r4n3 | Dorsal default mode | Posterior cingulate cortex/precuneus |
ROI = region of interest.
From a network perspective, patients with mild Alzheimer disease showed less anticorrelation between the right ECN and posterior SAL, LN and ventral DMN; a weaker negative correlation between the dorsal DMN and VSN/DAN; and a weaker positive correlation within the right ECN than controls. On the other hand, a stronger positive connectivity was found between the left ECN and dorsal DMN. The inversion of the correlation signal was observed mainly between anteroposterior areas (i.e., between the left inferior frontal area and the posterior cingulate cortex/precuneus) and largely affected the connection between the DMN and VSN/DAN and between the left ECN and the LN, which were anticorrelated in controls and positively correlated in patients with mild Alzheimer disease.
Patients with aMCI versus patients with mild Alzheimer disease
The ROI × ROI analysis showed that patients with aMCI did not present FDR-corrected statistically different results from patients with mild Alzheimer disease. Uncorrected results (p < 0.001), adjusted for years of education, showed that compared with patients with aMCI, those with mild Alzheimer disease had decreased internetwork connectivity in between the left ECN and the posterior SAL and dorsal DMN. Inversion of the correlation signal was found between the ventral DMN and left ECN and between the VSN/DAN and several ROIs of the DMN (Appendix 1, Fig. S2).
Association between functional connectivity and CSF values
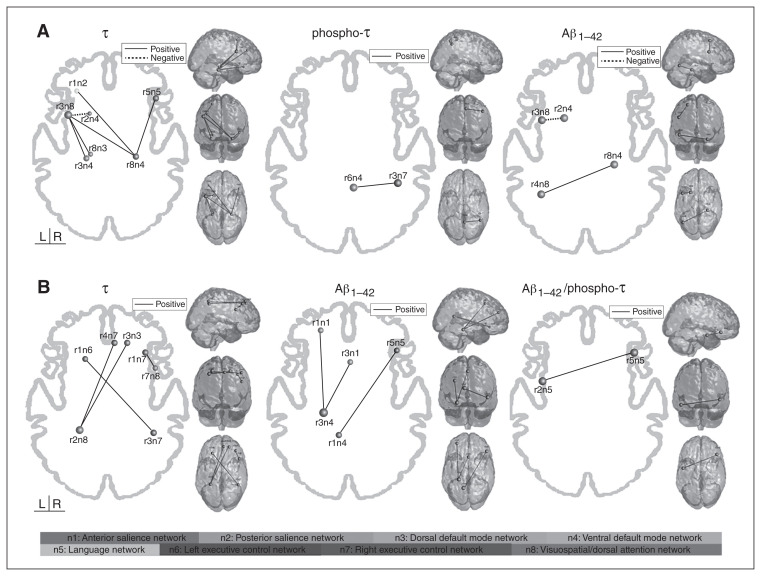
In the aMCI group, the functional connectivity of the following regions showed a positive association with CSF total τ levels: the ventral DMN (r8n4) and the LN (r5n5), the posterior SAL (r1n2) and the VSN/DAN (r3n8), and the VSN/DAN (r3n8) and both the dorsal DMN (r8n3) and the ventral DMN (r3n4). We found a negative correlation between the functional connectivity between the VSN/DAN (r3n8) and the ventral DMN (r2n4). Phospho-τ was positively associated with connectivity between the ventral DMN (r6n4) and the right ECN (r3n7). Aβ1–42 was positively associated with connectivity between the ventral DMN (r8n4) and the VSN/DAN (r4n8) and negatively associated with connectivity between the ventral DMN (r2n4) and the VSN/DAN (r3n8; Fig. 3A, Table 4).
Fig. 3.
Significant correlations between functional connectivity in the regions of interest and cerebrospinal fluid (CSF) protein levels in (A) patients with amnestic mild cognitive impairment (aMCI) and (B) patients with mild Alzheimer disease (AD). Solid lines represent positive correlation, and dotted lines represent negative correlation. See Table 1 for anatomic regions.
Table 4.
