Abstract
Objective
To examine cognitive and affective mechanisms underlying Mindfulness-Based Addiction Treatment (MBAT) versus Cognitive Behavioral Therapy (CBT) and Usual Care (UC) for smoking cessation.
Method
Participants in the parent study from which data were drawn (N = 412; 54.9% female; 48.2% African-American, 41.5% non-Latino White, 5.4% Latino, 4.9% other; 57.6% annual income < $30,000) were randomized to MBAT (n = 154), CBT (n = 155), or UC (n = 103). From quit date through 26 weeks post-quit, participants completed measures of emotions, craving, dependence, withdrawal, self-efficacy, and attentional bias. Biochemically-confirmed 7-day smoking abstinence was assessed at 4 and 26 weeks post-quit. Although the parent study did not find a significant treatment effect on abstinence, mixed-effects regression models were conducted to examine treatment effects on hypothesized mechanisms, and indirect effects of treatments on abstinence were tested.
Results
Participants receiving MBAT perceived greater volitional control over smoking and evidenced lower volatility of anger than participants in both other treatments. However, there were no other significant differences between MBAT and CBT. Compared to those receiving UC, MBAT participants reported lower anxiety, concentration difficulties, craving, and dependence, as well as higher self-efficacy for managing negative affect without smoking. Indirect effects of MBAT versus UC on abstinence occurred through each of these mechanisms.
Conclusions
Whereas several differences emerged between MBAT and UC, MBAT and CBT had similar effects on several of the psychosocial mechanisms implicated in tobacco dependence. Results help to shed light on similarities and differences between mindfulness-based and other active smoking cessation treatments.
Keywords: mindfulness, mechanisms, smoking cessation, nicotine dependence
Tobacco use is the leading preventable cause of illness and premature mortality in the U.S. (USDHHS, 2014), and quitting smoking significantly increases life expectancy (Jha et al., 2013). Although most smokers express interest in quitting, the vast majority are unsuccessful in their quit attempts (CDC, 2011). Mindfulness (defined as “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally;” Kabat-Zinn, 1994, p. 4) shows promise for improving psychological health (e.g., Gotink et al., 2015; Khoury et al., 2013) and has been incorporated into smoking cessation treatments with initial success (e.g., Brewer et al., 2011). However, the mechanisms underlying mindfulness-based interventions versus more traditional approaches for smoking cessation are unclear. The purpose of the present study was to investigate cognitive and affective mechanisms underlying mindfulness-based versus cognitive-behavioral and usual care smoking cessation treatments in a racially/ethnically diverse sample.
Mindfulness-based Treatments for Smoking Cessation
At least six trials support the use of in-person, multi-session mindfulness-based interventions for smoking cessation (Brewer et al., 2011; Davis, Fleming, Bonus, & Baker, 2007; Davis, Goldberg, et al., 2014; Davis, Manley, Goldberg, Smith, & Jorenby, 2014; Davis et al., 2013; Vidrine et al., 2016). Mindfulness-based programs have produced significantly higher abstinence rates than standard treatment (Brewer et al., 2011) and quitline-delivered treatment (Davis, Goldberg, et al., 2014). In the largest known trial of mindfulness treatment for smoking cessation, Vidrine et al. (2016) compared mindfulness-based addiction treatment (MBAT) to cognitive-behavioral therapy (CBT) and usual care (UC). MBAT did not differ significantly from CBT or UC in terms of post-treatment abstinence rates. However, mindfulness was superior in promoting lapse recovery. That is, among participants who were not abstinent at the end of treatment, those who received mindfulness-based treatment were more likely to regain abstinence at later time points (versus CBT or UC). The potential for mindfulness to promote lapse recovery is critical given that most smokers lapse early in the quit attempt (Hughes et al., 1992), and the majority of these lapses lead to full-blown relapse (Kenford et al., 1994).
Although mindfulness-based smoking cessation treatments show promise, the underlying mechanisms are yet to be well delineated. Investigating why and how treatments for addictive behaviors work is a critical question (Witkiewitz & Marlatt, 2008), and a number of researchers have called for studies to elucidate the mechanisms through which mindfulness-based treatments impact clinical outcomes (e.g., Davidson, 2016; Witkiewitz, Bowen, et al., 2014). Understanding mechanisms could lead to the development of more efficacious and cost-effective treatments. For example, interventions could have greater impact by intensifying the focus on key mechanisms and/or removing treatment aspects that do not directly target these mechanisms.
Potential Underlying Mechanisms
Emotions and Stress
Quitting smoking is associated with increased negative affect (Leventhal, Waters, Moolchan, Heishman, & Pickworth, 2010), and negative affect and stress are strong predictors of difficulty quitting (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). Positive emotions, on the other hand, may protect against relapse (Levine, Marcus, Kalarchian, Houck, & Cheng, 2010). Randomized controlled trials indicate that mindfulness training reduces negative emotions and stress (across healthy and clinical populations; Gotink et al., 2015), and increases positive emotions (among adults in partial remission from depression; Garland, Geschwind, Peeters, & Wichers, 2015) to a greater extent than wait-list controls or treatment as usual. Goyal et al.’s (2014) meta-analysis in adult clinical populations indicated that mindfulness meditation programs reduced anxiety and depression, but did not affect positive emotions when compared to nonspecific active controls. However, there was insufficient evidence regarding the effects of mindfulness vs. more specific active controls (e.g., CBT). Although there is a relative dearth of trials comparing mindfulness to CBT, some research suggests that mindfulness training and CBT are equally efficacious for reducing depression and anxiety (Sundquist et al., 2015; Tovote et al., 2014).
Affective Volatility
In addition to severity of negative affect, greater volatility (i.e., lability/scatter over time) of negative affect over the course of smoking cessation predicts lower likelihood of abstinence (Piasecki, Jorenby, Smith, Fiore, & Baker, 2003a, 2003b). Conceptually, mindfulness is integrally related to affective volatility, particularly with regard to negative emotions. That is, nonjudgmental observation of unpleasant internal and external stimuli is thought to lessen the tendency for extreme mood fluctuations in reaction to those stimuli (Chambers, Gullone, & Allen, 2009; Teasdale et al., 2002). Hill and Updegraff (2012) found that among college students, dispositional mindfulness predicted lower volatility of both negative and positive emotions. Adams et al. (2014) found that dispositional mindfulness predicted lower volatility of negative emotions and depressive symptoms among smokers attempting to quit, indicating higher stability in negative (but not positive) emotions. No study, to our knowledge, has examined the effect of mindfulness-based treatment (or CBT) on affective volatility.
