Abstract
Background
An animal model of gambling disorder, previously known as pathological gambling, could advance our understanding of the disorder and help with treatment development. We hypothesized that repeated exposure to uncertainty during gambling induces behavioural and dopamine (DA) sensitization — similar to chronic exposure to drugs of abuse. Uncertainty exposure (UE) may also increase risky decision-making in an animal model of gambling disorder.
Methods
Male Sprague Dawley rats received 56 UE sessions, during which animals responded for saccharin according to an unpredictable, variable ratio schedule of reinforcement (VR group). Control animals responded on a predictable, fixed ratio schedule (FR group). Rats yoked to receive unpredictable reward were also included (Y group). Animals were then tested on the Rat Gambling Task (rGT), an analogue of the Iowa Gambling Task, to measure decision-making.
Results
Compared with the FR group, the VR and Y groups experienced a greater locomotor response following administration of amphetamine. On the rGT, the FR and Y groups preferred the advantageous options over the risky, disadvantageous options throughout testing (40 sessions). However, rats in the VR group did not have a significant preference for the advantageous options during sessions 20–40. Amphetamine had a small, but significant, effect on decision-making only in the VR group. After rGT testing, only the VR group showed greater hyperactivity following administration of amphetamine compared with the FR group.
Limitations
Reward uncertainty was the only gambling feature modelled.
Conclusion
Actively responding for uncertain reward likely sensitized the DA system and impaired the ability to make optimal decisions, modelling some aspects of gambling disorder.
Introduction
The worldwide prevalence of problem gambling ranges from 0.2% to 5.3%.1 A significant positive association exists between the availability of gambling opportunities and gambling-related harm.2–4 As new gambling opportunities are emerging (including online gambling and electronic gaming machines), gambling disorder is a growing public health concern.
Previously referred to as pathological gambling, gambling disorder is an addictive disorder according to DSM-5.5 Although some behaviours present in patients with gambling disorder are unique to gambling (e.g., loss chasing), similarities between gambling disorder and substance addiction can be made, including withdrawal symptoms, irritability when attempting to stop or reduce the behaviour, and tolerance.5 Likewise, similar mechanisms and brain regions may be dysregulated in individuals with substance addiction and gambling disorder.
Preclinical studies of drug abuse have reported that repeated exposure to a variety of drugs of abuse, such as amphetamine, can lead to enhanced drug self-administration6–9 and induces both behavioural and neurochemical sensitization, particularly of the mesolimbic dopamine (DA) system. The sensitization effect is largely evidenced by greater DA release in the ventral striatum and a heightened locomotor response following a challenge dose of the drug.10–13 Similar neuroplasticity may also occur in gambling disorder. Compared with healthy controls, individuals with pathological gambling also exhibit greater DA release (54%–63%) in the striatum and midbrain in response to an oral amphetamine challenge.14
Using locomotor activity following a low dose of amphetamine as a noninvasive, indirect correlate of mesolimbic DA sensitization, recent studies have shown that repeated exposure to unpredictable reward delivery15 or responding for reinforcement on a variable schedule of reward delivery16 enhanced amphetamine’s ability to increase locomotor activity compared with control groups. As a result, both repeated exposure to psychostimulants and uncertainty exposure (UE) appear to induce a similar behavioural response that likely reflects DA sensitization.
Increased risky decision-making is also present in patients with gambling disorder and those with substance use disorder. The Iowa Gambling Task (IGT) is often used to assess decision-making in clinical populations.17 During the IGT, healthy participants learn to choose the advantageous options associated with smaller immediate gains but less long-term loss more often than disadvantageous options that yield greater immediate gains but larger long-term loss. Conversely, those classified as pathological gamblers choose disadvantageously more often than controls.18–23 A similar preference for the disadvantageous options is also present in individuals with substance use disorder.24–28 Therefore, the IGT may capture a bias in decision-making that is common to both gambling disorder and substance dependence.
Exposure to addictive drugs is an obvious cause of addiction-related neuroplasticity; however, the corresponding determinants of sensitization in gambling disorder remain unclear. This study determined whether repeated exposure to different aspects of reward uncertainty (passive or active receipt of uncertain reward) induces a sensitization-like effect and/or influences decision-making. Rats responded for saccharin according to a fixed ratio (FR) or variable ratio (VR) schedule of reinforcement. Animals responding on the FR schedule experienced certainty exposure (CE), because the exact number of responses required to receive saccharin could be learned. Rats responding on the VR schedule experienced UE — the number of responses required to obtain reinforcement was unknown. To determine whether passive, unexpected reward delivery (i.e., surprise) likewise influenced sensitization and decision- making, rats yoked to the FR or VR groups were also included. Following UE/CE, all rats were trained on the Rat Gambling Task (rGT),29 a rodent analogue of the IGT, to assess risky decision-making. Before and after rGT training, the animals’ locomotor response to a challenge dose of amphetamine was determined to indicate whether UE induced behavioural sensitization and whether sensitization remained or was further altered through the decision-making experience.
Methods
Aminals
Male Sprague Dawley rats (Charles River Laboratories), weighed 225–250 g at the beginning of the study. Rats were pair-housed in a colony room maintained at 21°C under a 12-hour reverse-light cycle (lights off at 8 am). Food and water were available ad libitum except throughout rGT testing, during which animals were food-restricted to 80%–90% of their free-feeding weight. Testing occurred 4–6 times per week during the dark phase of the light cycle. Experiments were conducted in accordance with the Canadian Council of Animal Care, and the Centre for Addiction and Mental Health Animal Care Committee approved all protocols.
Rats were separated into 4 groups according to the type of uncertainty exposure received. Animals responded for reward according to a predictable/certain FR (FR group, n = 8) or unpredictable/uncertain VR (VR group, n = 8) schedule of reinforcement. Rats yoked to receive unpredictable reward according to the FR or VR groups were also included. Rats were pair-housed with their yoked control.