Strength of the correlations between cerebrospinal fluid biomarkers and functional connectivity of regions of interest in patients with aMCI and mild Alzheimer disease
| Group | CSF biomarker | ROI | ROI | r |
|---|---|---|---|---|
| aMCI | Total τ | r1n2 | r8n4 | 0.63 |
| r3n8 | r2n4 | −0.601 | ||
| r3n8 | r8n3 | 0.602 | ||
| r3n8 | r3n4 | 0.637 | ||
| r3n8 | r8n4 | 0.612 | ||
| r8n4 | r5n5 | 0.621 | ||
| Phospho-τ | r6n4 | r3n7 | 0.55 | |
| Aβ1–42 | r3n8 | r2n4 | −0.65 | |
| r4n8 | r8n4 | 0.57 | ||
| Alzheimer disease | Total τ | r3n3 | r2n8 | 0.698 |
| r1n7 | r7n8 | 0.769 | ||
| r2n8 | r4n7 | 0.697 | ||
| r1n6 | r3n7 | 0.765 | ||
| Aβ1–42 | r1n1 | r3n4 | 0.724 | |
| r3n4 | r3n1 | 0.693 | ||
| r1n4 | r5n5 | 0.728 | ||
| Aβ1–42/phospho-τ | r2n5 | r5n5 | 0.693 |
aMCI = amnestic mild cognitive impairment; CSF = cerebrospinal fluid; ROI = region of interest.
In the Alzheimer disease group, the functional connectivity of the following networks showed a positive association with CSF total τ levels: the right ECN (r1n7) and the VSN/DAN (r7n8), the left ECN (r1n6) and the right ECN (r3n7), and the VSN/DAN (r2n8) and both the dorsal DMN (r3n3) and the right ECN (r4n7). The Aβ1–42 was positively associated with functional connectivity between the ventral DMN (r1n4) and the LN (r5n5) and between the ventral DMN (r3n4) and 2 regions of the anterior SAL (r1n1 and r3n1). The functional connectivity of 2 regions of the LN (r2n5 and r5n5) was positively associated with Aβ1–42/phospho-τ (Fig. 3B, Table 4).
It may be helpful to mention that lower levels of CSF Aβ1–42 and Aβ1–42/phospho-τ reflect a pattern of Alzheimer disease pathology. A reduced CSF level of Aβ1–42 may at least partly be due to a sequestration of amyloid in plaques, with lower levels diffusing to CSF.21 High levels of CSF total τ and phospho-τ, on the contrary, correlated with the presence of neurofibrillary tangles, predicting the presence of Alzheimer disease pathologic features with high accuracy.22 In other words, patients with Alzheimer disease are expected to have higher levels of total τ and phospho-τ and lower levels of Aβ1–42 and Aβ1–42/phospho-τ in the CSF than the healthy elderly population. For patients with aMCI, the higher the CSF total τ and phospho-τ levels (brain pathologic feature), the higher the functional connectivity mainly between the DMN, VSN/DAN and right ECN. On the other hand, a higher level of CSF total τ (brain pathologic feature) was associated with decreased functional connectivity between the VSN/DAN and the DMN. Lower Aβ1–42, which reflects higher amyloid burden in the brain (brain pathologic feature), was associated with both stronger and weaker functional connectivity between the VSN/DAN and the ventral DMN.
For patients with Alzheimer disease, the higher the level of CSF total τ, the stronger the functional connectivity of the bilateral ECN, DMN and VSN/DAN. Lower levels of Aβ1–42 and Aβ1–42/phospho-τ, in turn, were associated with decreased functional connectivity of the anterior SAL, LN and DMN.