Tobacco Dependence, Withdrawal, and Craving
Greater tobacco dependence and withdrawal symptoms predict more difficulty quitting (Kenford et al., 2002; Piasecki et al., 2003a). However, smokers with greater dispositional mindfulness tend to have lower levels of dependence (Vidrine et al., 2009). By promoting nonjudgmental awareness and purposeful (rather than impulsive) action, mindfulness training might reduce the likelihood of smoking as an automatic reaction to internal and external triggers (Brewer, Elwafi, & Davis, 2013). Given that automatic processes are thought to play a critical role in addictive behavior (Tiffany, 1990), mindfulness could be a useful strategy for reducing dependence. Mindfulness training might also help to attenuate craving (Davis, Manley, et al., 2014; Ruscio, Muench, Brede, & Waters, 2015) and lessen the negative emotional experience of withdrawal. Although the differential effects of mindfulness vs. CBT are unclear, CBT might also reduce tobacco dependence (Raja et al., 2014).
Agency
In terms of quitting smoking, a sense of “agency” can include self-efficacy for refraining from smoking in high-risk situations as well as expectations about one’s ability to regulate emotions without smoking (Vidrine et al., 2009). Smokers with greater agency are more likely to successfully quit (Businelle et al., 2010). By increasing awareness of present-moment experience, mindfulness interventions may help smokers to broaden their perceived array of possible coping strategies and resources, which could increase agency. Indeed, Vidrine et al. (2009) found that smokers with greater dispositional mindfulness indicated greater self-efficacy for abstaining from smoking and stronger expectations that they could regulate their emotions without smoking. Self-efficacy may also be a key mechanism through which CBT influences smoking cessation (Hendricks, Delucchi, & Hall, 2010).
Attentional Bias
Among smokers, abstinence increases attentional bias toward smoking-related cues (Leventhal et al., 2010), and attentional bias is associated with higher risk for early lapses to smoking (Waters et al., 2003). Greater ability to focus and redirect attention is hypothesized to be a key mechanism of mindfulness interventions (and has even been termed “attentional control training” in some early work; Teasdale, Segal, & Williams, 1995). Mindfulness meditation involves continual redirecting of attention to present-moment experience. In daily life, mindfulness practice can involve noticing when one’s attention is captured by problematic stimuli (e.g., smoking triggers) and disengaging from these cues. Through this practice over time, smokers might experience greater purposeful control over their attention and find that their attention is less automatically captured by smoking-related cues. Although no known research has examined the effect of mindfulness training on attentional bias specifically toward cigarettes, Davis, Goldberg, et al. (2014) found that a mindfulness-based smoking cessation intervention led to greater self-reported attentional control.
Current Study
Data were collected as part of a randomized controlled trial (Vidrine et al., 2016) comparing the efficacy of MBAT to CBT and UC for smoking cessation. In this parent trial, 7-day abstinence rates at 4 weeks post-quit were 34.4% in MBAT, 32.3% in CBT, and 24.3% in UC. At 26 weeks post-quit, rates were 13.0% in MBAT, 15.5% in CBT, and 11.7% in UC. There were no significant differences between treatment groups on overall abstinence rates over time. However, among participants who were smoking at the end of treatment, those in MBAT were more likely to recover abstinence by the following week (26.8%) and 26 weeks post-quit (7.1%) compared to participants in either CBT (7.0% and 3.5% by the following week and 26 weeks, respectively) or UC (13.2% and 0%). Despite the lack of significant effects on overall abstinence, it is possible that the treatments operate via different mechanisms. Mediation can occur in the absence of an overall effect of treatment on the outcome (Mackinnon & Fairchild, 2009) and can provide important information about mechanisms through which interventions might influence outcomes. Thus, the current study examined mechanisms underlying MBAT vs. CBT and UC. First, we sought to examine whether the three treatments had differential effects on proposed mechanisms. Second, we investigated indirect effects of treatments through hypothesized mechanisms.
We hypothesized that both MBAT and CBT would lead to greater improvements in most mechanisms compared to UC. In terms of differential effects of MBAT vs. CBT, we had three specific predictions. First, given that mindful observation of unpleasant experiences is thought to attenuate emotional reactivity, we expected that MBAT would more selectively impact affective volatility. Second, because mindfulness practice involves conscious, purposeful action (rather than “auto-pilot”), we hypothesized that MBAT would be more likely to reduce certain aspects of dependence (i.e., automaticity, sense of loss of control). Third, because a core component of mindfulness is attentional control training, we hypothesized that MBAT would reduce attentional bias relative to CBT. Given past research suggesting that both mindfulness and CBT might improve various mechanisms (e.g., emotions, agency), we did not have specific hypotheses about how MBAT vs. CBT might differentially influence other variables.
Method
Participants
Participants for the parent study were recruited in the greater Houston, Texas area using print media. Eligible participants were at least 18 years old, currently smoked cigarettes (at least 5 cigarettes/day for the past year), were motivated to quit smoking in the next month, had a viable home address and phone number, were able to read and write in English, produced an expired carbon monoxide (CO) level of ≥ 8 parts per million (ppm), and provided collateral contact information. Exclusion criteria were: contraindication for the nicotine patch, regular use of tobacco products other than cigarettes, use of bupropion or nicotine replacement other than the patches provided in the study, pregnancy or lactation, another household member enrolled in the study, active substance dependence, current psychiatric disorder or currently used psychotropic medications, or having received smoking cessation treatment in the previous three months. The study was approved by the institutional review board, and all participants provided written informed consent.
Procedures
Participants were randomized to MBAT (n = 154), CBT (n = 155), or UC (n = 103) using adaptive minimization. See Vidrine et al. (2016) for more details on participant flow through the study and session-by-session treatment outlines. The three treatments included some common elements. All participants were given self-help materials based on the Treating Tobacco Use and Dependence Clinical Practice Guideline (Fiore et al., 2008), psychoeducation about tobacco dependence/lapse/relapse, and nicotine patch therapy. In addition, all treatments incorporated cognitive-behavioral strategies, but only MBAT specifically taught mindfulness.
Mindfulness-Based Addiction Therapy (MBAT)
The MBAT manual (Wetter et al., 2009) created for this trial closely follows the content of Mindfulness-Based Cognitive Therapy (MBCT; Segal, Williams, & Teasdale, 2002), but replaces depression-related material with material pertinent to smoking cessation. The three primary aims of MBAT (based on MBCT) are to: 1) increase attention to present-moment experience (e.g., thoughts, feelings, physical sensations); 2) encourage nonjudgmental awareness of mental events (i.e., noticing thoughts as “just thoughts” without becoming caught up in their content); and 3) foster the ability to acknowledge difficult sensations (e.g., cravings, maladaptive thoughts), refocus attention on the present moment, and purposefully choose how to respond (rather than impulsively react). MBAT sessions involved 30-45 minutes of formal mindfulness practice per session (e.g., sitting meditation, yoga), as well as discussion. Participants were encouraged to practice mindfulness formally (e.g., body scan, sitting meditation) six days per week in addition to informal practice (e.g., mindfulness of routine activities) several times each day. MBAT was delivered in eight two-hour in-person group counseling sessions, and session 5 occurred on the quit date.
