Abstract
Helicobacter pylori α1–3/4-fucosyltransferase (Hp3/4FT) was expressed in Escherichia coli at a level of 30 mg/L culture and used as a diverse catalyst in a one-pot multienzyme (OPME) system for high-yield production of L-fucose-containing carbohydrates including Lewis antigens such as Lewis a, b, x, O-sulfated Lewis x, and sialyl Lewis x as well as human milk fucosides such as 3-fucosyllactose (3-FL), lacto-N-fucopentaose (LNFP) III, lacto-N-difuco-hexaose (LNDFH) II and III. Noticeably, while difucosylation of tetrasaccharides was readily achieved using an excess amount of donor, synthesis of LNFP III was achieved by Hp3/4FT-catalyzed selective fucosylation of the N-acetyllactoamine (LacNAc) component in lacto-N-neotetraose (LNnT).
Graphical abstract
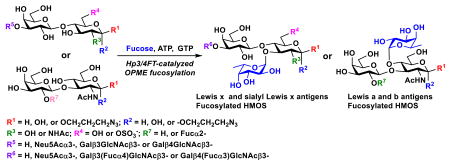
Lewis x, a, b, O-sulfated and sialyl Lewis x antigens, and fucosylated human milk oligosaccharides such as 3-FL, LNFP III, LNDFH II, and LNDFH III were efficiently produced using an Hp3/4FT-catalyzed one-pot multienzyme (OPME) fucosylation system.
Lewis antigens such as Lewis a (Lea), Leb, Lex, and Ley are Lfucose-containing glycans1 presented abundantly on the surface of human red blood cells, endothelium and epithelium of various tissues such as kidney, genitourinary, and gastrointestinal tract.2, 3 Many of them are the binding targets for pathogenic bacteria that led to infection.1 Lea and Leb are fucosylated type I chains containing a Galβ3GlcNAc disaccharide sequence and are mainly expressed in surface epithelia.4 In comparison, Lex and Ley are fucosylated type II chains containing a Galβ4GlcNAc disaccharide sequence5 and are expressed in deep glands.6 The α1–3- and α1–4-linked Lfucoses are essential structure components of Lex/Ley and Lea/Leb antigens, respectively.
α1–3/4-Linked fucosides are also abundant in human milk oligosaccharides (HMOS)7 where they have been found to have prebiotic, anti-adhesive antimicrobial, and immunomodulating activities which contribute significantly to the benefits of breast feeding.8-12 In general, α1–3-fucosylated HMOS were found to be protective for human immunodeficiency virus (HIV)-exposed uninfected children during breastfeeding.13 Lewis antigen-type HMOS were shown to reduce selectin-mediated cell-cell interactions.14, 15
Fucosylated glycans have received great attention due to their potential applications as nutraceuticals and pharmaceuticals. Various synthetic methods have been explored. Compared to chemical fucosylation approaches which require tedious hydroxyl group protection and deprotection, efficient and environmentally friendly enzymatic fucosylation via fucosyltransferase-catalyzed reactions is a preferred route.
Among reported α1–3, α1–4, and α1–3/4-fucosyltransferases,16-26 Helicobacter pylori UA948 α1–3/4-fucosyltransferase (Hp3/4FT) that can use both type I and type II glycans as acceptors22, 25 draw our attention as an attractive catalyst. Nevertheless, its application in synthesizing fucosylated glycans has not been fully explored. Here, we report the use of Hp3/4FT in a highly efficient one-pot three-enzyme (OP3E) fucosylation system for the synthesis of diverse α1–3- and α1–4-linked fucosides, including Lea, Leb, Lex, O-sulfated Lex, sialyl Lex, and human milk fucosides 3-FL, LNFP III, LNDFH II and III.
To obtain an active C-His6-tagged Hp3/4FT, the gene of C-terminal 34 amino acid-truncated Hp3/4FT from H. pylori strain UA948 (GenBank Accession number AAF35291.2, full length 462 aa) was codon optimized and cloned into pET22b(+) vector. Expression in E. coli BL21(DE3) cells led to about 30 mg of soluble protein that could be routinely purified from 1 liter culture using a Ni2+-column (Figure S1, ESI†). As other bacterial α1–3-, α1–4-, and α1–3-/4-fucosyltransferases, Hp3/4FT belongs to glycosyltransferase family 10 (GT10) in the Carbohydrate Active Enzyme (CAZy) database (www.cazy.org).27, 28 It shares more than 78% sequence identity with other FTs from H. pylori (Figure S2, ESI†).