Discussion
In the present work, we aimed to evaluate the differences of both intra- and internetwork functional connectivity of large-scale RSNs in the aMCI and Alzheimer disease groups and to correlate the imaging data with characteristic pathophysiological proteins obtained from CSF. Overall, although the DMN was the network with more disruptions in patients with Alzheimer disease, connectivity alterations were not limited to the DMN; other networks were also affected, extending the findings of previous studies. Nearly all RSNs analyzed in the present study were disrupted in patients with Alzheimer disease. Furthermore, these patients presented an inverted correlation (i.e., positive instead of negative) between the DMN and the VSN/DAN, between the DMN and the LN, and between the DMN and the left ECN. Pathological levels of CSF proteins were associated with the connectivity of several RSNs other than the DMN, such as the VSN/DAN, bilateral ECN, anterior SAL and LN, both in patients with aMCI and in those with Alzheimer disease.
Disruptions in RSNs in patients with mild Alzheimer disease have been reported extensively in the literature over the last decade. In the present work, we found that network disruptions in patients with mild Alzheimer disease go beyond intranetwork connectivity, but the associations between RSNs (i.e., internetwork connectivity) are also altered. The Alzheimer disease group showed decreased positive connectivity within the ECN and decreased anticorrelation between the ECN and the posterior SAL. A recent study23 that used pathophysiological interaction analysis to investigate modulatory interactions among RSNs reported an association between the SAL and executive networks, suggesting that they are functionally connected. Our work in turn, suggests that this connectivity is damaged in patients with Alzheimer disease, aligning with the findings of previous research reporting loss of correlation between the SAL and ECN in patients with Alzheimer disease5 and even in those with aMCI.1 Although the present study did not find statistically significant results for the aMCI group, functional connectivity disruptions within7 and between1,7 networks in these patients have been reported previously in the literature. Our lack of significant results for patients with aMCI could be due to the heterogeneity of the sample (early v. late aMCI), different cognitive reserve levels, which is known to influence the functional connectivity of networks,24 or our different methodological approach. The main methodological difference between the present study and previous internetwork functional connectivity studies lies in the analysis of the correlation signal between ROIs. In other words, we did not exclude negative correlations from the analysis (or we used absolute values) to avoid misleading interpretations about the directions of the alterations. By doing so, we could evaluate not only the strength of the correlations, but also their directions.
Among patients with Alzheimer disease, for example, we observed alterations between the DMN and other RSNs, such as a decreased negative connectivity between the ventral DMN and the LN and between the dorsal DMN and the VSN/DAN (which were less anticorrelated in patients with Alzheimer disease), as published previously.8 On the other hand, we observed an increased correlation between the DMN and the ECN in patients with Alzheimer disease (more positive correlation in Alzheimer disease). Similarly, patterns of inverted correlation were observed between the DMN and the VSN/DAN, between the DMN and the LN, and between the DMN and the left ECN (anticorrelated in controls and positively correlated in patients with Alzheimer disease).
The complex interactions between the DMN and other networks are of utmost importance for cognitive processing. It is known, for example, that some systems in the brain are intrinsically organized into anticorrelated networks, such as the DMN and external goal-oriented tasks systems.25 The DMN consists of a set of areas that are suppressed during goal-directed cognition (task-negative network),26 whereas other RSNs, such as the ECN and VSN/DAN, are recruited in tasks in which participants have to maintain external attention (task-positive networks).27 Furthermore, decreased suppression of the DMN is associated with poorer cognitive task performance in the healthy population28 and in cognitive decline groups.29 Taken together, these findings provide evidence of the complex and intrinsic association between the DMN and task-positive networks for cognition. In the present study, we found that the main connectivity abnormalities in patients with Alzheimer disease occur between the DMN and some task-positive RSNs: not only did patients with Alzheimer disease show less anticorrelation between some regions (between the ventral DMN and the LN, and between the dorsal DMN and the VSN/DAN), but also an inversion of the correlation signal between the DMN and task-positive networks (between the DMN and the VSN/DAN, between the DMN and the LN, and between the DMN and the left ECN, which were positively correlated instead of anticorrelated).
