Abstract
Background
Mounting evidence indicates the presence of structural brain alterations in individuals with obsessive–compulsive disorder (OCD). Findings are, however, rather heterogeneous, which may be partly because of differences in methodological approaches or clinical sample characteristics. The aim of the present study was to analyze the whole brain cortical volume, surface area and thickness in a large sample of patients with OCD compared with age- and sex-matched healthy controls.
Methods
We conducted whole brain surface-based analyses of grey matter measures using the automated FreeSurfer software in patients with OCD and matched controls. Group analyses were performed and corrected for multiple testing using Monte Carlo simulations (p < 0.05). Altered brain regions and their average morphological values were associated to symptom severity and type (Yale–Brown Obsessive Compulsive Scale scores).
Results
We included 75 patients and 75 controls in our analyses. Patients with OCD showed decreases in both volume and surface area compared with healthy controls in inferior-superior parieto-occipital regions. In addition, the precuneus, posterior cingulate areas, middle frontal and orbitofrontal areas, and middle inferior temporal areas extending to the fusiform gyrus were characterized by a reduced surface area only. There were no differences in grey matter thickness between the groups.
Limitations
The presence of comorbidities, medication usage and the multisymptomatic feature of OCD could have influenced our results to a certain degree.
Conclusion
Our results suggest decreased grey matter volume and surface area in several key regions in patients with OCD. Parietal regions showed reductions in both volume and surface area, which underlines the potential relevance of these regions for the pathophysiology of the disorder.
Introduction
Studying the brain anatomic structure offers a promising approach to improve our understanding of neural functional alterations often encountered in individuals with psychiatric disorders. In those with obsessive–compulsive disorder (OCD), a psychiatric disorder with a 2%–3% lifetime prevalence1 that causes strong impairment of daily life, findings up to now show disruptions on a functional and a structural neural level, mainly in a network including cortico-striato-thalamo-cortical (CSTC) areas. Overall, these findings are rather inconsistent and limited by confounding factors, such as differences in clinical characteristics of the study sample or varying methodological approaches.
Meta-analytic reviews showed that cortical areas, mainly the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC) and parietofrontal regions, seem to be predominantly affected by grey matter volume deficits in patients with OCD, whereas the lenticular nuclei and thalamus were found to be characterized by increases in grey matter volume.2–4 A recent review article5 confirmed these results and concluded that structural alterations in patients with OCD are widespread and occur most probably at a network level, with cortical tissue reductions and a tendency toward increases in grey matter volume of subcortical limbic areas. These subcortical tissue increases were also corroborated by a recent meta/mega-analysis of the ENIGMA OCD imaging consortium.6 Additionally, this meta/mega-analysis showed that the neuroplasticity of specific areas depends on the age of the studied sample (i.e., smaller hippocampal but larger pallidum volumes were prominent in adults with OCD, whereas only larger thalamic volumes were specific for pediatric patients). Besides grey matter volume alterations in patients with OCD, significantly decreased grey matter thickness in partly overlapping areas (i.e., limbic, parietal and temporal areas) was also found in another recent mega-analysis.7 It is interesting to note that these findings also partly confirmed a previous mega-analysis on volume by de Wit and colleagues,8 which described reductions in grey but also white matter in partly overlapping (i.e., frontostriatal limbic) areas.
Regarding these structural alterations in patients with OCD, most previous studies focused either on grey matter volume or thickness using voxel-based morphometry (VBM),9 with results being partly inconsistent in terms of the direction of alteration (increase v. decrease of cortical parameters), but also with respect to the cortical parameter itself (grey/white matter volume or thickness) or the anatomic region found to be altered.
It has been shown that, on the one hand, grey matter parameters (i.e., volume, surface area, cortical folding or thickness) are genetically and phenotypically independent from each other.10,11 On the other hand, changes in 1 parameter can contribute to changes in the others to varying degrees, as shown by longitudinal developmental studies.12 Therefore, focusing on only 1 grey matter parameter can obscure information about the others. Moreover, these parameters are known to differ strongly with regard to their developmental start and general course of development. Thus, cortical surface area develops as a consequence of cortical folding in prenatal brain development,13 whereas grey matter volume is fully developed only in later stages of postnatal development.14,15
Hence, studying these parameters of cortical structure can give more insight into the repeatedly discussed question of whether these morphological properties are independently affected in patients with OCD and whether they might help to explain the rather inconsistent findings.
Surface-based analysis (SBA) methods allow an exact determination of all these cortical parameters. Compared with VBM, SBA has several advantages. It is not prone to smoothing across neighbouring gyri as it uses smoothing on the inflated cortical surface. Moreover, it can assess these parameters of brain morphology and their contribution independently from each other and is not so sensitive to image registration as it computes the morphometric parameters in native space.16–18
To the best of our knowledge, only 2 studies have used the SBA approach in OCD samples to study gyrification, volume, surface area and thickness at the same time.19,20 Venkatasubramanian and colleagues19 applied SBA by using the automatic FreeSurfer software and provided first evidence of altered volume, surface area and thickness in a number of different regions comprising the ACC, OFC and occipital cortex in medication-naive patients with OCD. These alterations were partly associated with clinical characteristics (i.e., symptom severity, symptom type, disorder insight). Despite using the same method in an OCD sample with similar clinical characteristics (i.e., unmedicated patients), Fan and colleauges20 found alterations that partly diverged from the findings of Venkatasubramanian and colleagues,19 both regarding direction and location. Thus, the results by Fan and colleagues20 revealed a significant increase in thickness in parietal areas and gyrification increases in a network containing the insula and frontal and occipital areas in patients with OCD that were positively associated with symptom severity.