Uncertainty or certainty exposure
Animals were trained twice daily for 28 days (56 sessions) in 16 standard operant conditioning boxes (Med Associates). Each chamber, located within a light- and sound-attenuating cabinet, was equipped with a nosepoke response hole on the left side of a liquid receptacle in which reinforcement (0.1 mL, 0.3% saccharin in water) could be delivered. A house light centrally located at the top of the chamber on the opposite wall was illuminated for the entire 60-minute session. Rats in the FR or VR groups nosepoked for reinforcement. Similar to the study by Singer and colleagues,16 the reinforcement schedule increased in difficulty from FR/VR1 to FR/VR20, when an individual animal obtained at least 20 rewards across 2 consecutive sessions. Rats in the Y group passively received reinforcement according to their “master” rat. Animals remained in their home cage for 2 weeks after UE/CE.
Locomotor activity
Two weeks after UE/CE (locomotor test 1) and 1 week after rGT testing (locomotor test 2), activity was recorded using a custom-built system. Animals were tested in clear polycarbonate chambers measuring 25 cm × 20 cm × 45 cm3. An array of 11 externally mounted infrared photodetectors were spaced evenly 2 cm above the long axis of the chamber floor. As in the study by Singer and colleagues,16 animals received an injection of saline and were immediately placed in the locomotor chamber for 90 minutes. Rats then received an injection of amphetamine (0.5 mg/kg) and were promptly placed back into the same chamber for 90 minutes.
Rat Gambling Task
Following locomotor test 1 and 1 week of food restriction, rGT testing began. Testing occurred once daily according to previously described methods.29–31 Briefly, animals were trained in 12 standard 5-hole operant conditioning chambers (Med Associates). Chambers contained an array of 5 nose-poke holes on a curved back wall and a food tray located in the centre front wall connected to an external pellet dispenser. A house light was centrally located at the top of the chamber on the front wall. Prior to rGT testing, rats received 4 forced-choice training sessions in which only 1 option was available per trial to ensure all options were equally sampled initially, preventing the development of a bias due to low sampling.29 Animals were then tested on the rGT for the duration of the experiment.
During each 30-minute rGT session, rats initiated a trial by making a response into the illuminated food tray. The tray light was then extinguished and a 5-second intertrial interval (ITI) began. A response into any of the 5 holes during the ITI was classified as a premature response, a measure of impulsive action.29,32 Premature responses were signalled by illumination of the house light for 5 seconds, after which the tray-light was turned on and animals could initiate another trial. Following the ITI, 4 response holes (1, 2, 4 and 5) were illuminated. If the trial was omitted (no response after 10 s), the response lights were turned off and the tray light was reilluminated so that the animal could start another trial. A nosepoke response into an illuminated hole extinguished all lights and resulted in reward delivery (win) or initiated a timeout period (loss). On win trials, the tray light turned on and the corresponding number of pellets (45 mg, BioServ, F0021) were delivered. Collection of the reward initiated the next trial. On loss trials, the stimulus light within the chosen hole flashed at 0.5 Hz for the duration of the corresponding timeout period. Perseverative responses made at the array following a win or at the array and food tray during a timeout were recorded.
The reinforcement schedule for each option is shown in Table 1. As timeouts reduce the time animals have to obtain reward, rats must learn to maximize gains within each session. The optimal option is the 2-pellet option (P2), followed by P1. These advantageous options result in the most reward gained in each 30-minute session. The disadvantageous options are P3 and P4. Two versions of the rGT, which differ in the spatial location of the options, were counterbalanced across all animals.29
Table 1.
Rat Gambling Task parameters
| Parameter | P1 | P2 | P3 | P4 |
|---|---|---|---|---|
| Chance of a win trial, % | 90 | 80 | 50 | 40 |
| No. of pellets rewarded (win trial only) | 1 | 2 | 3 | 4 |
| Time-out duration, s (loss trial only) | 5 | 10 | 30 | 40 |
| Order of options from left to right (hole 1 to hole 5) in 5-hole chambers | ||||
| Version A | 1 | 4 | 5 | 2 |
| Version B | 2 | 5 | 4 | 1 |
Decision-making was assessed for 40 sessions, to assess the effects of UE/CE on acquisition of the rGT and the stability of the rats’ decision-making preference. After 40 sessions, animals received an injection of amphetamine 10 minutes before rGT testing. Doses (0, 0.5 or 1.0 mg/kg) were administered according to a Latin Square design. Drug testing took place on Tuesday and Friday; baseline sessions occurred on Monday, Wednesday and Thursday.
Amphetamine
d-amphetamine sulfate (US Pharmacopeia) was dissolved in sterile saline and administered intraperitoneally. Doses were calculated as a salt.
Statistical analysis
Analyses were conducted using SYSTAT for Windows version 12.00.08. A summary of the main analyses performed are provided in Table 2. Repeated-measures analyses of variance (ANOVAs) used group as a between-subjects factor and schedule, dose and/or session as within-subjects factors, depending on the data analyzed. Version was included as a between-subjects factor during rGT data analyses; however, no version × group interaction was observed during analyses. As such, data from animals tested on each rGT version were pooled.25 For the rGT, the percentage choice (no. of choices of an option ÷ total no. of choices × 100%) and percentage of premature responses (no. of premature responses ÷ [total no. of trials initiated – omissions] × 100%) were subjected to an arc-sine transformation before statistical analysis to limit the effect of the artificially imposed ceiling.33 The number of perseverative responses made during a timeout were calculated as a fraction of the total timeout duration.
Table 2.
Summary of variables for repeated-measures analyses of variance
| Data | Within-subjects factors | Between-subjects factors |
|---|---|---|
| Uncertainty exposure | Reinforcement schedule | Group |
| Locomotor | ||
| Test 1 and test 2 | Dose | Group |
| Test 1 v. test 2 | Test, dose | Group |
| rGT testing: sessions 1–40 in 5-session blocks | ||
| Advantageous choice | Session block | Group, version |
| rGT Testing: sessions 36–40 | ||
| Choice of each option | Choice, session | Group, version |
| Score, trials completed, omitted trials, latency to choose, latency to collect rewards, perseverative responses | Session | Group, version |
| rGT testing: response to amphetamine | ||
| Choice of each option | Choice, dose | Version |
| Score, trials completed, omitted trials, latency to choose, latency to collect rewards, perseverative responses | Dose | Version |
rGT = Rat Gambling Task.