The mechanisms behind this anticorrelation are not yet understood, but it has been suggested that anticorrelated RSNs might have opposed functional roles. For instance, an fMRI study in rats30 that found anticorrelations within the frontolimbic circuit (between the amygdala and the infralimbic cortex) suggested that their negative association could arise from an inhibitory interaction between these areas. The inversion of the correlation signal found in patients with Alzheimer disease (between regions that would be expected to be anticorrelated) could reflect an inappropriate recruitment of task-positive networks by the DMN to compensate for damage. Furthermore, converging evidence suggests that shifts in excitatory/inhibitory balance in vulnerable cortical circuits are significant drivers in the pathophysiological progression of Alzheimer disease.31
Interestingly, most of the correlation abnormalities involved the DMN regions, which are structures known to be hit by the pathophysiological process in Alzheimer disease.9 For instance, it has repeatedly been demonstrated in PiB-PET studies that the presence of amyloid plaques is sufficient for there to be functional connectivity changes not only in the DMN,10 but also in the SAL and ECN32 and even between networks.33 In the present study, we found that a lower level of CSF Aβ1–42 was associated with decreased functional connectivity between the ventral DMN and the LN and between the ventral DMN and the anterior SAL in patients with Alzheimer disease, whereas in patients with aMCI there was stronger functional connectivity between some regions of the ventral DMN and the VSN/DAN and decreased connectivity between other regions of the ventral DMN and the VSN/DAN. Amyloid burden in the DMN areas is a plausible explanation for the functional connectivity disruption between these RSNs; in addition, previous studies using PiB-PET amyloid imaging have demonstrated that amyloid accumulation occurs throughout the lateral frontoparietal cortex34 and extends to the attention network,35 affecting the functional connectivity of regions belonging to the VSN/DAN in patients with Alzheimer disease.36 Furthermore, a lower level of CSF Aβ1–42/phospho-τ was associated with decreased functional connectivity within regions of the LN in patients with Alzheimer disease, complementing the results of previous work that found that a lower level of CSF Aβ1–42/phospho-τ was associated with DMN disconnectivity.37
The extent to which other pathological proteins affect RSN connectivity, however, remains unclear. Among samples obtained from CSF, we showed that besides Aβ1–42, total τ and phospho-τ levels also affect the brain’s functional connectivity. For instance, both the aMCI and Alzheimer disease groups showed abnormalities in functional connectivity associated with the presence of total τ in the CSF: higher levels of total τ (which reflects neuronal degeneration38) in patients with aMCI were associated with disruptions between the DMN and the LN and between the DMN and the VSN/DAN, whereas in patients with Alzheimer disease higher levels of τ were associated with disruptions between the right ECN and the VSN/DAN, between the left and right ECN, between the VSN/DAN and the dorsal DMN, and between the VSN/DAN and the right ECN. Phospho-τ levels also had an impact in patients with aMCI: a higher level of CSF phospho-τ was associated with stronger functional connectivity between the ventral DMN and the right ECN. Although the direction of the association seems unexpected, our results suggest that the presence of a higher level of CSF total τ and phospho-τ implies an imbalance between several brain regions and affects the connectivity of many RSNs, either making them stronger or weaker. In this context, the association between CSF total τ levels and functional alterations in many brain regions should be interpreted in light of the new findings on the spread of the pathological proteins during the course of Alzheimer disease.
Phospho-τ is a protein considered to be an indicator of the pathological hyperphosphorylation process of total τ and the presence of neurofibrillary tangle formation in the brain.39 Both are concentrated mainly in temporal areas, but various in vitro and in vivo studies have reported the seeding and propagation of total τ aggregates in the brain along the neuroanatomical connections during onset of the disease as well as during its course.40 Therefore, it is highly likely that the pathological process associated with the τ protein affects the functional communication between brain areas. Furthermore, the tauopathy is highly correlated with cognitive decline and with the clinical progression of Alzheimer disease, suggesting a central role of τ during the course of the disease.41 In the present work, higher CSF levels of total τ and phospho-τ (i.e., indicators of brain degeneration) were associated with increased functional connectivity between many regions in both patients with aMCI and those with Alzheimer disease that extend beyond the temporal areas.