Using fluorophore 2-anthranilic acid (2AA)-labeled disaccharides (LacβPro2AA, LacNAcβPro2AA, and Galβ3GlcNAcβPro2AA) as acceptor substrates in high-performance liquid chromatography (HPLC) assays, pH profiles (Figure S3, ESI†) showed similar patterns although optimal activity of Hp3/4FT was observed in a broader pH range when LacNAcβ2AA was used as the acceptor (6.0–9.0) compared to that (pH 7.0–9.0) for LacβPro2AA and Galβ3GlcNAcβPro2AA. In addition, Hp3/4FT was more efficient in using LacNAcβPro2AA and Galβ3GlcNAcβPro2AA as acceptors and a lower amount of enzyme (0.24 μg) was used in the assays compared to LacβPro2AA (1.9 μg enzyme). The fucosyltransferase activity decreased dramatically when the pH was below 6.0 or above 9.0 (for Lacβ2AA and Galβ3GlcNAcβPro2AA) or 10.0 (for LacNAcβPro2AA). The thermal stability assays of Hp3/4FT indicated that the enzyme was stable at 30 °C or lower for at least 60 min. The enzyme activity decreased dramatically by incubation at 37 °C and an almost complete loss of activity was observed at 40 °C (Figure S4, ESI†). At 4 °C, Hp3/4FT was stable for at least two months without activity decrease.
A metal ion was not required for the fucosyltransferase activity of Hp3/4FT as 10 mM of EDTA did not alter its activity significantly (Figure S5, ESI†). Nevertheless, the presence of 10 mM of divalent metal ions such as Ca2+, Co2+, Mg2+, Mn2+, or Ni2+ increased the enzyme activity slightly while the presence of Cu2+ decreased the activity about three-fold. Although Hp3/4FT has three cysteine residues, the presence of 10 mM dithiothreitol (DTT) did not affect the activity of the enzyme.
As shown in Table 1 (kinetic plots in Figure S6, ESI†), among three 2AA-labelled acceptor substrates, Hp3/4FT showed the highest catalytic efficiency for LacNAcβ2AA (kcat/KM = 10.87 s−1 mM−1) due to the lowest KM value (0.46±0.07 mM) and a medium range kcat (5.00±0.18 s−1). Galβ3GlcNAcβ2AA had a medium catalytic efficiency (5.89 s−1 mM−1) with the worst KM (1.32±0.24 mM) and the highest kcat (7.77±0.45 s−1). Lacβ2AA had the lowest catalytic efficiency (kcat/KM = 0.55 s−1 mM−1) with a medium KM value (kcat/KM = 0.99±0.18 mM) and the lowest kcat value (0.54±0.03 s−1, more than 9-folder lower than the other two). Hp3/4FT also showed different catalytic efficiencies (0.80 s−1 mM−1, 7.01 s−1 mM−1, and 11.19 s−1 mM−1) for guanosine 5′-diphosphate fucose (GDP-Fuc) when different acceptors (Lacβ2AA, LacNAcβ2AA, and Galβ1–3GlcNAcβ2AA0 were used.
Table 1. Apparent kinetic parameters of Hp3/4FT.
| Substrate | kcat (s−1) | KM (mM) | kcat/KM (s−1 mM−1) |
|---|---|---|---|
| LacβPro2AA | 0.54±0.03 | 0.99±0.18 | 0.55 |
| aGDP-Fuc | 0.30±0.14 | 0.38±0.07 | 0.80 |
| LacNAcβPro2AA | 5.00±0.18 | 0.46±0.07 | 10.87 |
| bGDP-Fuc | 6.02±0.26 | 0.86±0.13 | 7.01 |
| Galβ3GlcNAcβPro2AA | 7.77±0.45 | 1.32±0.24 | 5.89 |
| cGDP-Fuc | 3.92±0.16 | 0.35±0.06 | 11.19 |
Determined using LacβPro2AA as an acceptor
Determined using LacNAcβPro2AA as an acceptor
Determined using Galβ3GlcNAcβPro2AA as an acceptor.