Several other OCD studies used the SBA approach. However, they analyzed only 1 measure of grey matter — thickness — with heterogeneous results.21–25 A more recent study by Kühn and colleagues26 explored cortical thickness using the same SBA method in a large sample of 101 patients with OCD and 95 controls and partly confirmed results of both previous studies. They reported cortical thinning in the bilateral subgenual and dorsal ACC as well as in middle frontal, inferior temporal, supramarginal and occipital areas of the left hemisphere and the right insula. They also found an increase in thickness in the left precentral gyrus.
Taken together, existing SBA and VBM studies in OCD samples are scarce and rather inconsistent, whereby the ACC appears to be most frequently reported as altered in thickness, surface and volume, and the middle frontal, insular and parieto-occipital areas are also found to be altered in patients with OCD, albeit with less consistency. Hence, previous findings need to be treated with caution and should be interpreted against the background of existing differences in sample characteristics and partly different methodological approaches.
In order to extend recent findings and to bring more light to the heterogeneous picture of the cortical grey matter alterations in patients with OCD, we performed a whole brain SBA of grey matter volume, surface area and thickness in a large and carefully selected sample of patients with OCD and matched healthy controls. Based on previous findings19,20 we expected a significantly altered cortical morphology in these various grey matter measures in patients with OCD compared with controls. Additionally, we hypothesized that these structural alterations would be significantly associated with OCD psychopathology, which we investigated by correlating the detected structural abnormalities with clinical scores (i.e., symptom severity).
Methods
Participants
We recruited patients from the Windach Institute and Hospital of Neurobehavioural Research and Therapy (WINTR), Germany, and the University Hospital for Psychiatry and Psychotherapy, Jena, Germany. All were in-house patients in wards specialized in OCD with a standardized admission process, psychopathological screenings and assessment of disorder history performed by an experienced psychiatrist. This sample has been described in detail elsewhere.27 The healthy controls were recruited in Jena and in Munich through local study announcements (e.g., blackboards, newspapers).
Both patients and healthy controls were screened using a standardized questionnaire, including questions on the presence of any (additional) psychiatric or neurologic illness, psychiatric or neurologic disorders in first-degree relatives, medication and MRI compatibility. Exclusion criteria for both groups were a history of clinically important head injuries, seizures or neurologic diseases. Healthy controls with a history of psychiatric illness were excluded. Exclusion criteria for patients were schizophrenia, autism, substance and alcohol abuse/dependency, mental retardation, pregnancy and severe medical conditions.
After a complete description of the study aims, we obtained written informed consent from all participants. The study protocol was in compliance with the Declaration of Helsinki and approved by the ethics committees of the Klinikum rechts der Isar and the University of Jena. Prior to the scanning session we assessed demographic characteristics and symptom severity using the Yale–Brown Obsessive Compulsive Scale (Y-BOCS).28
Image acquisition
Controls and patients recruited from WINTR were scanned at the Department of Neuroradiology, Klinikum rechts der Isar, Technische Universität München, Germany. Controls and patients from Jena were scanned at the University Hospital Jena.
High-resolution anatomic T1-weighted scans from Jena were acquired using a 3 T whole body system equipped with a 12-element receive-only head matrix coil (MAGNETOM TIM Trio, Siemens Medical Solutions). High-resolution anatomic T1-weighted volume scans (magnetization-prepared rapid gradient-echo [MPRAGE]) were obtained in sagittal orientation under the following parameters: repetition time (TR) 2300 ms, echo time (TE) 3.03 ms, inversion time (TI) 900 ms, flip angle 9°, field of view (FOV) 256 × 256 mm2, matrix 256 × 256 mm2, 192 sagittal slices, acceleration factor (PAT) of 2, with an isotropic resolution of 1 × 1 × 1 mm3.
Data from Munich were collected using a 3 T whole body system equipped with a 12-element receive-only head matrix coil (INGENIA, Philips Healthcare). High-resolution anatomic T1-weighted volume scans (MPRAGE) were obtained in sagittal orientation under the following parameters: TR 9 ms, TE 4 ms, TI 900 ms, flip angle 8°, FOV 240 × 240 mm2, matrix 240 mm × 240 mm2, 170 sagittal slices, with an isotropic resolution of 1 × 1 × 1 mm3.
Image processing and computation of surface measures
Using the framework of the general linear modeling (GLM), implemented as an automated function in FreeSurfer (glm_fit), we assessed the regional grey matter differences with respect to volume, surface area and thickness between patients and controls at the level of each vertex for each hemisphere separately, and included age, sex and scanner type (Siemens v. Philips) as covariates to correct for their potential confounding effects. The reconstructed surfaces for each participant were visually inspected, and minor defects were manually corrected as recommended by the software guidelines.
To account for multiple testing across the whole brain, we performed Monte Carlo simulations29 with 10 000 iterations in order to identify significant contiguous clusters of vertex-wise group differences (p < 0.05).
In addition to analysis of covariance (ANCOVA), we assessed the potentially confounding effect of the 2 scanner types (Siemens v. Phillips) and their different sequences. Hence, we performed another glm_fit analysis with age and sex as covariates in which we evaluated whether the 2 scanner groups differed significantly in volume, surface area or thickness.