Limitations
This work has some limitations that must be acknowledged. First, all presented data are cross-sectional, and longitudinal studies would enable investigation of which interactions among RSNs are first disrupted and of possible effects on the connectivity in other RSNs. Second, our findings depend on our choice of seed regions (though our chosen regions had been studied previously20), and whole brain functional connectivity analysis or the selection of different ROIs could provide different results. Third, because there was no statistical difference in age among the groups, we decided not to include it as a nuisance variable in our analysis; however, we recognize that aging could be associated with both CSF and functional connectivity results. Regardless of these limitations, the present work provides reliable evidence that the connectivity between several RSNs is disrupted in patients with Alzheimer disease and that CSF protein levels in both patients with aMCI and those with Alzheimer disease are associated with RSN abnormalities.
Conclusion
According to previous studies, the functional alterations in patients with Alzheimer disease extend beyond the connectivity within networks, and the interaction between RSNs are also affected (i.e., internetwork connectivity). We found that the main connectivity abnormalities in patients with Alzheimer disease occur between the DMN and some task-positive RSNs: not only did patients with Alzheimer disease show less anticorrelation between some regions, they also showed an inversion of the correlation signal (positively correlated instead of anticorrelated) between some areas. Abnormal levels of CSF proteins were associated with functional disconnectivity between several regions in both patients with aMCI and those with mild Alzheimer disease, extending well beyond the DMN or temporal areas.
Acknowledgements
The study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo grant #2013/10431-9.
Footnotes
Competing interests: None declared.
Contributors: M. Weiler, B. de Campos and M. Balthazar designed the study. M. Weiler, C. Teixeira, A. Carletti-Cassani, J. Vicentini and T. Magalhães acquired the data, which M. Weiler, B. de Campos, R. Casseb, L. Talib, O. Forlenza and M. Balthazar analyzed. M. Weiler, B. de Campos, R. Casseb, A. Carletti-Cassani, J. Vicentini, T. Magalhães and L. Talib wrote the article, which all authors reviewed and approved for publication.
References
- 1.He X, Qin W, Liu Y, et al. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp. 2014;35:3446–64. doi: 10.1002/hbm.22414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agosta F, Pievani M, Geroldi C, et al. Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiol Aging. 2012;33:1564–78. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Weiler M, Fukuda A, Massabki LH, et al. Default mode, executive function, and language functional connectivity networks are compromised in mild Alzheimer’s disease. Curr Alzheimer Res. 2014;11:274–82. doi: 10.2174/1567205011666140131114716. [DOI] [PubMed] [Google Scholar]
- 4.Greicius MD, Kimmel DL. Neuroimaging insights into network-based neurodegeneration. Curr Opin Neurol. 2012;25:727–34. doi: 10.1097/WCO.0b013e32835a26b3. [DOI] [PubMed] [Google Scholar]
- 5.Dai Z, Yan C, Li K, et al. Identifying and mapping connectivity patterns of brain network hubs in Alzheimer’s disease. Cereb Cortex. 2015;25:3723–42. doi: 10.1093/cercor/bhu246. [DOI] [PubMed] [Google Scholar]
- 6.Song J, Qin W, Liu Y, et al. Aberrant functional organization within and between resting-state networks in AD. PLoS One. 2013;8:e63727. doi: 10.1371/journal.pone.0063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang P, Zhou B, Yao H, et al. Aberrant intra- and inter-network connectivity architectures in Alzheimer’s disease and mild cognitive impairment. Sci Rep. 2015;5:14824. doi: 10.1038/srep14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brier MR, Thomas JB, Snyder AZ, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–7. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKhann GM. Changing concepts of Alzheimer disease. JAMA. 2011;305:2458–9. doi: 10.1001/jama.2011.810. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 13.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association work-groups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–36. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 15.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–7. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 16.Fazekas F, Chawluk J, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJNR Am J Neuroradiol. 1987;149:351–6. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 17.Olsson A, Vanderstichele H, Andreasen N, et al. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51:336–45. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 18.De Campos BM, Coan AC, Lin Yasuda C, et al. Large-scale brain networks are distinctly affected in right and left mesial temporal lobe epilepsy. Hum Brain Mapp. 2016;37:3137–52. doi: 10.1002/hbm.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirer WR, Ryali S, Rykhlevskaia E, et al. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–65. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blennow K, Zetterberg H. Cerebrospinal fluid biomarkers for Alzheimer’s disease. J Alzheimers Dis. 2009;18:413–7. doi: 10.3233/JAD-2009-1177. [DOI] [PubMed] [Google Scholar]