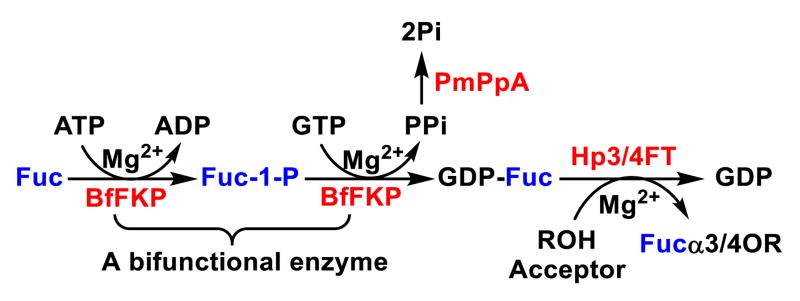
With no noticeable fucosidase activity observed when tested using LexβProN3, LexβpNP, or LeaβProN3 as a substrate, Hp3/4FT was readily applied in a one-pot three-enzyme (OP3E) fucosylation system for the synthesis of various α1–3- and/or α1– 4-linked fucosides (Scheme 1). In this system, GDP-Fuc was formed from L-fucose, adenine 5′-triphosphate (ATP), and guanosine 5′-triphosphate (GTP) using a bifunctional Bacteroides fragilis enzyme (BfFKP) with both L-fucokinase and GDP-Fuc pyrophosphorylase activities.29 Pasteurella multocida inorganic pyrophosphatase (PmPpA)30 was used to break down the pyrophosphate byproduct to drive the formation of GDPFuc which was used as the donor substrate for Hp3/4FT for the formation of fucosides.
Scheme 1.
Hp3/4FT-catalyzed one-pot three-enzyme (OP3E) synthesis of fucosides. Enzyme abbreviations: FKP, Bacteroides fragilis strain NCTC9343 bifunctional L-fucokinase/GDP-fucose pyrophosphorylase; PmPpA, Pasteurella multocida inorganic pyrophosphatase; Hp3/4FT, H. pylori α1–3/4-fucosyltransferase.
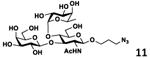
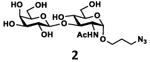
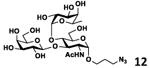
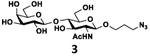
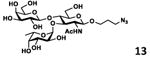
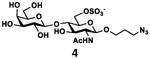
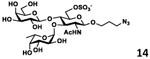
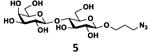
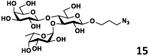
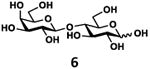
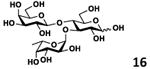
As shown in Table 2, all disaccharides (1–6), and tetrasaccharides (7-9) tested were well suited acceptor substrates for Hp3/4FT for the production of mono- or difucosylated products (11–19). These included type I disaccharide Galβ3GlcNAcβOR (1)31 and its analog Galβ3GlcNAcαOR (2)31 where R was a propyl azide (ProN3 aglycon). They were α1–4-fucosylated by Hp3/4FT to produce mono-fucosylated products Galβ3(Fucα4)GlcNAcβProN3 (11, Lea antigen) and Galβ3(Fucα4)GlcNAcαProN3 (12) in excellent 95% and 96% yields, respectively. Type II disaccharide Galβ4GlcNAcβOR (3)30 and its 6-O-sulfated analog Galβ4GlcNAc6SβOR (4)32 were also excellent acceptors for α1– 3-fucosylation by Hp3/4FT to produce Galβ4(Fucα3)GlcNAcβProN3 (13, Lex antigen) and Galβ4(Fucα3)GlcNAc6SβProN3 (14) in 98% and 93% yields, respectively. Mono-fucosylation of Type VI disaccharide Galβ4GlcβOR (5)33-35 and lactose (6) led to the formation of Galβ4(Fucα3)GlcβProN3 (15) and 3-fucosyllactose [16, 3-FL, Galβ4(Fucα3)Glc] in 92% and 90% yields, respectively. 3-FL is one of the most abundant fucosylated HMOS that could inhibit bacterial or viral adhesion to human epithelial cells.36
Table 2. One-pot three-enzyme (OP3E) synthesis of α1–3/4-linked fucosides.