Data from the rGT were compared within each group to determine if animals expressed a significant preference for the advantageous options (P1+P2) or if amphetamine significantly altered decision-making within each group; rGT data were compared between groups using the percent of advantageous choices. During rGT training (sessions 1–40), choice data were analyzed in blocks of 5 sessions and as the average of the last 5 sessions (sessions 36–40). Planned comparisons were conducted using paired sample t tests to compare data within each group (i.e., saline v. amphetamine dose, advantageous choices v. disadvantageous choices) or 2-sample t tests to compare data between 2 groups. For 2-sample t tests, the p value was determined from either the pooled or separate variance, depending on the outcome of a hypothesis test of variance. We considered results to be significant at p < 0.05, and p values between 0.07 and 0.06 were considered to represent the trend level.
No significant differences were observed between the yoked groups during uncertainty exposure, their locomotor response to amphetamine (test 1: FR Y 4459.1 ± 665.3; VR Y 5391.1 ± 553.0, t14 = 1.077, p = 0.3; test 2: FR Y 6381.5 ± 412.9; VR Y 6277.1 ± 613.2, t14 = 0.141, p = 0.9) or throughout rGT testing (all sessions, choice × group: F3,36 = 0.325, p = 0.8) or combined preference for the advantageous options, particularly during the last 5 rGT sessions (sessions 36–40) (FR Y: 67.5% ± 10.3%; VR Y: 63.4% ± 8.3%; group: F1,12 = 2.771, p = 0.1). Therefore, these groups were subsequently combined into a single yoked group (Y group, n = 16).
Results
Uncertainty exposure
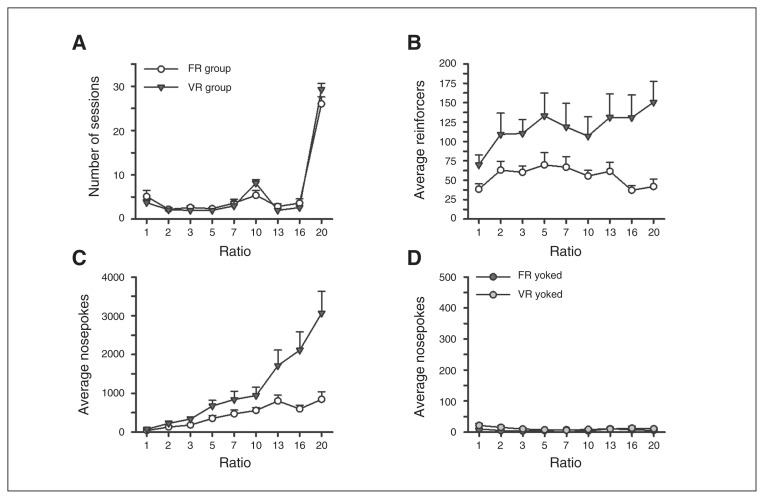
The FR and VR groups were trained on a similar number of sessions for each schedule of reinforcement (Fig. 1A; schedule × group: F8,112 = 1.811, p = 0.07). Animals in the VR group earned a greater number of reinforcers (Fig. 1B; schedule × group: F8,112 = 2.853, p = 0.006) and made more nosepokes while earning reinforcers (Fig. 1C; schedule × group: F8,112 = 10.717, p < 0.001) across the testing ratios compared with the FR group.
Fig. 1.
Uncertainty or certainty exposure. (A) The total number of sessions animals were tested on at each ratio (i.e., schedule of reinforcement) did not differ between the 2 groups. Rats in the variable ratio schedule of reinforcement (VR group) (B) obtained a significantly greater number of reinforcers and (C) made more nosepokes to obtain the reinforcers than rats in the fixed ratio schedule (FR group). (D) Rats yoked to receive unpredictable reward (Y group) made a minimal number of nosepokes throughout testing.
Locomotor test 1
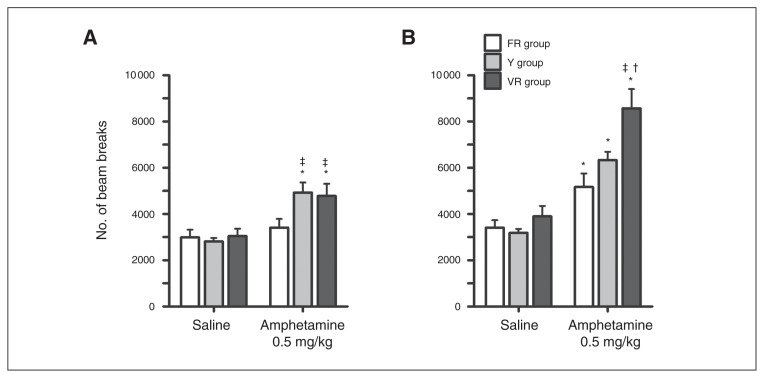
Locomotor activity following uncertainty exposure tended to differ between groups following administration of amphetamine compared with saline (Fig. 2A; dose: F1,29 = 21.818, p < 0.001; dose × group: F2,29 = 2.873, p = 0.07). No differences were observed between groups following saline administration. Planned comparisons showed that 0.5 mg/kg of amphetamine significantly increased locomotor activity compared with saline in the VR and Y groups, but not the FR group. Additionally, locomotor activity following administration of amphetamine was greater in the VR and Y groups than the FR group.
Fig. 2.
Locomotor activity. (A) Following uncertainty/certainty of exposure (UE/CE), rats in all 3 groups responded similarly to saline, and locomotor activity significantly increased similarly following amphetamine in the fixed ratio schedule (FR) and yoked to receive unpredictable reward (Y) groups. (B) Locomotor activity was reassessed following Rat Gambling Task (rGT) training. All groups responded similarly to saline, and amphetamine increased activity in all groups. Amphetamine increased locomotor activity significantly more in the variable ratio schedule of reinforcement (VR) group than the FR and Y groups. *p ≤ 0.05 comparing activity following saline or amphetamine within each group. †p ≤ 0.05 comparing the VR group to the Y group. ‡p ≤ 0.05 comparing the VR or Y groups to the FR group following amphetamine.