Cognitive Behavioral Treatment (CBT)
CBT taught problem-solving/coping skills for smoking cessation based on relapse prevention theory (Marlatt & Gordon, 1985) and the Guideline (Fiore et al., 2008). Primary topics included: 1) planning to quit smoking (e.g., recognizing triggers); 2) learning about nicotine addiction; 3) practicing stress management techniques; 4) preparing for quit day and using the nicotine patch; 5) learning skills to cope with cravings and negative emotions; 6) enlisting social support; 7) managing nutrition and exercise; and 8) reviewing skills and planning to maintain abstinence. Like MBAT, CBT involved eight two-hour in-person group counseling sessions, and session 5 occurred on the quit date.
Usual Care (UC) Intervention
The UC intervention taught coping and problem-solving strategies based on the Guideline (Fiore et al., 2008). UC was delivered in four 5- to 10-minute individual counseling sessions, with session 3 occurring on the quit date (i.e., week 5 of the protocol as in MBAT and CBT). UC, which was less intensive in terms of both time and attention, was designed to be consistent with what smokers requesting help with cessation might receive in a healthcare setting.
Measures
(See Figure 1 for timeline of study procedures and assessments).
Figure 1.
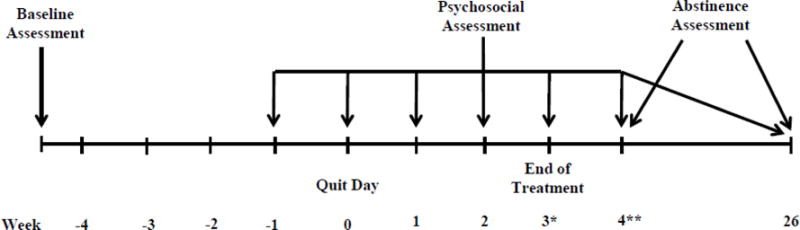
Timeline of study procedures and assessments.
Notes:
*Mechanisms for indirect effects analyses predicting Week 4 abstinence were assessed at Week 3 post-quit.
**Mechanisms for indirect effects analyses predicting Week 26 abstinence were assessed at Week 4 post-quit.
Covariates
Demographic variables (assessed at baseline) included age, gender, race/ethnicity, partner status, and education. The Heaviness of Smoking Index (HSI; Kozlowski, Porter, Orleans, Pope, & Heatherton, 1994) was administered at baseline to assess pre-treatment smoking behavior. The two HSI items are cigarettes per day and time to first cigarette after waking.
Potential Mechanisms
Emotions and stress
The 20-item Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) yields subscale scores for positive affect (PA; e.g., enthusiastic, proud) and negative affect (NA; e.g., upset, irritable). The current study utilized PANAS scores from the quit date and 1, 2, 3, 4, and 26 weeks post-quit, with Cronbach’s alphas ranging from .92–.95 for the PA and NA subscales. The Center for Epidemiological Studies - Depression (CES-D; Radloff, 1977), a 20-item measure of past-week depressive symptoms, was administered on the quit date and at 4 and 26 weeks post-quit (α = .91–.93 in the current sample). The 4-item Perceived Stress Scale - Short Form (PSS-SF; Warttig, Forshaw, South, & White, 2013) was administered on the quit date and 1, 2, 3, 4, and 26 weeks post-quit (α = .71–.81).
Dependence, withdrawal, and craving
The 68-item Wisconsin Inventory of Smoking Dependence Motives (WISDM; Piper et al., 2004) yields total and subscale scores for “primary dependence” (i.e., automaticity, craving, tolerance, loss of control) and “secondary dependence” (e.g., cognitive enhancement, positive and negative reinforcement). The WISDM was administered at 4 and 26 weeks post-quit (α = .86–.99). The Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999) is a 28-item measure yielding a total score and 7 subscale scores (i.e., anger, anxiety, concentration difficulty, craving, hunger, sadness, sleep problems). It was administered on the quit date and 1, 2, 3, 4, and 26 weeks post-quit (α = .72–.94).
Agency
Two components of agency, self-efficacy and affect regulation expectancies (Vidrine et al., 2009), were assessed. The 9-item version of the Self-Efficacy Scale (SES; Velicer, Diclemente, Rossi, & Prochaska, 1990), administered on the quit date and 1, 2, 3, 4, and 26 weeks post-quit, assesses self-efficacy for avoiding smoking in positive affect/social, negative affect, and habitual/craving situations (α = 80–.97). The Affective Information Processing Questionnaire (AIPQ: Wetter, Brandon, & Baker, 1992) assessed smokers’ expectations that they could regulate their emotions by smoking and by other means in response to 10 vignettes. The AIPQ was administered on the quit date and 4 and 26 weeks post-quit (α = .93–.97).
Subjective bias toward cigarettes
The Subjective Bias Questionnaire (SBQ) assesses attentional bias toward cigarettes. Three items, drawn from Leventhal et al. (2007), ask participants to rate the extent to which their attention has been drawn to cigarettes, other people smoking, and cigarette smoke on a 1 (not at all) to 5 (extreme amount) Likert scale (α = .83–.86).
Outcome Variable
Abstinence
Seven-day point prevalence abstinence from smoking was assessed at 4 and 26 weeks post-quit and biochemically confirmed with CO level < 6 ppm (Vidrine et al., 2016).
Analyses
First, mixed-effects regression models were conducted using SAS PROC MIXED to examine effects of treatments on hypothesized mechanisms from the quit date through 26 weeks post-quit (6 time points total). Models specified an unstructured covariance matrix for the vector of random intercept and slope of time for each participant. Group by time interactions were not significant and were not retained in final models. Variables tested were positive and negative affect (PANAS), depressive symptoms (CES-D), stress (PSS-SF), withdrawal symptoms (WSWS), smoking dependence motives (WISDM), craving (WSWS, WISDM), self-efficacy (SES), affect regulation expectancies (AIPQ), and subjective bias toward cigarettes (SBQ). Analyses controlled for demographics, baseline smoking, and abstinence at each time point.
Second, volatility indices were created for key affective variables expected to fluctuate over the course of treatment. These variables were positive and negative affect (PANAS), perceived stress (PSS-SF), and WSWS subscales for anger, anxiety, sadness, and craving. The time points selected were those that all groups attended, beginning one week prior to the quit date (week 1 pre-quit, quit day, and weeks 3, 4, and 26 post-quit). Volatility indices were calculated using the mean square successive difference (MSSD) approach (Jahng, Wood, & Trull, 2008), which captures both affective variability and temporal instability. Given the unequal time intervals, an adjustment in the calculation of the successive difference (lambda = 0.25) was used (Jahng et al., 2008). Analyses of covariance (ANCOVAs) were conducted to examine group effects on volatility surrounding the quit date and through follow-up, controlling for baseline demographic variables, baseline smoking, and abstinence at each time point.