Statistical analysis
Further statistical analyses were performed using the SPSS Inc. software version 11.5.1. Differences in age between the groups were assessed using the Student t test, and differences in sex were assessed using χ2 square tests. Within the patient group we performed multiple linear regression to assess the association between 4 clinical characteristics (Y-BOCS total score, obsessions, compulsions and duration of illness) and alteration in parameters of the cortical structure (mean volume, surface area or thickness of altered brain regions). Each of the 4 clinical scores was hereby taken as a separate criterion, with mean volume or surface area extracted from the clusters that emerged from the group comparison as predictors, and age, sex and scanner type as covariates. We conducted the regression analyses separately for each hemisphere, for each of the 4 symptom scores and for each of the cortical parameters showing a group difference. In case of a significant association, the corresponding partial correlation coefficient was reported.
To control for the potential effect of medication or comorbidity on the brain clusters that were found to be different in patients than controls, we performed multivariate ANCOVAs (MANCOVA). Medication and comorbidity were used as independent variables, and average volume and surface area values (extracted from the clusters found to be different in the group comparison) were used as dependent variables. Age, sex and scanner type were entered as covariates. We performed the analyses separately for each hemisphere. We addressed the question of whether medication status (medicated v. unmedicated) or comorbidity (comorbidity v. no comorbidity) affected the surface structure in the clusters that were found to be different in patients than in controls.
Our statistical analyses were Bonferroni-corrected with an α of p < 0.0025 for the total of 20 models (10 for each hemisphere and each measure).
In addition, to evaluate if there was a potential difference between medicated and unmedicated patients, we performed separate whole brain GLM analyses for the cortical parameters (volume, surface area, thickness) with age, sex and scanner type as covariates, and corrected for multiple testing using a false discovery rate (FDR) of p < 0.05.
Results
Participants
The study sample comprised 75 right-handed patients who met the DSM-IV criteria for OCD and 75 right-handed healthy controls matched for age (t148 = 0.54, p = 0.58) and sex (χ21 = 0.1, p = 0.73). Forty-two of the patients were recruited from WINTR and 33 were recruited from the University Hospital in Jena. Slightly more than half (57%) of all patients were medicated, and one-third (32%) had 1 or more comorbid psychiatric disorder (Table 1).
Table 1.
Demographic and clinical characteristics of the sample
| Characteristic | Group; mean ± SD (range) or no. | |
|---|---|---|
|
| ||
| OCD (n = 75) | Control (n = 75) | |
| Age, yr | 30.99 ± 9.55 (19–63) | 30.17 ± 8.99 (18–57) |
| Sex, male:female | 27:48 | 30:45 |
| Age at onset, yr | 16.90 ± 6.64 | — |
| Medication, yes:no | 43:32 | — |
| Medication type | ||
| SSRI | 24 | — |
| SNRI | 5 | — |
| TrA | 2 | — |
| ≥ 1 medication | 12 | — |
| Comorbidities, yes:no | 24:51 | — |
| Comorbidity type | ||
| Depression | 13 | — |
| Anxiety disorder | 3 | — |
| Personality disorder | 1 | — |
| Impulse control disorder NOS | 1 | — |
| ≥ 1 comorbid disorder | 6 | — |
| Y-BOCS score | ||
| Total score | 20.77 ± 6.08 (9–38) | — |
| Obsessions | 10.45 ± 3.47 (1–19) | — |
| Compulsions | 10.29 ± 3.96 (0–19) | — |
NOS = not otherwise specified; OCD = obsessive–compulsive disorder; SD = standard deviation; SNRI = serotonin–norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor; TrA = tricyclic antidepressant; Y-BOCS = Yale–Brown Obsessive Compulsive Scale.
Differences in surface measures
Volume
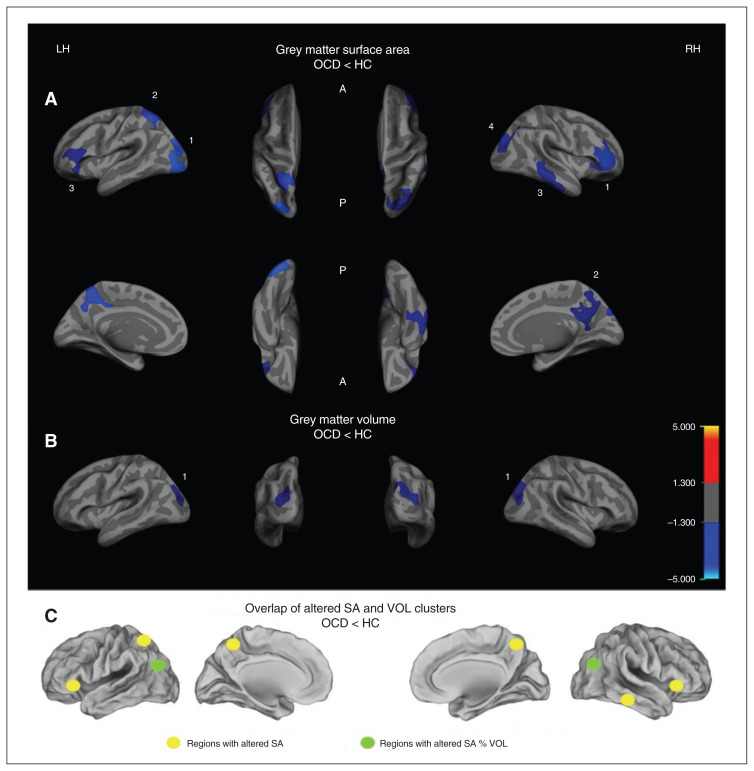
The whole brain analysis to investigate differences in cortical volume between the groups revealed a significantly (p < 0.01) decreased volume in patients with OCD compared with healthy controls in a cluster of the right hemisphere comprising the superior and inferior parietal areas as well as small parts of the lateral occipital cortex and in a similar cluster in the left hemisphere comprising superior-inferior parietal and lateral occipital regions (p < 0.05; Table 2 and Fig. 1B).