Baseline rGT performance
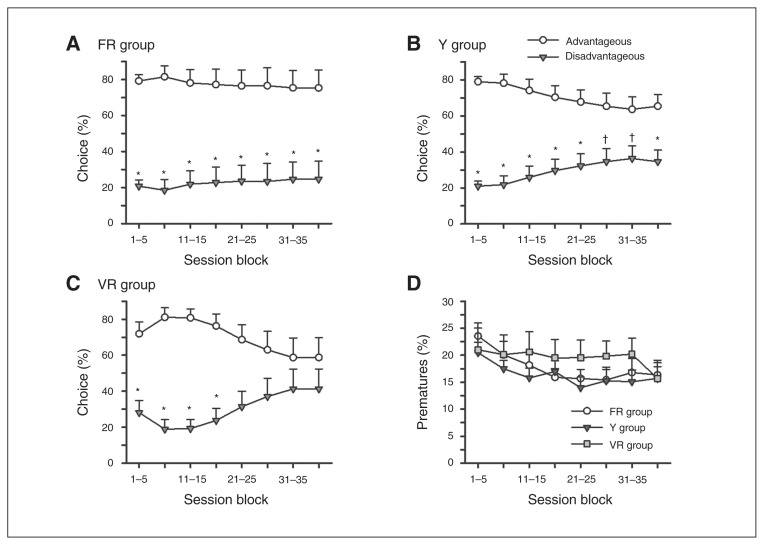
To determine if differences in preference for the advantageous options (P1+P2) existed between groups, average choice of the advantageous options were compared across every 5 sessions. This analysis showed significant differences among the 3 groups in their preference for the advantageous options (session block × group: F14,182 = 1.773, p = 0.05).
To determine if animals preferred the advantageous options on the rGT within each group, average choice of the advantageous options (P1+P2) was compared with choice of the disadvantageous options (P3+P4) across every 5 sessions. Planned comparisons showed that the FR and Y groups maintained a strong preference for the advantageous options throughout training (Fig. 3A–B). Although rats in the VR group initially preferred the advantageous options, planned comparisons showed that this preference diminished and was no longer significant after 20 training sessions (Fig. 3C). Premature responses did not differ between the FR, VR and Y groups throughout rGT training (Fig. 3D; group: largest F2,26 = 1.079, p = 0.38). Therefore, differences in impulsive action likely did not influence the differences in decision-making observed between groups.
Fig. 3.
Decision-making preference and premature responses made during the Rat Gamling Task (rGT) testing. Rats in the (A) fixed ratio schedule (FR) and (B) yoked to receive unpredictable reward (Y) groups showed a strong preference for the advantageous options over the disadvantageous options throughout rGT testing. (C) Rats in the variable ratio schedule of reinforcement (VR) group showed a significant preference for the advantageous options only during the first 4 session blocks. (D) The percentage of premature responses made during the rGT task did not differ between the groups. *p ≤ 0.05 and †p ≤ 0.07 comparing choice of the advantageous options to the disadvantageous options during each block.
Baseline performance on the rGT was further compared between groups using an average of the different variables measured across the last 5 sessions (Table 3). Although there was a main effect of choice (F3,36 = 10.125, p < 0.001), there was no choice × group interaction (F3,36 = 0.459, p = 0.72). Although rGT score was clearly lower in the VR group than the FR group, a significant score × group interaction was not observed (F8,104 = 0.464, p = 0.88). Groups did not differ in the number of trials completed (101.5 ± 6.5), the number of omitted trials (0.34 ± 0.12), latency to choose an option (0.85 ± 0.09 s) or collect rewards (1.09 ± 0.03 s), or perseverative responses made (0.70 ± 0.10 responses/timeout duration) (average of the last 5 sessions for all rats ± standard error of the mean; group: largest F2,26 = 1.734, p = 0.20).
Table 3.
Performance on the Rat Gambling Task as an average of the last 5 sessions
| Baseline variables | Group; mean ± SEM | ||
|---|---|---|---|
|
| |||
| FR | Y | VR | |
| P1 percent choice | 15.6 ± 7.6 | 10.1 ± 3.0 | 6.1 ± 1.3 |
| P2 percent choice | 59.8 ± 13.7 | 55.3 ± 6.9 | 52.6 ± 11.0 |
| P3 percent choice | 12.6 ± 7.9 | 14.0 ± 4.3 | 21.0 ± 12.2 |
| P4 percent choice | 12.0 ± 7.2 | 20.6 ± 6.7 | 20.3 ± 7.5 |
| Score | 50.7 ± 19.9 | 30.8 ± 12.8 | 17.5 ± 22.1 |
FR = fixed ratio schedule; SEM = standard error of the mean; VR = variable ratio schedule of reinforcement; Y = yoked to receive unpredictable reward.