| Entry | Acceptor | Product | Yield (%) |
|---|---|---|---|
| a |

|
|
95 |
| b |
|
|
96 |
| c |
|
|
98 |
| d |
|
|
93 |
| e |
|
|
92 |
| f |
|
|
90 |
| g |
|
|
98 |
| h |
|
|
88 |
| i |
|
|
99 |
| j |
|
|
84 |
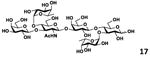
Both Galβ3/4GlcNAc and Galβ4Glc components in lacto-N-tetraose (7, LNT, Galβ3GlcNAcβ3Galβ4Glc) and lacto-N-neotetraoside (9, LNnTβProN3, Galβ4GlcNAcβ3Galβ4GlcβProN3)37, 38 are suitable fucosylation sites for Hp3/4FT. Fucosylation with an excess amount of Lfucose, ATP, and GTP (3 equivalents each) led to the formation of difucosylated hexasaccharides Galβ3(Fucα4)GlcNAcβ3Galβ4(Fucα3)Glc (17) and Galβ4(Fucα3)GlcNAcβ3Galβ4(Fucα3)GlcβProN3 (19) in excellent 98% and 99% yields, respectively. Compound 17 (lacto-N-difuco-hexaose II, or LNDFH II)39 is a hexasaccharide containing both α1–3- and α1–4-linked fucose and is presented in human milk at a level of 0.08–0.1 g L−1.40 While compound 19 is the glycoside of another human milk hexasaccharide lacto-N-neodifucohexaose II (LNnDFH II or LNDFH III).12 Quite remarkably, if the ratio of GDP-Fuc to lacto-N-neotetraose (8, LNnT) was set to 1.2:1, Hp3/4FT selectively catalyzed the transfer of fucose to the LacNAc (instead of lactose) component in LNnT and led to the formation of LNFP III (18) as characterized by MALDI-TOF tandem mass spectrometry and 2D nuclear magnetic resonance (NMR) spectroscopy (Figure S7, ESI†). This acceptor site preference was consistent with the kinetics study results.
Sialylated type II glycan Neu5Acα3Galβ4GlcNAcβOR was also a good acceptor for β1–3-fucosylation by Hp3/4FT to produce Neu5Acα3Galβ4(Fucα3)GlcNAcβProN3 (20, sialyl Lex antigen) in 84% yield. In comparison, Neu5Acα3Galβ4GlcβOR and sialyl type I glycan Neu5Acα3Galβ3GlcNAcβOR were poor acceptors for Hp3/4FT.
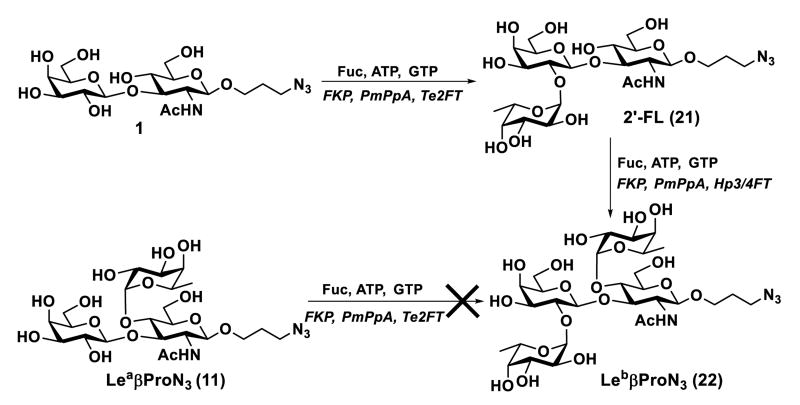
The Hp3/4FT was also used for the synthesis of a Leb antigen which has both α1–2- and α1–4-linked fucose residues (Scheme 2). The α1–2-fucose was installed to type I disaccharide Galβ3GlcNAcβProN3 (1) to produce H type I trisaccharide22 Fucα2Galβ3GlcNAcβProN3 (21) by a one-pot three-enzyme fucosylation system containing BfFKP, PmPpA, and a highly efficient α1–2-fucosyltransferase cloned from Thermosynechococcus elongatus (Te2FT).41 Leb tetrasaccharide Fucα2Galβ3(Fucα4)GlcNAcβProN3 (22) was successfully obtained in 98% yield, indicating α1–2-fucosylation of type I glycan did not affect its recognition by Hp3/4FT as an acceptor. However, attempts to alter the fucosylation sequence by performing Te2FT-catalyzed α1–2-fucosylation of Lea (11) was not successful, indicating that α1–3-fucosylation of type I glycan blocked Te2FT activity. The result was similar to that reported previously.22
Scheme 2.