Third, indirect effects of MBAT vs. CBT vs. UC on abstinence were examined. Although treatment did not directly impact overall abstinence rates, indirect effects analyses can provide important information about underlying mechanisms (e.g., treatments could achieve the same outcomes via different mechanisms). Further, there may be less statistical power to detect overall effects than to detect other links within the mediation chain (Mackinnon & Fairchild, 2009). Potential mechanisms that were identified in the first phase of analyses as significantly differing between treatments were tested, controlling for baseline demographics, number of cigarettes per day, and time to first cigarette. In predicting abstinence at 4 weeks post-quit, mechanisms were assessed at 3 weeks post-quit. In predicting abstinence at 26 weeks post-quit, mechanisms were assessed at 4 weeks post-quit. Because the WISDM was only administered at 4 and 26 weeks post quit, week 4 WISDM scores were examined as mechanisms predicting week 26 abstinence, but WISDM scores were not used as mediators predicting 4-week abstinence.
For indirect effects analyses, sensitivity analyses were conducted to examine the robustness of effects under different assumptions regarding missing data. Although a common practice in smoking cessation research is to assume that participants with missing data are smoking, this “missing = smoking” approach can lead to biased estimates (Blankers et al., 2016; Hedeker, Mermelstein, & Demirtas, 2007). The statistical analyses using the available data are based on full-likelihood estimation, and hence are valid under the missing at random assumption (Laird, 1988). In order to study the sensitivity of results to the missing at random assumption, we performed additional sensitivity analyses based on the multiple-model multiple imputation approach outlined by Siddique, Harel, Crespi, and Hedeker (2014). The abstinence outcomes at weeks 4 and 26 were imputed from a number of assumptions varying from missing at random (k = 1) to missing = smoking (k = .01). The mediator variables were imputed from a conditional multivariate normal distribution given the imputed abstinence outcomes. This two-step approach was repeated five times to generate five imputed data sets. The final sensitivity analyses combined the results from the five imputed data sets using the multiple imputation formula.
Among participants who lapsed early in treatment in the parent trial, those who received MBAT were more likely to recover abstinence than those who received CBT or UC (Vidrine et al., 2016). Thus, we planned to conduct indirect effects analyses specifically among early lapsers. However, these analyses were not conducted because of the small sample size and highly unbalanced outcome division (4.2% abstinent at 26 weeks [n = 4], 65.3% smoking [n = 62], and 30.5% missing data [n = 29]).
Results
Participants
Participants were 412 smokers (48.2% African-American, 41.5% non-Latino White, 5.4% Latino, 4.9% other). Over half (54.9%) were female, most (57.6%) reported a total annual household income of less than $30,000, and one third had ≤ high school education/GED. Participants smoked an average of 19.9 (SD = 10.1) cigarettes per day.
Effects of Treatments on Hypothesized Mechanisms over Time
Compared to those receiving CBT, MBAT participants scored lower on WISDM – Loss of Control (β = .21, p = .046). The effects of MBAT vs. CBT on WISDM – Primary Dependence (β = .19, p = .059), WISDM – Craving (β = .19, p = .066), and WISDM – Tolerance (β = .19, p = .059) each trended as expected, with MBAT participants indicating less primary dependence, craving, and tolerance than those receiving CBT. In addition, the effect of MBAT vs. CBT on subjective bias trended as expected, β = .16, p = .059, with participants in MBAT reporting less attentional bias toward cigarettes than those receiving CBT (See Table 1).
Table 1.
Differential Effects of MBAT, CBT, and UC on hypothesized mechanisms over time.
| MBAT vs. CBT (MBAT coded as 1, CBT as 0) | MBAT vs. UC (MBAT coded as 1, UC as 0) | CBT vs. UC (CBT coded as 1, UC as 0) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p |
|
| ||||||
| Emotions | ||||||
| Positive Affect (PANAS PA) | −.09 (−.29, .10) | .337 | .09 (−.13, .30) | .444 | .18 (−.04, .40) | .109 |
| Negative Affect (PANAS NA) | .08 (−.11, .27) | .403 | −.19 (−.40, .03) | .086 | −.27 (−.48, −.05) | .015 |
| Depressive Symptoms (CES−D) | .03 (−.16, .22) | .762 | −.18 (−.40, .03) | .099 | −.21 (−.43, .005) | .056 |
|
| ||||||
| Perceived Stress (PSS−SF) | .09 (−.10, .28) | .362 | −.16 (−.38, .05) | .141 | −.25 (−.47, −.03) | .024 |
|
| ||||||
| Withdrawal and Craving (WSWS) | ||||||
| Anger | .02 (−.15, .20) | .779 | −.22 (.42, .02) | .031 | −.24 (−.45, −.04) | .017 |
| Anxiety | −.06 (−.23, .12) | .526 | −.28 (.48, .07) | .007 | −.25 (−.43, −.07) | .008 |
| Sadness | −.003 (−.18, .18) | .973 | −.23 (.44, .02) | .032 | −.23 (−.44, −.02) | .035 |
| Concentration Difficulties | .003 (−.18, .19) | .976 | −.26 (.48, .05) | .014 | −.27 (−.48, −.06) | .013 |
| Craving | −.09 (−.26, .08) | .294 | −.24 (.44, .05) | .016 | −.15 (−.35, .05) | .138 |
| Hunger | .05 (−.14, .23) | .636 | .002 (−.21, .22) | .986 | −.04 (−.26, .17) | .692 |
| Sleep | −.12 (−.30, .07) | .227 | −.009 (−.22, .21) | .934 | .11 (−.11, .32) | .331 |
|
| ||||||
| Smoking Dependence Motives (WISDM) | ||||||
| Total | −.14 (−.34, .06) | .172 | −.46 (−.69, −.23) | .0001 | −.32 (−.55, −.08) | .008 |
| Primary | −.19 (−.39, .007) | .059 | −.43 (−.66, −.21) | .0002 | −.24 (−.47, −.01) | .039 |
| Secondary | −.11 (−.31, .10) | .307 | −.46 (−.69, −.22) | .0001 | −.35 (−.59, −.12) | .004 |