Effect of amphetamine on rGT performance
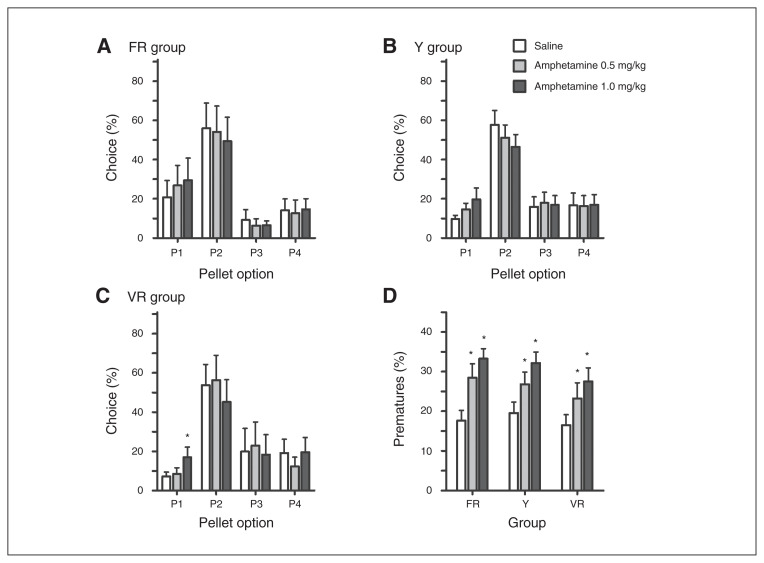
In the FR and Y groups, amphetamine did not alter choice preference, as there was no significant dose × choice interaction (Fig. 4A–B; FR group: F6,36 = 0.643, p = 0.70; Y group: F6,84 = 1.679, p = 0.14). However, in the VR group, administration of amphetamine significantly altered rGT performance (Fig. 4C; dose × choice: F6,36 = 2.427, p = 0.05). In the VR group, compared with saline, there was no effect of 0.5 mg/kg amphetamine (dose × choice: F3,18 = 0.697, p = 0.57), but there was an effect of 1.0 mg/kg amphetamine (dose × choice: F3,18 = 3.247, p = 0.05). The t tests showed that this effect was driven by an increased choice of P1, similar to previous reports.29,30,34,35
Fig. 4.
Effect of amphetamine on Rat Gambling Task (rGT) performance. Amphetamine did not significantly alter decision-making preference in the (A) fixed ratio schedule (FR) or (B) yoked to receive unpredictable reward (Y) groups, but (C) significantly increased choice of P1 in the variable ratio schedule of reinforcement (VR) group following the 1.0 mg/kg dose. (D) At both doses tested, amphetamine significantly increased premature responding in all 3 groups during rGT performance. *p ≤ 0.05 comparing the response following saline to amphetamine within each group.
Also, similar to previous studies, compared with saline amphetamine increased premature responding in all 3 groups (Fig. 4D; FR group: F2,12 = 11.419, p = 0.002; Y group: F2,28 = 14.018, p < 0.001; VR group: F2,12 = 8.854, p = 0.004). Compared with saline, FR and Y groups performed fewer trials after 0.5 mg/kg, and all groups completed fewer trials following 1.0 mg/kg (FR group: F2,12 = 9.921, p = 0.003; Y group: F2,28 = 8.911, p = 0.001; VR group: F2,12 = 3.593, p = 0.06, data not shown). Amphetamine did not alter the number of trials omitted, latency to choose an option or collect reward, or perseverative responding (data not shown).
Locomotor test 2
Locomotor activity again differed between groups following administration of amphetamine compared with saline (Fig. 2B; dose: F1,29 = 97.458, p < 0.001; dose × group: F2,29 = 5.632, p = 0.009). Amphetamine significantly increased locomotor activity compared with saline in all 3 groups. Furthermore, although locomotor activity following administration of amphetamine did not differ between the FR and Y groups, the VR group, which displayed the most disadvantageous decision-making on the rGT, had a greater response to amphetamine than both the FR and Y groups.
Analyses of the 2 locomotor tests showed that animals behaved differently on the 2 tests (test: F1,29 = 47.721, p < 0.001; test × group: F2,29 = 4.622, p = 0.018, test × dose: F1,29 = 20.594, p < 0.001). Although the animals’ response to saline differed between the 2 tests, this effect was not dependent on group (test: F1,29 = 10.825, p = 0.003; test × group: F2.29 = 0.832, p = 0.45). However, the animals’ response to amphetamine did significantly differ by group across the 2 tests (test: F1,29 = 40.019, p < 0.001; test × group: F2.29 = 4.081, p = 0.027). Post hoc analyses showed that the animals’ response to amphetamine was greater during the second locomotor test for all groups; however, this effect was greatest in the VR group (FR group: t7 = 2.921, p = 0.022; Y group: t15 = 2.727, p = 0.016; VR group: t7 = 5.347, p = 0.001). A greater locomotor response to amphetamine during the second test could result from uncertainty experienced during rGT testing or from receiving additional injections of amphetamine.
Correlations with reinforcers earned during UE/CE
Pearson correlations were performed using the total number of reinforcers earned during UE/CE and locomotor response following amphetamine as well as the average rGT score during the last 5 test sessions. There was no significant correlation with the number of reinforcers earned during UE/CE following administration of amphetamine for any group during the first (FR group: r2 = 0.178, p = 0.30; Y group: r2 = 0.090, p = 0.26; VR group: r2 = 0.130, p = 0.38) or second (FR group: r2 = 0.231, p = 0.23; Y group: r2 = 0.091, p = 0.26; VR group: r2 = 0.092, p = 0.47) locomotor tests. Additionally, there was no correlation with the number of reinforcers earned during UE/CE and final score on the rGT (FR group: r2 = 0.1590, p = 0.33; Y group: r2 = 0.005, p = 0.79; VR group: r2 = 0.089, p = 0.48).
Discussion
These experiments demonstrated that UE results in behavioural sensitization, which may reflect an underlying DA sensitization. Animals that responded for reward according to a variable schedule of reinforcement (VR group) or animals that passively received reward (Y group) showed a greater locomotor response to amphetamine than control animals that received CE (FR group). Subsequently, only the VR group showed increased risky decision-making on the rGT, illustrated by a loss of preference for the advantageous options over the disadvantageous options later in training (sessions 21–40). Although all groups of rats showed greater locomotor activity in response to amphetamine compared with saline following rGT testing, this effect was largest in the VR group. Therefore, exposure to reward uncertainty — either through UE or through performance of a decision-making task — results in a sensitization-like effect, similar to chronic exposure to drugs of abuse. However, only actively responding for uncertain rewards significantly increased risky decision-making on the rGT.