Enzymatic synthesis of Leb antigen (22) by Hp3/4FT-catalyzed OP3E fucosylation of H type I trisaccharide (21) formed by Te2FT-catalyzed OP3E fucosylation reaction.
In conclusion, the recombinant Hp3/4FT was readily expressed in E. coli and purified with a high expression level (30 mg/L culture). It was used as an efficient catalyst in an OP3E fucosylation system for synthesizing various α1–3- and/or α1–4-linked mono- and difucosylated compounds including Lewis x, a, and b antigens, sialyl Lex, and fucosylated human milk oligosaccharides such as 3-FL, LNFP III, LNDFH II, and LNDFH III. These fucosides are important probes for biological studies.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) Common Fund grants U01GM120419 and U01GM0116263 as well as NIH grant R01HD065122. Bruker Avance-800 NMR spectrometer was funded by National Science Foundation grant DBIO-722538. Y.L., H.Y., and X.C. are co-founders of Glycohub, Inc., a company focused on the development of carbohydrate-based reagents, diagnostics, and therapeutics. Glycohub, Inc. played no role in the design, execution, interpretation, or publication of this study.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental details for enzyme characterization and glycan synthesis, NMR and HRMS data, and NMR spectra.
References
- 1.Becker DJ, Lowe JB. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 2.Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Ruvoen N, Clement M, Le Pendu J. Biochimie. 2001;83:565–573. doi: 10.1016/s0300-9084(01)01321-9. [DOI] [PubMed] [Google Scholar]
- 3.Holgersson J, Breimer ME, Samuelsson BE. APMIS Supplementum. 1992;27:18–27. [PubMed] [Google Scholar]
- 4.Inagaki H, Sakamoto J, Nakazato H, Bishop AE, Yura J. J Surg Oncol. 1990;44:208–213. doi: 10.1002/jso.2930440404. [DOI] [PubMed] [Google Scholar]
- 5.Green C. FEMS Microbiol Immunol. 1989;1:321–330. doi: 10.1111/j.1574-6968.1989.tb02417.x. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi K, Sakamoto J, Kito T, Yamamura Y, Koshikawa T, Fujita M, Watanabe T, Nakazato H. Am J Gastroenterol. 1993;88:919–924. [PubMed] [Google Scholar]
- 7.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. J Proteome Res. 2010;9:4138–4151. doi: 10.1021/pr100362f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newburg DS, Ruiz-Palacios GM, Morrow AL. Annu Rev Nutr. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 9.Fei Y, Sun YS, Li Y, Yu H, Lau K, Landry JP, Luo Z, Baumgarth N, Chen X, Zhu X. Biomolecules. 2015;5:1480–1498. doi: 10.3390/biom5031480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Annu Rev Nutr. 2000;20:699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 11.Smilowitz JT, Lebrilla CB, Mills DA, German JB, Freeman SL. Annu Rev Nutr. 2014;34:143–169. doi: 10.1146/annurev-nutr-071813-105721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X. Adv Carbohydr Chem Biochem. 2015;72:113–190. doi: 10.1016/bs.accb.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn L, Kim HY, Hsiao L, Nissan C, Kankasa C, Mwiya M, Thea DM, Aldrovandi GM, Bode L. J Nutr. 2015;145:66–72. doi: 10.3945/jn.114.199794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. Thromb Haemost. 2004;92:1402–1410. doi: 10.1160/TH04-01-0055. [DOI] [PubMed] [Google Scholar]
- 15.Bode L, Rudloff S, Kunz C, Strobel S, Klein N. J Leukoc Biol. 2004;76:820–826. doi: 10.1189/jlb.0304198. [DOI] [PubMed] [Google Scholar]
- 16.Dumon C, Samain E, Priem B. Biotechnol Prog. 2004;20:412–419. doi: 10.1021/bp0342194. [DOI] [PubMed] [Google Scholar]
- 17.Choi YH, Kim JH, Park BS, Kim BG. Biotechnol Bioeng. 2016;113:1666–1675. doi: 10.1002/bit.25944. [DOI] [PubMed] [Google Scholar]