- 22.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–9. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 23.Di X, Biswal BB. Modulatory interactions between the default mode network and task positive networks in resting-state. PeerJ. 2014;2:e367. doi: 10.7717/peerj.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bozzali M, Dowling C, Serra L, et al. The impact of cognitive reserve on brain functional connectivity in Alzheimer’s disease. J Alzheimers Dis. 2015;44:243–50. doi: 10.3233/JAD-141824. [DOI] [PubMed] [Google Scholar]
- 25.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anticevic A, Cole MW, Murray JD, et al. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–92. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–60. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 28.Sala-Llonch R, Bosch B, Arenaza-Urquijo EM, et al. Greater default-mode network abnormalities compared to high order visual processing systems in amnestic mild cognitive impairment: an integrated multi-modal MRI study. J Alzheimers Dis. 2010;22:523–39. doi: 10.3233/JAD-2010-101038. [DOI] [PubMed] [Google Scholar]
- 29.Hansen NL, Lauritzen M, Mortensen EL, et al. Subclinical cognitive decline in middle-age is associated with reduced task-induced deactivation of the brain’s default mode network. Hum Brain Mapp. 2014;35:4488–98. doi: 10.1002/hbm.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang Z, King J, Zhang N. Anticorrelated resting-state functional connectivity in awake rat brain. Neuroimage. 2012;59:1190–9. doi: 10.1016/j.neuroimage.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kann O. The interneuron energy hypothesis: implications for brain disease. Neurobiol Dis. 2016;90:75–85. doi: 10.1016/j.nbd.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Lim HK, Nebes R, Snitz B, et al. Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects. Brain. 2014;137:3327–38. doi: 10.1093/brain/awu271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elman JA, Madison CM, Baker SL, et al. Effects of beta-amyloid on resting state functional connectivity within and between networks reflect known patterns of regional vulnerability. Cereb Cortex. 2016;26:695–707. doi: 10.1093/cercor/bhu259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sepulcre J, Sabuncu MR, Becker A, et al. In vivo characterization of the early states of the amyloid-beta network. Brain. 2013;136:2239–52. doi: 10.1093/brain/awt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers N, Pasquini L, Gottler J, et al. Within-patient correspondence of amyloid-beta and intrinsic network connectivity in Alzheimer’s disease. Brain. 2014;37:3137–52. doi: 10.1093/brain/awu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch K, Myers NE, Gottler J, et al. Disrupted intrinsic networks link amyloid-beta pathology and impaired cognition in prodromal Alzheimer’s disease. Cereb Cortex. 2015 doi: 10.1093/cercor/bhu151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Li TQ, Andreasen N, et al. Ratio of Abeta42/P-tau181p in CSF is associated with aberrant default mode network in AD. Sci Rep. 2013;3:1339. doi: 10.1038/srep01339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreasen N, Vanmechelen E, Vanderstichele H, et al. Cerebrospinal fluid levels of total-tau, phospho-tau and A beta 42 predicts development of Alzheimer’s disease in patients with mild cognitive impairment. Acta Neurol Scand Suppl. 2003;179:47–51. doi: 10.1034/j.1600-0404.107.s179.9.x. [DOI] [PubMed] [Google Scholar]
- 39.Buerger K, Ewers M, Pirttila T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129:3035–41. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 40.Walker LC, Diamond MI, Duff KE, et al. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013;70:304–10. doi: 10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han SD, Gruhl J, Beckett L, et al. Beta amyloid, tau, neuroimaging, and cognition: sequence modeling of biomarkers for Alzheimer’s disease. Brain Imaging Behav. 2012;6:610–20. doi: 10.1007/s11682-012-9177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]