Although gambling itself is quite complex, reward uncertainty is an important feature of many, if not all, forms of gambling. For example, in slot machines — which are associated with high problem-gambling rates36 — the probability of receiving a reward is just under 50%.37 The maximum uncertainty of reward delivery is indeed 50%: anything above or below a 50% probability can in theory be predicted (i.e., more likely to win or lose). In the present experiment, we used a variable schedule of reinforcement, which also has a high level of uncertainty (e.g., at a VR20 schedule, any given nose-poke from 1–40 could be reinforced, with an average of 20 nosepokes required to receive a reward). Therefore, it is unlikely that animals predicted whether any given nosepoke would be rewarded. The Y group received passive UE, as saccharin was delivered without any forewarning. Unpredictable reward delivery may influence the mesolimbic DA pathway through anticipation. For example, neurons in the ventral tegmental area fire more rapidly before reward delivery when reward delivery is uncertain compared with trials in which reward delivery is certain, with the largest increase in firing rate coinciding with maximal uncertainty (50%).38
Previous research has shown that UE, either through passive reward delivery15 or actively responding for reinforcement,16 resulted in greater locomotor activity following a low dose of amphetamine compared to control groups. The present study showed that behavioural sensitization was similar in animals responding for unpredictable reward (VR group) or passively receiving unpredictable reward (Y group). Although animals in the VR group received more reward than rats in the FR group, the 2 yoked groups did not differ in their locomotor response to amphetamine or decision-making preferences. Likewise, there was no significant correlation with the amount of reward received and decision-making preferences or locomotor activity. Therefore, the amount of reward received during UE likely does not impact these measures.
Actively responding for uncertain rewards or passively receiving uncertain rewards may produce different amounts of uncertainty. If the amount of uncertainty was arranged on a continuum — with the FR and VR groups receiving the least and most uncertainty, respectively — we hypothesize that the Y group would be placed in the middle. We suggest this possibility because, although rewards are delivered with uncertainty in the Y group, the VR group could potentially have experienced more uncertainty, as there was uncertainty associated with every response the animals made. Although both Y and VR groups initially exhibited a similar locomotor response following amphetamine, this behavioural sensitization measurement may not have been able to detect smaller differences in DA sensitization between these groups, or perhaps there was a ceiling effect. However, when the second locomotor test was given, the effect of amphetamine on locomotor activity was significantly greater only in the VR group. This increase may have been caused by the additional UE the VR group experienced as a result of making more risky choices on the rGT. Alternatively, greater locomotor activity on the second test could be attributed to prior amphetamine exposure. Regardless, it is unlikely that the locomotor challenge to amphetamine was not sensitive enough to detect differences in behavioural sensitization or that the animals reached a ceiling effect. As such, both forms of UE — active (VR group) or passive (Y group) — initially resulted in a similar level of behavioural sensitization.
Only rats in the VR group showed a large increase in risky decision-making on the rGT, indicated by the absence of a significant difference between the advantageous and disadvantageous options during the last 20 sessions. Animals in the Y group showed a trend toward increased choice of the disadvantageous options near the end of rGT testing, but still maintained a greater preference for the advantageous options than the VR group. Interestingly, both the Y and VR groups showed behavioural sensitization before rGT testing, but this effect was greatly enhanced in the VR group after rGT testing. As such, both types of UE appear to influence choice on the rGT; however, actively responding for uncertain rewards, rather than passively receiving them, appeared to have a greater influence on risky decision-making. The interaction between actively responding during the UE may have had a greater influence on decision-making, as animals are also required to make responses during the rGT. These findings suggest that in addition to sensitization, the type of UE also influences risky decision-making. We are currently exploring the contribution of sensitization alone by comparing psychostimulant-induced sensitization to sensitization through UE.
Although the VR group failed to show a significant preference for the advantageous options at the end of rGT testing, there was no difference in choice of each individual option (P1, P2, P3, or P4) available on the rGT between the FR, Y or VR groups at the end of training. Yet, a significant preference for the advantageous (P1+P2) over the disadvantageous (P3+P4) options was maintained throughout testing in the FR group; a similar effect was observed in the Y group. Therefore, increased preference for the disadvantageous options in the VR group cannot be attributed to increased choice of a single risky option. Likewise, this finding also cannot be attributed to decreased preference for a single advantageous option. Additionally, increased preference for the risky, disadvantageous options in the VR group cannot be attributed to an inability to learn the optimal strategy on the rGT since animals initially preferred the advantageous options. Therefore, actively responding for uncertain reward negatively influences future decision-making.
On the IGT, individuals with gambling disorder and healthy controls also initially do not differ from each other, often initially showing a preference for the disadvantageous options. With more trials, a large preference for the advantageous options emerges in controls, whereas those with gambling disorder fail to improve on the task.18–23 In the present study, rats in the VR group initially preferred the advantageous options (similar to controls), but then an increased preference for the disadvantageous options emerged with repeated testing. Therefore, in both the rGT and IGT, decision-making preferences in the experimental/patient group are initially similar to controls, and a decision-making deficit emerges as animals/participants gain more experience with the task. A potential explanation for this effect may be familiarity with the potential gains and losses associated with each option. Performance of participants with gambling disorder on the IGT has been associated with explicit knowledge of the outcomes.39 Furthermore, patients with gambling disorder prefer riskier options on tasks in which the gains and losses are explicit.40 Therefore, familiarity with the outcomes of each option may be required before a stable preference emerges.
Animals in the VR group could have increased their choice of the risky, disadvantageous options for numerous reasons. Although we did not directly conduct any experiments to analyze this issue, we have formulated some hypotheses. One possibility could be related to the magnitude of the potential losses. Larger losses could have become less frustrating or punishing for the VR group (i.e., animals became more tolerant of the timeouts) compared with the control groups over time. Or perhaps animals became more tolerant of the reduced reward frequency associated with the disadvantageous options compared with the advantageous options (50% and 40% compared with 80% and 90%, respectively). This tolerance could have been precipitated by the UE; as with any given response, animals in the VR group were unable to predict whether reward would be delivered (similar to being unable to predict if reward would be delivered following choice of the disadvantageous options on the rGT).