- 18.Baisch G, Ohrlein R, Streiff M, Kolbinger F. Bioorg Med Chem Lett. 1998;8:755–758. doi: 10.1016/s0960-894x(98)00092-4. [DOI] [PubMed] [Google Scholar]
- 19.Martin SL, Edbrooke MR, Hodgman TC, van den Eijnden DH, Bird MI. J Biol Chem. 1997;272:21349–21356. doi: 10.1074/jbc.272.34.21349. [DOI] [PubMed] [Google Scholar]
- 20.Sugiarto G, Lau K, Yu H, Vuong S, Thon V, Li Y, Huang S, Chen X. Glycobiology. 2011;21:387–396. doi: 10.1093/glycob/cwq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin SW, Yuan TM, Li JR, Lin CH. Biochemistry. 2006;45:8108–8116. doi: 10.1021/bi0601297. [DOI] [PubMed] [Google Scholar]
- 22.Rasko DA, Wang G, Palcic MM, Taylor DE. J Biol Chem. 2000;275:4988–4994. doi: 10.1074/jbc.275.7.4988. [DOI] [PubMed] [Google Scholar]
- 23.Leonard R, Lhernould S, Carlue M, Fleurat P, Maftah A, Costa G. Glycoconj J. 2005;22:71–78. doi: 10.1007/s10719-005-0404-4. [DOI] [PubMed] [Google Scholar]
- 24.Rabbani S, Miksa V, Wipf B, Ernst B. Glycobiology. 2005;15:1076–1083. doi: 10.1093/glycob/cwj004. [DOI] [PubMed] [Google Scholar]
- 25.Ma B, Audette GF, Lin S, Palcic MM, Hazes B, Taylor DE. J Biol Chem. 2006;281:6385–6394. doi: 10.1074/jbc.M511320200. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Pandey RP, Kim D, Sohng JK. Biotechnol Bioproc E. 2013;18:843–849. [Google Scholar]
- 27.Campbell JA, Davies GJ, Bulone V, Henrissat B. Biochem J. 1997;326(Pt 3):929–939. doi: 10.1042/bj3260929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coutinho PM, Deleury E, Davies GJ, Henrissat B. J Mol Biol. 2003;328:307–317. doi: 10.1016/s0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- 29.Yi W, Liu X, Li Y, Li J, Xia C, Zhou G, Zhang W, Zhao W, Chen X, Wang PG. Proc Natl Acad Sci U S A. 2009;106:4207–4212. doi: 10.1073/pnas.0812432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau K, Thon V, Yu H, Ding L, Chen Y, Muthana MM, Wong D, Huang R, Chen X. Chem Commun. 2010;46:6066–6068. doi: 10.1039/c0cc01381a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Thon V, Lau K, Cai L, Chen Y, Mu S, Li Y, Wang PG, Chen X. Chem Commun. 2010;46:7507–7509. doi: 10.1039/c0cc02850a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santra A, Yu H, Tasnima N, Muthana MM, Li Y, Zeng J, Kenyon NJ, Louie AY, Chen X. Chem Sci. 2016;7:2827–2831. doi: 10.1039/c5sc04104j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Mattos LC. Rev Bras Hematol Hemoter. 2016;38:331–340. doi: 10.1016/j.bjhh.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye J, Liu X, Peng P, Yi W, Chen X, Wang F, Cao H. ACS Catal. 2016;6:8140–8144. [Google Scholar]
- 35.Yu H, Chokhawala H, Karpel R, Yu H, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. J Am Chem Soc. 2005;127:17618–17619. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]
- 36.Montreuil J. C R Hebd Seances Acad Sci. 1956;242:192–193. [PubMed] [Google Scholar]
- 37.McArthur JB, Yu H, Zeng J, Chen X. Org Biomol Chem. 2017;15:1700–1709. doi: 10.1039/c6ob02702d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H, Zeng J, Li Y, Thon V, Shi B, Chen X. Org Biomol Chem. 2016;14:8586–8597. doi: 10.1039/c6ob01706a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhn R, Gauhe A. Chem Ber-Recl. 1960;93:647–651. [Google Scholar]
- 40.Chaturvedi P, Warren CD, Altaye M, Morrow AL, Ruiz-Palacios G, Pickering LK, Newburg DS. Glycobiology. 2001;11:365–372. doi: 10.1093/glycob/11.5.365. [DOI] [PubMed] [Google Scholar]
- 41.Zhao C, Wu Y, Yu H, Shah IM, Li Y, Zeng J, Liu B, Mills DA, Chen X. Chem Commun. 2016;52:3899–3902. doi: 10.1039/c5cc10646j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.