| Affiliative Attachment | −.13 (−.34, .08) | .237 | −.36 (−.60, −.12) | .004 | −.23 (−.47, .01) | .060 |
| Automaticity | −.12 (−.32, .08) | .234 | −.27 (−.50, −.04) | .021 | −.15 (−.38, .08) | .203 |
| Loss of Control | −.21 (−.41, −.004) | .046 | −.43 (−.66, −.20) | .0003 | −.23 (−.46, .004) | .053 |
| Behavioral Choice – Melioration | −.14 (−.34, .07) | .198 | −.45 (−.68, −.21) | .0002 | −.31 (−.55, −.08) | .010 |
| Cognitive Enhancement | −.11 (−.32, .09) | .284 | −.41 (−.64, −.17) | .0007 | −.29 (−.53, −.06) | .015 |
| Craving | −.19 (−.39, .01) | .066 | −.44 (−.68, −.21) | .0002 | −.25 (−.49, −.02) | .032 |
| Cue Exposure – Associative Processes | −.15 (−.35, .06) | .159 | −.43 (−.67, −.20) | .0003 | −.28 (−.52, −.05) | .018 |
| Negative Reinforcement | −.09 (−.29, .11) | .391 | −.45 (−.68, −.22) | .0002 | −.36 (−.59, −.13) | .003 |
| Positive Reinforcement | −.05 (−.26, .15) | .629 | −.49 (−.73, −.26) | <.0001 | −.44 (−.68, −.21) | .0002 |
| Social−Environmental Goads | .04 (−.17, .25) | .691 | −.07 (−.30, .17) | .585 | −.11 (−.35, .13) | .373 |
|
| ||||||
| Smoking Dependence Motives (WISDM), continued | ||||||
| Taste-Sensory | −.14 (−.34, .07) | .198 | −.46 (−.69, −.22) | .0001 | −.32 (−.56, −.09) | .007 |
| Tolerance | −.19 (−.39, .007) | .059 | −.44 (−.66, −.21) | .0002 | −.24 (−.47, −.01) | .038 |
| Weight Control | −.05 (−.26, .17) | .681 | −.29 (−.54, −.04) | .022 | −.25 (−.50, .004) | .054 |
|
| ||||||
| Self-efficacy (SES) | ||||||
| Total | −.02 (−.19, .16) | .864 | .16 (−.05, .36) | .128 | .17 (−.03, .38) | .096 |
| Positive Affect/Social Situations | −.05 (−.23, .13) | .594 | .10 (−.11, .31) | .336 | .15 (−.06, .36) | .155 |
| Negative Affect Situations | −.008 (−.18, .17) | .926 | .20 (.004, .40) | .045 | .21 (.01, .41) | .038 |
| Habitual/Craving Situations | .007 (−.17, .18) | .935 | .14 (−.06, .33) | .174 | .13 (−.07, .33) | .201 |
|
| ||||||
| Affect Regulation Expectancies (AIPQ) | ||||||
| By Not Smoking | −.08 (−.26, .11) | .421 | .11 (−.10, .31) | .310 | .18 (−.03, .39) | .085 |
| By Smoking | .06 (−.12, .24) | .514 | −.17 (−.38, .03) | .090 | −.23 (−.44, −.03) | .023 |
|
| ||||||
| Subjective Bias toward Cigarettes (SBQ) | −.16 (−.33, .006) | .059 | −.23 (−.42, −.04) | .016 | −.07 (−.27, .12) | .448 |
Notes.
Standardized parameter estimates are shown (i.e., continuous variables were rescaled for analysis). Hypothesized mechanisms were measured from the quit date through 26 weeks post-quit.
MBAT – Mindfulness-Based Addiction Treatment; CBT = Cognitive Behavioral Therapy; UC = Usual Care.
PANAS PA = Positive Affect subscale of the Positive and Negative Affect Schedule; PANAS NA = Negative Affect subscale of the Positive and Negative Affect Schedule; CES-D = Center for Epidemiological Studies – Depression; PSS-SF = Perceived Stress Scale - Short Form; WSWS = Wisconsin Smoking Withdrawal Scale; WISDM = Wisconsin Inventory of Smoking Dependence Motives; SES = Self-Efficacy Scale; AIPQ = Affective Information Processing Questionnaire; SBQ = Subjective Bias Questionnaire.
All models controlled for demographics, baseline smoking (cigarettes per day and time to first cigarette), and abstinence at each time point. Results shown in bold represent significant effects based on alpha = .05.
Compared to participants receiving UC, participants receiving MBAT reported lower levels of anger, anxiety, sadness, craving, concentration difficulties, craving, dependence motives (all subscales of the WISDM except social-environmental goads), subjective bias toward cigarettes, and higher self-efficacy for avoiding smoking when experiencing negative affect (ps < .05; see Table 1). Compared to UC participants, those who received CBT reported lower negative affect, stress, anger, anxiety, sadness, concentration difficulties, dependence motives (most subscales), expectations of regulating affect by smoking, and higher self-efficacy for avoiding smoking when experiencing negative affect (ps < .05; see Table 1).
The ANCOVA predicting volatility of anger from treatment group was significant (see Table 2). Post-hoc tests indicated that participants receiving MBAT evidenced lower volatility of anger than participants in CBT (p = .015) and UC (p = .005). The ANCOVA predicting volatility of craving trended as expected (p = .056), with MBAT participants evidencing the lowest volatility. However, MBAT did not differ significantly from CBT (p = .349) or UC (p = .109).
Table 2.
Effects of Treatment Group on Volatility of Emotions and Craving.
| Adjusted for demographics and baseline smoking | Adjusted for demographics, baseline smoking, and abstinence | Estimated Marginal Means* (Standard Error) | |||||
|---|---|---|---|---|---|---|---|
| Variable | F | p | F | p | UC | CBT | MBAT |
| Positive Affect (PANAS PA) | .45 | .638 | .81 | .445 | 45.95 (10.22) |
34.44 (7.83) |
47.98 (7.75) |
| Negative Affect (PANAS NA) | .92 | .400 | 1.24 | .293 | 65.13 (13.15) |
38.93 (10.07) |
50.04 (9.97) |
| Perceived Stress (PSS-SF) | .95 | .949 | .65 | .523 | 7.22 (1.19) |
5.67 (.91) |
5.67 (.90) |
| Anger (WSWS) | 5.87 | .003 | 6.61 | .002 |
.98a (.12) |
.85a (.09) |
.48b (.09) |
| Anxiety (WSWS) | .44 | .647 | .80 | .451 | .69 (.10) |
.58 (.07) |
.54 (.07) |
| Sadness (WSWS) | 2.97 | .053 | .68 | .509 | .52 (.08) |
.43 (.06) |
.40 (.06) |
| Craving (WSWS) | 2.33 | .099 | 2.94 | .056 | 1.15 (.18) |
.97 (.14) |
.64 (.13) |
Notes.
MBAT – Mindfulness-Based Addiction Treatment; CBT = Cognitive Behavioral Therapy; UC = Usual Care.
PANAS PA = Positive Affect subscale of the Positive and Negative Affect Schedule; PANAS NA = Negative Affect subscale of the Positive and Negative Affect Schedule; PSS-SF = Perceived Stress Scale - Short Form; WSWS = Wisconsin Smoking Withdrawal Scale.