It is also possible that, compared to avoiding losses or increased reward frequency, the magnitude of the potential reward had a greater influence on decision-making. Smaller reinforcer amounts associated with the advantageous options (1 or 2 pellets) may have diminished in value in the VR group, which biased animals toward the riskier options associated with larger immediate reward on win trials (3 or 4 pellets). Interestingly, compared with controls, individuals with gambling disorder show a higher sensitivity to larger gains on the IGT.41 Likewise, in control rats, animals preferred the largest reward option on a modified rGT in which the frequency of reward delivery was equalized across all options, essentially increasing the importance of reward magnitude over loss frequency or timeout magnitude.29
Despite the differences in locomotor activity in response to amphetamine, there were only minor differences between the groups in their response to amphetamine on the rGT. Similar to previous studies,29,30,34,35 administration of amphetamine increased premature responses on the rGT. However, the only group that changed their decision-making preferences was the VR group, increasing choice of P1 (an effect also similar to those reported in previous studies,29,30,34,35 but much smaller in magnitude). The reduced effects of amphetamine on decision-making in our study compared with others may result from the animals’ UE/CE or previous experience with amphetamine in the locomotor challenge test.
Several preclinical tests of gambling-like behaviour have been developed that focus on different aspects of gambling behaviour, such as loss chasing42 or betting.43 Additionally, paradigms modelled after slot machines have also been established.44,45 These tests are useful for understanding the neurobiological basis of gambling-like behaviour. In contrast, the findings presented here demonstrated a novel model of gambling disorder itself. We used an experimental manipulation (UE) that resulted in behavioural sensitization, likely indicative of DA sensitization, and increased risky decision-making. These features in our rodent model are similar to those of patients with gambling disorder; these patients show greater DA release14 and a progressive risky decision-making bias on a comparable laboratory-based task.18
Limitations
This work has some limitations. First, we modelled only 1 aspect of gambling: the uncertain receipt of reward. There are many different types of gambling behaviours, all of which can involve many different features (e.g., betting, risk of loss, light and sound stimuli). Repeated exposure to these features of gambling should be studied in future models. Second, it is important to note that we used only male rats in the present study; therefore, it would be worthwhile to determine whether similar neurochemical and behavioural abnormalities occur following UE in female animals. Third, we did not measure DA directly to assess sensitization. Finally, individuals with gambling disorder experience tremendous losses (personally and financially) that in no way can be appropriately modelled in lab rats.
Conclusion
Although UE through passive receipt of uncertain reward (Y group) or actively responding for uncertain reinforcement (VR group) resulted in behavioural sensitization, increased risky decision-making was observed only in rats the VR group. Moreover, additional UE during rGT testing appeared to further increase sensitization in this group. We propose that UE could be used to model some neurochemical and decision-making deficits present in gambling disorder. An animal model of gambling disorder is a significant advance that could further the development of effective treatment strategies for patients with gambling disorder and provide a greater understanding of additional neurochemical and behavioural abnormalities in affected patients.
Acknowledgements
This work was funded by an operating grant from the National Center for Responsible Gaming (NCRG) to F. Zeeb, M. Zack and P. Fletcher. F. Zeeb was also supported by a Canadian Institutes for Health Research (CIHR) Postdoctoral Fellowship.
Footnotes
Competing interests: F. Zeeb previously consulted for Intervivo Solutions on an unrelated matter. None declared by the other authors.
Contributors: F. Zeeb, M. Zack and P. Fletcher designed the study F. Zeeb, Z. Li and D. Fisher acquired the data, which F. Zeeb, M. Zack and P. Fletcher analyzed. F. Zeeb wrote the article, which all authors reviewed and approved for publication.
References
- 1.Hodgins DC, Stea JN, Grant JE. Gambling disorders. Lancet. 2011;378:1874–84. doi: 10.1016/S0140-6736(10)62185-X. [DOI] [PubMed] [Google Scholar]
- 2.Cox BJ, Yu N, Afifi TO, et al. A national survey of gambling problems in Canada. Can J Psychiatry. 2005;50:213–7. doi: 10.1177/070674370505000404. [DOI] [PubMed] [Google Scholar]
- 3.Hodgins DC. Commentary on Markham et al. (2014): Huffing and puffing our way to accurate gambling-related harm prevalence estimates. Addiction. 2014;109:1517. doi: 10.1111/add.12678. [DOI] [PubMed] [Google Scholar]
- 4.Markham F, Young M, Doran B. Gambling expenditure predicts harm: evidence from a venue-level study. Addiction. 2014;109:1509–16. doi: 10.1111/add.12595. [DOI] [PubMed] [Google Scholar]
- 5.Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Publishing; 2013. [Google Scholar]
- 6.Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology (Berl) 1992;107:271–6. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- 7.Valadez A, Schenk S. Persistence of the ability of amphetamine preexposure to facilitate acquisition of cocaine self-administration. Pharmacol Biochem Behav. 1994;47:203–5. doi: 10.1016/0091-3057(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 8.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–39. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Vezina P, Lorrain DS, Arnold GM, et al. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–62. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher PJ, Tenn CC, Sinyard J, et al. A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacology. 2007;32:1122–32. doi: 10.1038/sj.npp.1301221. [DOI] [PubMed] [Google Scholar]
- 11.Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–98. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 13.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- 14.Boileau I, Payer D, Chugani B, et al. In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: a positron emission tomography study with [(11)C]-(+)-PHNO. Mol Psychiatry. 2014;19:1305–13. doi: 10.1038/mp.2013.163. [DOI] [PubMed] [Google Scholar]