Table 2.
Clusters showing significantly reduced grey matter volume in patients with obsessive–compulsive disorder*
| Cluster annotation | Max t-value | VtxMax | Cluster size, mm2 | VtxMax Talairach coordinates, x, y, z | CWP p value (90% CI) | NVtxs |
|---|---|---|---|---|---|---|
| Left hemisphere | ||||||
| Superior-inferior parietal cortex extending to lateral occipital cortex | −4.549 | 112 174 | 1031.24 | −26.5, −82.4, 17.9 | 0.044 (0.041–0.046) | 1492 |
| Right hemisphere | ||||||
| Superior-inferior parietal cortex | −4.289 | 157 870 | 1432.89 | 24.7, −79.7, 25 | 0.006 (0.005–0.007) | 2118 |
CI = confidence interval; CWP = cluster-wise probability; NVtx = number of vertices in cluster; VtxMax = no. of peak vertices of the significant cluster.
Annotation of clusters according to FreeSurfer atlas.
Fig.1.
Group differences in (A) clusters with significantly reduced grey matter surface area (SA) and (B) clusters with significantly reduced grey matter volume (VOL), shown separately for the right (RH) and left hemispheres (LH). Clusters are displayed on the FreeSurfer main surface of the participants’ average brain (lateral, medial, dorsal or ventral view). The colour bar indicates the t-value after cluster-wise correction for multiple comparisons using Monte Carlo simulations (p < 0.05). (C) Overlap between the altered SA and VOL clusters. Cluster numbers correspond to those in Table 3 and in the article text. A = anterior; HC = healthy controls; OCD = obsessive–compulsive disorder; P = posterior.
Surface area
Patients with OCD had a significantly (p < 0.05) decreased surface area in 4 clusters of the right hemisphere (parietal cortex, rostral middle frontal cortex, inferior temporal cortex, precuneus) compared with healthy controls. When applying a stricter threshold (p < 0.01), only the parietal and the rostral middle frontal cluster remained significant (Table 3). Likewise, 3 clusters of the left hemisphere showed a significantly decreased surface area (p < 0.05) in patients with OCD compared with healthy controls, comprising mainly the left lateral occipital, superior parietal and rostral middle frontal regions. When applying a stricter threshold (p < 0.01), only the lateral occipital and superior parietal clusters remained significant (Table 3 and Fig. 1A).
Table 3.
Clusters showing significant changes in grey matter surface area in patients with obsessive–compulsive disorder
| Cluster | Max t-value | VtxMax | Cluster size, mm2 | VtxMax Talairach coordinates, x, y, z | CWP p value (90% CI) | NVtxs |
|---|---|---|---|---|---|---|
| Left hemisphere | ||||||
| Lateral occipital cortex, superior-inferior parietal cortex | −3.941 | 87 140 | 2971.33 | −26.9, −85.3, 18.4 | 0.0006 (0.0003–0.0009) | 4014 |
| Superior parietal cortex, precuneus, posterior cingulate | −3.568 | 14 332 | 2422.88 | −7.4, −48.9. 59.8 | 0.002 (0.001–0.003) | 5152 |
| Rostral middle frontal cortex, pars triangularis, pars orbitalis, pars opercularis | −2.681 | 84 578 | 1701.91 | −46.6, 30.5, 9.2 | 0.025 (0.023–0.027) | 2384 |
| Right hemisphere | ||||||
| Rostral middle frontal cortex, pars triangularis, pars orbitalis, lateral orbitofrontal cortex | −4.278 | 58 399 | 2293.91 | 42.0, 41.8, 0.0 | 0.006 (0.005–0.007) | 3159 |
| Precuneus, isthmus cingulate | −4.237 | 156 055 | 1690.44 | 8.6, −50.4, 24.6 | 0.030 (0.028–0.032) | 3705 |
| Middle-inferior temporal cortex, banks of the superior temporal sulcus, fusiform gyrus | −3.844 | 59 310 | 2007.95 | 66.6, −31.2, −5.9 | 0.012 (0.010–0.013) | 3124 |
| Superior-inferior parietal cortex, lateral occipital cortex, cuneus | −2.720 | 104 620 | 2201.18 | 34.3, −78.3, 19.9 | 0.007 (0.006–0.008) | 3436 |
CI = confidence interval; CWP = cluster-wise probability; NVtx = number of vertices in cluster; VtxMax = no. of peak vertices of the significant cluster.
Annotation of clusters according to the FreeSurfer atlas.
Thickness
The whole brain analyses of cortical thickness revealed no significant differences between the groups.
Correlation between surface area and volume
As the parietal cortex showed both decreased surface area and volume in patients compared with controls (Fig. 1), we investigated whether there was a direct association between both parameters in this area. The correlation of volume and surface area in this specific overlapping cluster showed a positive association between these measures in both hemispheres and both groups (left hemisphere: r = 0.83, p = 0.013 in patients and r = 0.86, p = 0.012 in controls; right hemisphere: r = 0.82, p = 0.014 in patients and r = 0.86, p = 0.012 in controls).
Association between cortical measures and clinical variables
There was no association between the cortical parameters and symptom severity or duration of illness (i.e., the analyses did not survive correction for multiple comparisons).
Influence of medication, comorbidity and scanner sequence
After Bonferroni correction, neither medication nor comorbidity showed a significant effect on the altered volume or surface area regions. Furthermore, no group differences in the whole brain analysis comparing medicated and unmedicated patients could be found in any of the grey matter measures.