Estimated marginal means are based on models controlling for demographics, baseline smoking (cigarettes per day and time to first cigarette), and abstinence at each time point. Significant differences are based on Tukey HSD. Results shown in bold represent significant effects based on alpha = .05.
Indirect Effects of Treatment on Abstinence through Hypothesized Mechanisms
The indirect effect of MBAT vs. CBT through WISDM – Loss of Control was not significant under any of the missing data assumptions (for k = 1, βind. effect = .02 [−.03, .11]).
Indirect effects of MBAT vs. UC through hypothesized mechanisms are shown in Table 3. In predicting abstinence at 4 weeks post-quit, indirect effects were significant for WSWS Anxiety and Self-efficacy-Negative Affect. Compared to UC, MBAT predicted lower anxiety and higher self-efficacy for managing negative affect, which predicted higher odds of abstinence at week 4. Results were consistent across all three missing data assumptions. In predicting week 26 abstinence, indirect effects for MBAT vs. UC were significant for WSWS Concentration, WSWS Craving, and WISDM Total, Secondary Dependence Motives, Cognitive Enhancement, Craving, Cue Exposure – Associative Processes, Negative Reinforcement, Positive Reinforcement, and Taste-Sensory. Compared to UC, MBAT predicted lower concentration difficulties, craving, and dependence, which predicted higher odds of abstinence at 26 weeks post-quit. The above results were consistent across all three missing data mechanisms.
Table 3.
Indirect Effects of MBAT vs. UC in Predicting Week 4 and 26 Abstinence.
| Dependent Variable: Week 4 Abstinence
| |||||||
|---|---|---|---|---|---|---|---|
| Variable | a path | b path | Direct Effect (95% CI) | Indirect Effect (95% CI) | |||
|
| |||||||
| Withdrawal (WSWS) | |||||||
| Anxiety | −.29 | −.26 | .25 (−.33, .83) | .08 (.001, .21) | |||
| Sadness | −.21 | −.35 | .26 (−.29, .82) | .08 (−.004, .22) | |||
| Concentration Difficulties | −.18 | .13 | .36 (−.19, .93) | −.02 (−.09, .03) | |||
| Craving | −.23 | −.70 | .19 (−.39, .76) | .16 (−.01, .39) | |||
|
| |||||||
| Self-efficacy for Negative Affect Situations (SES) | .28 | .50 | .22 (−.32, .79) | .14 (.03, .31) | |||
|
| |||||||
| Subjective Bias (SBQ) | −.20 | −.53 | .26 (−.30, .86) | .11 (−.01, .28) | |||
|
| |||||||
| Dependent Variable: Week 26 Abstinence | |||||||
|
| |||||||
| Variable | a path | b path | Direct Effect (95% CI) | Indirect Effect (95% CI) | |||
|
| |||||||
| Withdrawal and Craving (WSWS) | |||||||
| Anxiety | −.25 | −.07 | −.14 (−.77, .49) | .02 (−.06, .12) | |||
| Sadness | −.19 | −.36 | −.20 (−.86, .43) | .07 (−.02, .24) | |||
| Concentration Difficulties | −.28 | −.36 | −.22 (−.87, .41) | .10 (.002, .28) | |||
| Craving | −.31 | −.50 | −.28 (−.93, .35) | .16 (.03, .38) | |||
|
| |||||||
| Smoking Dependence Motives (WISDM) | |||||||
| Total | −.42 | −.37 | −.28 (−.92, .33) | .16 (.02, .38) | |||
| Primary | −.39 | −.31 | −.24 (−.87, .37) | .12 (−.008, .36) | |||
| Secondary | −.41 | −.39 | −.28 (−.92, .33) | .16 (.03, .37) | |||
| Affiliative Attachment | −.33 | −.16 | −.17 (−.79, .44) | .05 (−.06, .21) | |||
| Automaticity | −.22 | −.24 | −.16 (−.78, .44) | .06 (−.02, .20) | |||
| Loss of Control | −.40 | −.16 | −.19 (−.81, .43) | .07 (−.06, .26) | |||
| Behavioral Choice - Melioration | −.42 | −.18 | −.20 (−.80, .42) | .08 (−.04, .25) | |||
| Cognitive Enhancement | −.41 | −.40 | −.28 (−.90, .33) | .16 (.03, .38) | |||
| Craving | −.37 | −.51 | −.30 (−.93, .31) | .19 (.04, .45) | |||
| Cue Exposure – Associative Processes | −.36 | −.45 | −.30 (−.94, .31) | .17 (.04, .38) | |||
| Negative Reinforcement | −.45 | −.39 | −.29 (−.94, .31) | .18 (.05, .37) | |||
| Positive Reinforcement | −.43 | −.34 | −.27 (−.90, .34) | .15 (.03, .33) | |||
| Taste-Sensory | −.34 | −.42 | −.25 (−.87, .37) | .15 (.03, .34) | |||
| Tolerance | −.43 | −.22 | −.21 (−.85, .41) | .10 (−.05, .31) | |||
| Weight Control | −.22 | −.02 | −.13 (−.76, .49) | .01 (−.07, .12) | |||
|
| |||||||
| Self-efficacy for Negative Affect Situations (SES) | .29 | .21 | −.18 (−.84, .46) | .06 (−.03, .22) | |||
|
| |||||||
| Subjective Bias (SBQ) | −.21 | −.62 | −.23 (−.87, .40) | .13 (−.01, .36) | |||
Notes. Standardized estimates are shown (i.e., using rescaled mediators). “a path” = effect of treatment on mediator; “b path” = effect of mediator on abstinence, controlling for treatment; “indirect effect” = indirect effect of treatment on abstinence through mediator; “direct effect” = effect of treatment on abstinence, controlling for mediator. Mindfulness-Based Addiction Treatment (MBAT) coded as 1, Usual Care (UC) as 0. Models controlled for demographics and baseline smoking. Variables in bold represent significant indirect effects based on alpha = .05.
For indirect effects of CBT vs. UC on week 4 abstinence, WSWS Anxiety was the only significant mechanism across all three missing data assumptions (under k = 1, βind. effect = .15 [.03, .31]). Compared to UC, CBT predicted lower anxiety, which predicted higher odds of abstinence at 4 weeks post-quit. In predicting week 26 abstinence, indirect effects for CBT vs. UC were significant across all three missing data mechanisms for PSS-SF Perceived Stress (βind. effect = .09 [.01, .24]), WSWS Concentration (βind. effect = .09 [.01, .24]), and Self-Efficacy-Negative Affect (βind. effect = .11 [.01, .28]). Compared to UC, CBT predicted lower stress and concentration difficulties, and higher self-efficacy for managing negative affect without smoking, which predicted higher odds of abstinence at 26 weeks post-quit. See online supplemental table for estimates of all of the component paths for the CBT vs. UC indirect effects analysis.