- 15.Zack M, Featherstone RE, Mathewson S, et al. Chronic exposure to a gambling-like schedule of reward predictive stimuli can promote sensitization to amphetamine in rats. Front Behav Neurosci. 2014;8:36. doi: 10.3389/fnbeh.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer BF, Scott-Railton J, Vezina P. Unpredictable saccharin reinforcement enhances locomotor responding to amphetamine. Behav Brain Res. 2012;226:340–4. doi: 10.1016/j.bbr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bechara A, Damasio AR, Damasio H, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 18.Ciccarelli M, Griffiths MD, Nigro G, et al. Decision making, cognitive distortions and emotional distress: a comparison between pathological gamblers and healthy controls. J Behav Ther Exp Psychiatry. 2017;54:204–10. doi: 10.1016/j.jbtep.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Goudriaan AE, Oosterlaan J, de Beurs E, et al. Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Brain Res Cogn Brain Res. 2005;23:137–51. doi: 10.1016/j.cogbrainres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Krmpotich T, Mikulich-Gilbertson S, Sakai J, et al. Impaired decision-making, higher impulsivity, and drug severity in substance dependence and pathological gambling. J Addict Med. 2015;9:273–80. doi: 10.1097/ADM.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linnet J, Møller A, Peterson E, et al. Inverse association between dopaminergic neurotransmission and Iowa Gambling Task performance in pathological gamblers and healthy controls. Scand J Psychol. 2011;52:28–34. doi: 10.1111/j.1467-9450.2010.00837.x. [DOI] [PubMed] [Google Scholar]
- 22.Power Y, Goodyear B, Crockford D. Neural correlates of pathological gamblers preference for immediate rewards during the Iowa Gambling Task: an fMRI study. J Gambl Stud Springer US. 2011;28:623–36. doi: 10.1007/s10899-011-9278-5. [DOI] [PubMed] [Google Scholar]
- 23.Wiehler A, Peters J. Reward-based decision making in pathological gambling: the roles of risk and delay. Neurosci Res. 2015;90:3–14. doi: 10.1016/j.neures.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Bechara A, Dolan S, Denburg N, et al. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–89. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 25.Bechara A. Risky business: emotion, decision-making, and addiction. J Gambl Stud. 2003;19:23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- 26.Businelle MS, Apperson MR, Kendzor DE, et al. The relative impact of nicotine dependence, other substance dependence, and gender on Bechara Gambling Task performance. Exp Clin Psychopharmacol. 2008;16:513–20. doi: 10.1037/a0013510. [DOI] [PubMed] [Google Scholar]
- 27.Dolan SL, Bechara A, Nathan PE. Executive dysfunction as a risk marker for substance abuse: the role of impulsive personality traits. Behav Sci Law. 2008;26:799–822. doi: 10.1002/bsl.845. [DOI] [PubMed] [Google Scholar]
- 28.Rotheram-Fuller E, Shoptaw S, Berman SM, et al. Impaired performance in a test of decision-making by opiate-dependent tobacco smokers. Drug Alcohol Depend. 2004;73:79–86. doi: 10.1016/j.drugalcdep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–43. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]
- 30.Zeeb FD, Winstanley CA. Functional disconnection of the orbitofrontal cortex and basolateral amygdala impairs acquisition of a rat gambling task and disrupts animals’ ability to alter decision-making behavior after reinforcer devaluation. J Neurosci. 2013;33:6434–43. doi: 10.1523/JNEUROSCI.3971-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeeb FD, Winstanley CA. Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. J Neurosci. 2011;31:2197–204. doi: 10.1523/JNEUROSCI.5597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–80. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 33.McDonald J. Handbook of biological statistics. 3rd ed. Baltimore (Md): Sparky House Publishing; 2014. [Google Scholar]
- 34.Baarendse PJJ, Counotte DS, O’Donnell P, et al. Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology. 2013;38:1485–94. doi: 10.1038/npp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baarendse PJJ, Winstanley CA, Vanderschuren LJMJ. Simultaneous blockade of dopamine and noradrenaline reuptake promotes disadvantageous decision making in a rat gambling task. Psychopharmacology (Berl) 2013;225:719–31. doi: 10.1007/s00213-012-2857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowling N, Smith D, Thomas T. Electronic gaming machines: Are they the “crack-cocaine” of gambling? Addiction. 2005;100:33–45. doi: 10.1111/j.1360-0443.2005.00962.x. [DOI] [PubMed] [Google Scholar]
- 37.Tremblay A-M, Desmond RC, Poulos CX, et al. Haloperidol modifies instrumental aspects of slot machine gambling in pathological gamblers and healthy controls. Addict Biol. 2011;16:467–84. doi: 10.1111/j.1369-1600.2010.00208.x. [DOI] [PubMed] [Google Scholar]
- 38.Fiorillo CD. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 39.Ochoa C, Alvarez-Moya EM, Penelo E, et al. Decision-making deficits in pathological gambling: the role of executive functions, explicit knowledge and impulsivity in relation to decisions made under ambiguity and risk. Am J Addict. 2013;22:492–9. doi: 10.1111/j.1521-0391.2013.12061.x. [DOI] [PubMed] [Google Scholar]
- 40.Brand M, Kalbe E, Labudda K, et al. Decision-making impairments in patients with pathological gambling. Psychiatry Res. 2005;133:91–9. doi: 10.1016/j.psychres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Brevers D, Koritzky G, Bechara A, et al. Cognitive processes underlying impaired decision-making under uncertainty in gambling disorder. Addict Behav. 2014;39:1533–6. doi: 10.1016/j.addbeh.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers RD, Wong A, McKinnon C, et al. Systemic administration of 8-OH-DPAT and eticlopride, but not SCH23390, alters loss-chasing behavior in the rat. Neuropsychopharmacology. 2013;38:1094–104. doi: 10.1038/npp.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tremblay M, Cocker PJ, Hosking JG, et al. Dissociable effects of basolateral amygdala lesions on decision making biases in rats when loss or gain is emphasized. Cogn Affect Behav Neurosci. 2014;14:1184–95. doi: 10.3758/s13415-014-0271-1. [DOI] [PubMed] [Google Scholar]
- 44.Peters H, Hunt M, Harper D. An animal model of slot machine gambling: the effect of structural characteristics on response latency and persistence. J Gambl Stud. 2010;26:521–31. doi: 10.1007/s10899-010-9183-3. [DOI] [PubMed] [Google Scholar]
- 45.Winstanley CA, Cocker PJ, Rogers RD. Dopamine modulates reward expectancy during performance of a slot machine task in rats: evidence for a “near-miss” effect. Neuropsychopharmacology. 2011;36:913–25. doi: 10.1038/npp.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]