There was no difference in surface area, volume or thickness between the 2 scanner groups (Siemens v. Phillips).
Discussion
Our study results showed a reduced grey matter surface area and volume mainly in parieto-occipital areas of both hemispheres in patients with OCD. Additionally, we found only the surface area to be reduced in a network comprising frontal, temporal, precuneus and cingulate areas (Fig. 1C). However, our results did not show any significant difference in grey matter thickness between the groups, and none of the alterations showed a significant association with clinical scores.
Comparison with surface-based studies
To the best of our knowledge, the present study is only the third OCD study to analyze several cortical parameters in the same sample using the SBA approach. Our results show a certain overlap with previous SBA studies in OCD samples: alterations in similar areas (i.e., parieto-occipital alterations, including the precuneus) partly overlap with the findings of Fan and colleagues,20 and the same direction of alteration (i.e., decreases in volume and surface area) has been reported by Venkatasubramanian and colleagues.19
However, whereas we found decreased grey matter surface area and volume in similar regions as Fan and colleagues,20 their results showed mostly increased gyrification and thickness in those areas.
The divergence in the direction of alteration may be partly driven by methodological differences in terms of clinical sample characteristics (i.e., previous studies investigated smaller samples and mainly unmedicated and comorbidity-free20 or medication-naive patients19).
It is compelling that although other SBA studies21,23,25,26,30,31 found grey matter thickness to be affected in patients with OCD, our results did not show such alterations in this measure, but we did find partly similar alterations in other measures. However, when interpreting these divergent results one needs to be aware that previous studies presented rather heterogeneous results as well. They reported increased but also decreased grey matter thickness in distinct cortical regions, with some of the resulting heterogeneity probably being a consequence of methodological differences among the studies (i.e., SBA approach v. whole brain v. ROI-based analyses) or differences in sample characteristics (e.g., symptom profile, symptom severity, duration of illness, medication, sample size, presence of comorbidities). All this makes a valid comparison of the various findings rather difficult.
This heterogeneity regarding alterations in different structural parameters in partly similar areas may also be explained by the fact that, although these parameters are partly associated, they still can change independently from each other and influence changes in another parameter to a significant degree.32
In this context, it is interesting to note that we found similar areas (i.e., inferior-superior parietal regions) to be hypogyrified in the same sample, as reported in a recently published paper by our group.27
According to previous studies, gyrification reaches its developmental peak before early toddlerhood.33–35 Therefore, variations in gyrification are often discussed as useful markers for processes that evolved during a crucial period of early brain development.
Against this background one could speculate that these early changes in brain development could have laid the basis for a certain vulnerability in these areas. Hence, it is possible that these changes, which most probably happened during early brain development, made other grey matter properties (e.g., volume or surface area) more prone to alterations. This would also explain why similar areas were altered with regard to gyrification as well as volume and surface area. It should be noted, however, that this conclusion is speculative, and the precise association between these grey matter properties needs to be disentangled in future studies.
Another notable finding from the present study was the reduced surface area in the bilateral rostral middle frontal areas extending to the pars opercularis, pars triangularis and pars orbitalis. This finding is consistent with the main findings of 2 recent mega-analyses,7,8 which discussed the possibility that similar pathological processes might underlie reductions in cortical morphology of these frontal areas. Thinking a step further, these frontal areas, which are mainly responsible for cognitive control, have been shown to be linked to OCD-specific repetitive behaviours (compulsions), and fMRI studies show evidence of functional alterations in similar areas in patients with OCD, which lets us speculate that there may be a link between these functional and structural alterations.
Comparison with VBM studies
Compared with previous VBM studies, we likewise found structural alterations in inferior frontal areas, such as reported by de Wit and colleagues;8 alterations in small parts of the lateral orbitofrontal cortex, such as shown by Radua and Mataix-Cols;2 and parietofrontal alterations, such as reported by Rotge and colleagues.4 However, despite the spatial overlaps these alterations affected different cortical parameters, and we could not replicate any increases in cortical grey matter. These differences could have been driven by different methodological approaches; VBM might conceal certain changes that become manifest when analyzing cortical characteristics in a surface-based way,32 but they may also be triggered by clinical characteristics of the studied sample, such as medication, co-morbidities, symptom dimensions or sample size.
More specifically, when comparing our results to earlier findings, it is striking that we tended to detect a reduced surface area in brain regions that were reported in other studies to show a reduced volume.8,36 Moreover, other areas that showed alterations or even increases in grey matter in these previous studies (e.g., putamen, cerebellum, prefrontal cortex) did not show any significant difference to healthy controls in the present results. A potential reason for these differences may be that the grey matter volume as measured by VBM is a conglomerate of grey matter features that can be influenced by cortical folding properties of the surface area.16,32,37 Hence, an exclusive VBM analysis of grey matter volume could obscure potential differences in other features, such as surface area or folding, which may have significantly contributed to the volume changes reported in previous studies. Moreover, evidence showing a — to some degree — genetically and phenotypically independent development of some of these grey matter parameters,10–12 and the fact that SBA methods account for the folded surface when computing volume, supports the conclusion that SBA methods, such as those used in the Free-Surfer software, may contribute to a better understanding of the precise characteristics of grey matter alterations. However, exact interdependencies and potential effects of the grey matter parameters on each other remain unknown12 and should be evaluated by future studies using combined methodological approaches (VBM and SBA).32
Associations of grey matter parameters
Although the way grey matter is measured by the various approaches is relatively clear and varies only slightly, little evidence exists about the precise characteristics and physiologic implications of each grey matter measure that would allow for a meaningful interpretation of the results. There is evidence from basic research that the various neuroanatomical features of the cortex (i.e., thickness, surface area, gyrification), which are all to a greater or lesser degree included in the volume parameter, are highly heritable.11,38–40 Nevertheless, each of them underlies distinct genetic and evolutionary processes.11,41,42 Hence, environmental influences, such as diseases, can also have different effects on these subcomponents of grey matter. Therefore, it is important to highlight that the exclusive investigation of grey matter volume might obscure changes in brain morphology.