Discussion
Although mindfulness-based treatments show promise for smoking cessation, there are few data addressing the differential effects of mindfulness versus other active treatments on specific hypothesized mechanisms. In the parent trial (Vidrine et al., 2016), there was no effect of treatment type on overall abstinence rates, but MBAT did significantly increase lapse recovery rates relative to CBT and UC. The current study examined potential cognitive and affective mechanisms underlying MBAT versus CBT and UC for smoking cessation. As hypothesized, participants receiving MBAT perceived greater volitional control over smoking and evidenced lower volatility of anger than participants in both other treatments. However, there were no other significant differences between MBAT and CBT, nor were there significant indirect effects of MBAT vs. CBT, suggesting that mindfulness and cognitive-behavioral approaches may similarly influence several of the psychosocial mechanisms implicated in tobacco dependence. Compared to those receiving UC, however, MBAT participants reported lower anxiety, attentional bias toward cigarettes, concentration difficulties, craving, and smoking dependence motives, as well as higher self-efficacy for managing negative affect. Indirect effects of MBAT versus UC occurred through lower anxiety, concentration difficulties, craving, and dependence motives, as well as greater self-efficacy for managing negative affect without smoking.
Although a number of differences emerged between MBAT and UC, this study did not reveal many differences between MBAT and CBT. MBAT and CBT were matched in intensity and included common elements (e.g., MBAT incorporated cognitive-behavioral strategies in addition to its unique focus on mindfulness), which could partially explain the overall lack of differences. The lack of differences between MBAT and CBT is consistent with Goyal et al.’s (2014) meta-analysis, which found that although mindfulness-based therapies were superior to nonspecific active controls, there was insufficient evidence for differences between mindfulness training and specific active controls (including CBT). Similarly, Khoury et al.’s (2013) meta-analysis concluded that mindfulness-based therapy was superior to some other active treatments (e.g., supportive therapy) but not CBT. Recently, Goldin et al. (2016) reported that mindfulness training and CBT were equally efficacious for treating social anxiety disorder and that there were more similarities than differences in terms of underlying mechanisms.
CBT and mindfulness-based treatments certainly have commonalities. Both promote awareness of and exposure to internal sensations, which might lessen tendencies toward automatic and avoidant responses (Baer, 2003). However, whereas CBT involves efforts to change irrational thinking, mindfulness involves non-evaluative observation of thoughts (i.e., changing the way one relates to his/her thoughts rather than directly attempting to change the content of thoughts; Baer, 2003; Teasdale et al., 2002). Some studies have revealed meaningful differences between these two approaches. For example, mindfulness-based relapse prevention predicted fewer days of substance use than cognitive-behavioral relapse prevention (Witkiewitz, Warner, et al., 2014), especially at 12-month follow-up (Bowen et al., 2014). More research is needed to examine potential differences between mindfulness and CBT for various outcomes and populations, their underlying mechanisms, and with longer follow-up periods.
Two significant differences between MBAT and CBT did emerge. First, MBAT participants perceived greater volitional control over smoking than those receiving CBT or UC. Mindfulness involves purposeful, present-focused attention, which might increase the tendency for purposeful action and help people to feel more in control of their behavior. Second, MBAT predicted lower volatility of anger than both CBT and UC. Although no known research has examined the effects of mindfulness treatment on volatility, dispositional mindfulness predicts lower volatility of negative affect (Adams et al., 2014), and mindfulness training shows promise for reducing anger (e.g., Amutio et al., 2014). Mindfulness meditation may promote “metacognitive awareness,” or “decentering,” whereby individuals learn to view thoughts and feelings as mental events rather than facts (Bishop et al., 2004; Teasdale et al., 2002). This decentered perspective may reduce the likelihood of automatic reactions to experiences of anger, thus preventing its escalation. By helping smokers to observe the cognitive, emotional and physiological sensations of anger without reacting or becoming “stuck” in them, mindfulness training might prevent anger from cycling out of control (and thus reduce volatility of anger). Although we were not able to examine mediators of the effect of MBAT vs. CBT on lapse recovery because of small sub-samples, greater perceived volitional control over smoking and lower affective volatility could be mechanisms explaining why MBAT was superior to both CBT and usual care for promoting lapse recovery in the parent trial. For example, among participants who lapse early in a quit attempt, those learning to practice mindfulness may perceive greater control over their smoking behavior and maintain more emotional equilibrium in the context of this slip-up, which could help them to regain abstinence. Future research with larger samples might examine mediators of the effect of MBAT vs. other active treatments specifically on lapse recovery.
A side-by-side comparison of the MBAT vs. UC and CBT vs. UC analyses further sheds light on similarities and differences between MBAT and CBT. Effect sizes for the MBAT vs. UC and CBT vs. UC comparisons are similar overall, with a few notable differences. MBAT seems to have produced greater reductions in several aspects of dependence (including automaticity), craving, and subjective bias toward cigarettes. On the other hand, CBT appears to have produced greater reductions in negative affect and stress. Similarly, whereas indirect effects of MBAT vs. UC on week 26 abstinence occurred through reduced craving and dependence, indirect effects of CBT vs. UC occurred through reduced stress and higher self-efficacy for managing negative affect without smoking. Given the relatively large array of mechanisms examined and the general lack of significant differences between MBAT and CBT, these findings should be interpreted with caution and require replication. In addition, UC was less intensive than both MBAT and CBT, involved individual rather than group counseling, and was not balanced for time/attention; differences between UC and MBAT/CBT could be partially due to dose effects.
Results revealed a number of differences between MBAT and UC. MBAT both impacted the following variables to a greater extent than did UC, and there were significant indirect effects through these mechanisms for either week 4 or week 26 abstinence: 1) anxiety, 2) concentration difficulties, 3) craving, 4) self-efficacy for managing negative emotions, and 5) smoking dependence motives. First, consistent with extant research comparing mindfulness-based treatment to nonspecific active controls (Goyal et al., 2014; Khoury et al., 2013), MBAT was associated with lower anxiety compared to UC, and MBAT produced an indirect effect on week 4 abstinence via anxiety. Second, MBAT resulted in less difficulty concentrating compared to UC, which predicted a higher likelihood of abstinence 26 weeks post-quit. By improving present-focused attention, mindfulness meditation might minimize difficulties with concentration, thus lessening withdrawal symptoms.