Interestingly, the parietal cortex showed a reduced surface area and a reduced volume. Moreover, the correlation between both parameters showed a significant association in both patients and healthy controls. Although this result speaks in favour of a direct link between the different characteristics of brain morphology, it is still unknown how exactly these parameters influence each other. Some more recent evidence from longitudinal SBA data suggests that grey matter volume changes may emerge as a result of age- and sex- dependent interaction between grey matter thickness and surface area. These data also suggest that changes in grey matter surface area reflect mainly the more complex interaction between cortical gyrification and the size changes in the exposed convex hull area during brain development, which can vary strongly depending on age or sex.12
Associations between structural alterations and clinical scores
The present findings raise the question whether the alterations we detected in brain morphology of patients with OCD constitute a predisposing developmental abnormality or a consequence of disorder progression. Longitudinal studies of brain development in healthy individuals indicate that these cortical parameters (i.e., surface area and volume) can change in the course of life, whereas gyrification seems to constitute a rather stable parameter after early childhood and is therefore often discussed as a potential neurodevelopmental marker.27,43 Therefore, there is reason to assume that symptom severity, duration of illness or age of onset might be associated with the structural alterations found in the present study. Although several of the discussed studies in adult and pediatric samples of OCD showed associations between morphological changes of grey matter and clinical scores,6,7,19,20 the present results did not show this on a corrected significance threshold. Hence, in light of the study limitations, such as a potential influence of medication or comorbidities, which may also have confounded these associations, our results need to be treated with caution. Nevertheless, the hypothesis that alterations in cortical morphology could be linked to clinical factors, such as early age of onset, higher symptom severity, poor treatment response or delayed start of treatment, is still a matter of debate and needs further examination in longitudinal studies.
Relating structural alterations to functional alterations
A frequently discussed hypothesis is that structural alterations might constitute the basis for functional impairments in the affected brain. In patients with OCD, this hypothesis is sustained by the fact that partly similar areas are often reported to be altered on a functional as well as on a structural level. In that respect, the present findings as well as previous ones revealed structural alterations in mainly parieto-occipital as well as temporal and frontal regions in patients with OCD.4,21 Functional OCD studies showed alterations in some of these structurally altered regions during tasks that required visual, emotional or executive processing; inhibitory control; or working memory processing.44–50 Moreover, behavioural OCD studies also reported impairments in functions that are known to involve similar brain regions and networks.51,52 Furthermore, the alteration in surface area in the bilateral posterior cingulate goes in line with resting-state studies of functional connectivity in OCD samples. These studies found alterations in the attention and the default mode network,53,54 of which the posterior cingulate constitutes an important hub. Based on these findings, one can assume that these functional and structural impairments in patients with OCD are linked and may lead to network disruptions in addition to the reported alterations in specific regions.
Limitations
Psychiatric disorders share certain neural correlates to some degree,55 and the presence of comorbidities and/or medical treatment can alter or reinforce these morphological commonalities.7,56,57 However, although our sample was partly medicated and not comorbidity-free, these factors did not show any significant effect on the present results after correcting for multiple comparisons. Considering that on an uncorrected threshold an effect was found in certain clusters, it cannot be excluded that comorbidity or medical treatment had a certain influence on grey matter structure. Of note, the 2 previous SBA studies evaluated either unmedicated20 or medication-naive patients,19 with partly similar as well as divergent results compared with our study. Hence, the degree to which these cortical characteristics are really affected by medication or comorbidities needs to be further elucidated.
Another important point is that patients with OCD often experience a broad spectrum of symptoms, and previous imaging studies indicate that OCD may be conceptualized as a spectrum of multiple and potentially overlapping syndromes that might be — both from a functional and a structural perspective — mediated by distinct components of the CSTC.7,58,59 Against this background, the fact that our sample was multi-symptomatic limits our study’s explanatory power to some degree. Hence, further studies with even larger samples are needed to allow for a valid differentiation between patients with specific symptom types. Another limitation of our study is the fact that no standardized interview, such as the Structured Clinical Interview for DSM disorders or the M.I.N.I. International Neuropsychiatric Interview, was performed. However, before study inclusion patients had been extensively screened by experienced psychiatrists from the WINTR and Jena hospital, who confirmed the diagnosis of OCD.
Even though we tried to control for the effect of scanner type in the best possible way (i.e., by introducing scanner type as a covariate in the analyses and by comparing data from the 2 scanner groups), we cannot rule out that this variable may have influenced our results to a certain degree.
Another methodological limitation may be the fact that SBA methods limit analyses to cortical regions, whereas structural properties of subcortical brain areas, such as the basal ganglia or amygdala, which are assumed to be psychopathologically relevant for OCD, are not taken into consideration.
Conclusion
Despite these limitations, the present study is one of the few exploring volume, surface area and thickness in patients with OCD. Our results clearly indicate that aside from the frequently reported functional alterations grey matter surface area seems to be altered in patients with OCD and suggest that parieto-occipital and rostral middle frontal regions should be considered in the neurobiological model of the disorder. Mega-analyses should further investigate these whole brain cortical changes by accounting for all possible confounders in order to further isolate the central hubs of structural alterations in patients with OCD. Moreover, more data are needed to elucidate the exact interrelation between these grey matter parameters and their relevance for OCD.
Acknowledgements
The authors thank the Windach Institute and Hospital of Neurobehavioural Research and Therapy, Windach, Germany, for the opportunity to recruit patients at their institution. This work was supported by the German Research Foundation (DFG) grant number (KO 3744/2-1) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program. The authors also thank the Graduate School of Systemic Neurosciences (GSN) for making it possible to share the results with the neuroscientific community at various occasions, such as conferences, retreats and symposia.
Footnotes
Competing interests: None declared.
Contributors: G. Rus, T. Reess, G. Wagner and K. Koch designed the study. G. Rus. T. Reess, M. Zaudig, C. Zimmer and K. Koch acquired the data, which G. Rus, T. Reess and K. Koch analyzed. G. Rus wrote the article, which all authors reviewed and approved for publication.
References
- 1.Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey replication. Mol Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 3.Rotge JY, Guehl D, Dilharreguy B, et al. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry. 2009;65:75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Rotge JY, Langbour N, Guehl D, et al. Gray matter alterations in obsessive-compulsive disorder: an anatomic likelihood estimation meta-analysis. Neuropsychopharmacology. 2010;35:686–91. doi: 10.1038/npp.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piras F, Piras F, Chiapponi C, et al. Widespread structural brain changes in OCD: a systematic review of voxel-based morphometry studies. Cortex. 2015;62:89–108. doi: 10.1016/j.cortex.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Boedhoe PS, Schmaal L, Abe Y, et al. Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta- and mega-analysis. Am J Psychiatry. 2017;174:60–9. doi: 10.1176/appi.ajp.2016.16020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fouche JP, du Plessis S, Hattingh C, et al. Cortical thickness in obsessive-compulsive disorder: multisite mega-analysis of 780 brain scans from six centres. Br J Psychiatry. 2017;210:67–74. doi: 10.1192/bjp.bp.115.164020. [DOI] [PubMed] [Google Scholar]
- 8.de Wit SJ, Alonso P, Schweren L, et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 2014;171:340–9. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- 9.Ashburner J, Friston KJ. Voxel-based morphometry — the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 10.Winkler AM, Kochunov P, Blangero J, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–46. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panizzon MS, Fennema-Notestine C, Eyler LT, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–35. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011;31:7174–7. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapellou O, Counsell SJ, Kennea N, et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006;3:e265. doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frye RE, Liederman J, Malmberg B, et al. Surface area accounts for the relation of gray matter volume to reading-related skills and history of dyslexia. Cereb Cortex. 2010;20:2625–35. doi: 10.1093/cercor/bhq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutton C, Draganski B, Ashburner J, et al. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage. 2009;48:371–80. doi: 10.1016/j.neuroimage.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarkson MJ, Cardoso MJ, Ridgway GR, et al. A comparison of voxel and surface based cortical thickness estimation methods. Neuroimage. 2011;57:856–65. doi: 10.1016/j.neuroimage.2011.05.053. [DOI] [PubMed] [Google Scholar]
- 18.Helms G. Segmentation of human brain using structural MRI. MAGMA. 2016;29:111–24. doi: 10.1007/s10334-015-0518-z. [DOI] [PubMed] [Google Scholar]
- 19.Venkatasubramanian G, Zutshi A, Jindal S, et al. Comprehensive evaluation of cortical structure abnormalities in drug-naive, adult patients with obsessive-compulsive disorder: a surface-based morphometry study. J Psychiatr Res. 2012;46:1161–8. doi: 10.1016/j.jpsychires.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Fan Q, Palaniyappan L, Tan L, et al. Surface anatomical profile of the cerebral cortex in obsessive-compulsive disorder: a study of cortical thickness, folding and surface area. Psychol Med. 2013;43:1081–91. doi: 10.1017/S0033291712001845. [DOI] [PubMed] [Google Scholar]
- 21.Shin YW, Yoo SY, Lee JK, et al. Cortical thinning in obsessive compulsive disorder. Hum Brain Mapp. 2007;28:1128–35. doi: 10.1002/hbm.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallucca E, MacMaster FP, Haddad J, et al. Distinguishing between major depressive disorder and obsessive-compulsive disorder in children by measuring regional cortical thickness. Arch Gen Psychiatry. 2011;68:527–33. doi: 10.1001/archgenpsychiatry.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamae T, Narumoto J, Sakai Y, et al. Reduced cortical thickness in non-medicated patients with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:90–5. doi: 10.1016/j.pnpbp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Kim SG, Jung WH, Kim SN, et al. Disparity between dorsal and ventral networks in patients with obsessive-compulsive disorder: evidence revealed by graph theoretical analysis based on cortical thickness from MRI. Front Hum Neurosci. 2013;7:302. doi: 10.3389/fnhum.2013.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw P, Sharp W, Sudre G, et al. Subcortical and cortical morphological anomalies as an endophenotype in obsessive-compulsive disorder. Mol Psychiatry. 2015;20:224–31. doi: 10.1038/mp.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kühn S, Kaufmann C, Simon D, et al. Reduced thickness of anterior cingulate cortex in obsessive-compulsive disorder. Cortex. 2013;49:2178–85. doi: 10.1016/j.cortex.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Rus OG, Reess TJ, Wagner G, et al. Hypogyrification in obsessive-compulsive disorder. Psychol Med. 2017;47:1053–1061. doi: 10.1017/S0033291716003202. [DOI] [PubMed] [Google Scholar]
- 28.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 29.Hagler DJ, Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayan VM, Narr KL, Phillips OR, et al. Greater regional cortical gray matter thickness in obsessive-compulsive disorder. Neuroreport. 2008;19:1551–5. doi: 10.1097/WNR.0b013e3283112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fouche JP, du Plessis S, Hattingh C, et al. Cortical thickness in obsessive-compulsive disorder: multisite mega-analysis of 780 brain scans from six centres. Br J Psychiatry. 2017;210:67–74. doi: 10.1192/bjp.bp.115.164020. [DOI] [PubMed] [Google Scholar]
- 32.Palaniyappan L, Liddle PF. Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. Neuroimage. 2012;60:693–9. doi: 10.1016/j.neuroimage.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong E, Schleicher A, Omran H, et al. The ontogeny of human gyrification. Cereb Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- 34.Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013;36:275–84. doi: 10.1016/j.tins.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Magnotta VA, Andreasen NC, Schultz SK, et al. Quantitative in vivo measurement of gyrification in the human brain: changes associated with aging. Cereb Cortex. 1999;9:151–60. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- 36.Pujol J, Soriano-Mas C, Alonso P, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–30. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 37.Hutton C, De Vita E, Ashburner J, et al. Voxel-based cortical thickness measurements in MRI. Neuroimage. 2008;40:1701–10. doi: 10.1016/j.neuroimage.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atkinson EG, Rogers J, Mahaney MC, et al. Cortical folding of the primate brain: an interdisciplinary examination of the genetic architecture, modularity, and evolvability of a significant neurological trait in pedigreed baboons (genus Papio) Genetics. 2015;200:651–65. doi: 10.1534/genetics.114.173443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kochunov P, Glahn DC, Fox PT, et al. Genetics of primary cerebral gyrification: heritability of length, depth and area of primary sulci in an extended pedigree of Papio baboons. Neuroimage. 2010;53:1126–34. doi: 10.1016/j.neuroimage.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers J, Kochunov P, Zilles K, et al. On the genetic architecture of cortical folding and brain volume in primates. Neuroimage. 2010;53:1103–8. doi: 10.1016/j.neuroimage.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–8. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 42.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–9. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 43.Mills KL, Tamnes CK. Methods and considerations for longitudinal structural brain imaging analysis across development. Dev Cogn Neurosci. 2014;9:172–90. doi: 10.1016/j.dcn.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Heuvel OA, Veltman DJ, Groenewegen HJ, et al. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 2005;62:301–9. doi: 10.1001/archpsyc.62.3.301. [DOI] [PubMed] [Google Scholar]
- 45.Van Velzen LS, Vriend C, de Wit SJ, et al. Response inhibition and interference control in obsessive-compulsive spectrum disorders. Front Hum Neurosci. 2014;8:419. doi: 10.3389/fnhum.2014.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Vries FE, de Wit SJ, Cath DC, et al. Compensatory fronto-parietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry. 2014;76:878–87. doi: 10.1016/j.biopsych.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 47.Rus OG, Reess TJ, Wagner G, et al. Functional and structural connectivity of the amygdala in obsessive-compulsive disorder. Neuroimage Clin. 2017;13:246–55. doi: 10.1016/j.nicl.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olatunji BO, Ferreira-Garcia R, Caseras X, et al. Predicting response to cognitive behavioral therapy in contamination-based obsessive-compulsive disorder from functional magnetic resonance imaging. Psychol Med. 2014;44:2125–37. doi: 10.1017/S0033291713002766. [DOI] [PubMed] [Google Scholar]
- 49.Menzies L, Chamberlain SR, Laird AR, et al. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonçalves OF, Marques TR, Lori NF, et al. Obsessive-compulsive disorder as a visual processing impairment. Med Hypotheses. 2010;74:107–9. doi: 10.1016/j.mehy.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 51.Bannon S, Gonsalvez CJ, Croft RJ, et al. Executive functions in obsessive-compulsive disorder: State or trait deficits? Aust N Z J Psychiatry. 2006;40:1031–8. doi: 10.1080/j.1440-1614.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 52.Penadés R, Catalan R, Rubia K, et al. Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry. 2007;22:404–10. doi: 10.1016/j.eurpsy.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Zhang T, Wang J, Yang Y, et al. Abnormal small-world architecture of top-down control networks in obsessive-compulsive disorder. J Psychiatry Neurosci. 2011;36:23–31. doi: 10.1503/jpn.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meunier D, Ersche KD, Craig KJ, et al. Brain functional connectivity in stimulant drug dependence and obsessive-compulsive disorder. Neuroimage. 2012;59:1461–8. doi: 10.1016/j.neuroimage.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Radua J, van den Heuvel OA, Surguladze S, et al. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 2010;67:701–11. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- 56.Hoexter MQ, de Souza Duran FL, D’Alcante CC, et al. Gray matter volumes in obsessive-compulsive disorder before and after fluoxetine or cognitive-behavior therapy: a randomized clinical trial. Neuropsychopharmacology. 2012;37:734–45. doi: 10.1038/npp.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoexter MQ, Dougherty DD, Shavitt RG, et al. Differential pre-frontal gray matter correlates of treatment response to fluoxetine or cognitive-behavioral therapy in obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2013;23:569–80. doi: 10.1016/j.euroneuro.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Mataix-Cols D, Wooderson S, Lawrence N, et al. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–76. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 59.van den Heuvel OA, Remijnse PL, Mataix-Cols D, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–68. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]