Third, in line with the findings of Davis, Manley et al. (2014), MBAT (vs. UC) was associated with lower craving, which predicted 26-week abstinence. “Urge surfing,” a core MBAT practice (which has also been incorporated into other mindfulness-based interventions and cognitive-behavioral relapse prevention), teaches people to bring present-focused, nonjudgmental awareness to cravings (e.g., Bowen & Marlatt, 2009). Participants are asked to imagine their cravings as waves in the ocean that naturally rise but also dissipate with time. This way of observing cravings, without smoking or fighting against them, may prevent further escalations in craving, which could support prolonged abstinence. Fourth, MBAT participants indicated greater self-efficacy for coping with negative emotions without smoking than those in UC, which predicted abstinence at 4 weeks post-quit. This is consistent with Vidrine et al.’s (2009) finding that smokers with greater dispositional mindfulness were more confident in their ability to regulate emotions without smoking, and extends this finding to mindfulness treatment.
Finally, MBAT (vs. UC) reduced a variety of aspects of tobacco dependence, which predicted greater 26-week abstinence. MBAT influenced several smoking dependence motives that are consistent with the conceptual underpinnings of mindfulness-based treatment for addictive behaviors. Mindfulness is thought to foster awareness and purposeful responding rather than impulsive, automatic reactions to cues associated with addictive behavior (Brewer et al., 2013). Thus, it makes sense that mindfulness training would reduce the following particular subscales: Automaticity (smoking without awareness or purposeful choice), Loss of Control (the feeling that cigarettes control one’s life, rather than the sense of personal control over smoking), Cue Exposure-Associative Processes (strong links between external cues and smoking behavior), and Negative Reinforcement (smoking in attempt to reduce unpleasant feelings).
This study is limited by a reliance on repeated administration of self-report questionnaires with significant time lapses between assessments. Future research could utilize ecological momentary assessment (Shiffman, Stone, & Hufford, 2008) to examine how mindfulness might modulate cognitive and affective variables on a real-time, real-world basis. In addition, it is possible that MBAT might differentially impact other mechanisms (e.g., interpersonal or physiological indicators) not measured in the current study. Furthermore, adherence to between-session formal meditation practice recommendations in this study was low (Vidrine et al., 2016), and more informal mindfulness practices (e.g., urge surfing) were not assessed. More differences between MBAT and other active treatments could emerge with greater adherence to regular mindfulness practice. In a similar vein, continued mindfulness practice over a longer time period may be necessary for unique effects of mindfulness practice to develop. Finally, because this is the first known study to examine mechanisms underlying mindfulness-based versus cognitive-behavioral and usual care treatments for smoking cessation, a relatively large number of analyses were conducted to examine multiple potential mechanisms. Given the potential for this approach to inflate Type I error, we suggest that readers examine standardized estimates in addition to p-values. In addition, abstinence rates were higher at 4-weeks post-quit than at 26-weeks post-quit, which could have limited statistical power for analyses predicting the later time point. Replication will be needed to increase confidence in the findings.
This is the first known study to directly compare mechanisms underlying the effects of mindfulness-based versus cognitive-behavioral and usual care treatments for smoking cessation. Whereas numerous differences emerged between MBAT and UC, the effects of MBAT vs. CBT on the psychosocial mechanisms implicated in tobacco dependence appear very similar. Future research on the mechanisms underlying MBAT vs. other active treatments may need to utilize a wider array of assessments and methodologies in order to uncover (or not) differential effects on specific mechanisms. Finally, even in the absence of many indirect effects of MBAT vs. another intensive active treatment on abstinence, future research might investigate whether mindfulness-based treatments are more effective for specific subgroups of individuals, whereas other treatment approaches such as CBT may be more appropriate with other groups.
Supplementary Material
Public Health Statement.
Compared to standard smoking cessation treatment, mindfulness-based interventions may produce more favorable cognitive and emotional outcomes, which could improve chances of quitting. Mindfulness and cognitive-behavioral treatments appear to have similar effects on several of the psychosocial mechanisms implicated in tobacco dependence.
Acknowledgments
Funding Statement: This work was supported by the National Institute on Drug Abuse (R01DA018875), the National Center for Complementary and Integrative Health (K23AT008442); the National Cancer Institute (P30CA016672), and the National Institute on Minority Health and Health Disparities (K99MD010468). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Adams CE, Chen M, Guo L, Lam CY, Stewart DW, Correa-Fernandez V, Wetter DW. Mindfulness predicts lower affective volatility among African Americans during smoking cessation. Psychology of Addictive Behaviors. 2014;28(2):580–585. doi: 10.1037/a0036512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amutio A, Franco C, Perez-Fuentes Mde C, Gazquez JJ, Mercader I. Mindfulness training for reducing anger, anxiety, and depression in fibromyalgia patients. Front Psychol. 2014;5:1572. doi: 10.3389/fpsyg.2014.01572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: A conceptual and empirical review. Clinical Psychology-Science and Practice. 2003;10(2):125–143. doi: 10.1093/clipsy/bpg015. [DOI] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Devins G. Mindfulness: A proposed operational definition. Clinical Psychology: Science and Practice. 2004;11:230–241. doi: 10.1093/clipsy/bph077. [DOI] [Google Scholar]
- Blankers M, Smit ES, van der Pol P, de Vries H, Hoving C, van Laar M. The Missing=Smoking Assumption: A Fallacy in Internet-Based Smoking Cessation Trials? Nicotine & Tobacco Research. 2016;18(1):25–33. doi: 10.1093/ntr/ntv055. [DOI] [PubMed] [Google Scholar]
- Bowen S, Marlatt GA. Surfing the urge: brief mindfulness-based intervention for college student smokers. Psychology of Addictive Behaviors. 2009;23(4):666–671. doi: 10.1037/a0017127. [DOI] [PubMed] [Google Scholar]
- Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, Larimer ME. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psychiatry. 2014;71(5):547–556. doi: 10.1001/jamapsychiatry.2013.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Elwafi HM, Davis JH. Craving to quit: psychological models and neurobiological mechanisms of mindfulness training as treatment for addictions. Psychology of Addictive Behaviors. 2013;27(2):366–379. doi: 10.1037/a0028490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, Rounsaville BJ. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug and Alcohol Dependence. 2011;119:72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businelle MS, Kendzor DE, Reitzel LR, Costello TJ, Cofta-Woerpel L, Li Y, Wetter DW. Mechanisms linking socioeconomic status to smoking cessation: a structural equation modeling approach. Health Psychology. 2010;29(3):262–273. doi: 10.1037/a0019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Quitting smoking among adults–United States, 2001–2010. Morbidity and Mortality Weekly Reports. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- Chambers R, Gullone E, Allen NB. Mindful emotion regulation: An integrative review. Clinical Psychology Review. 2009;29(6):560–572. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Mindfulness-Based Cognitive Therapy and the Prevention of Depressive Relapse: Measures, Mechanisms, and Mediators. JAMA Psychiatry. 2016;73(6):547–548. doi: 10.1001/jamapsychiatry.2016.0135. [DOI] [PubMed] [Google Scholar]
- Davis JM, Fleming MF, Bonus KA, Baker TB. A pilot study on mindfulness based stress reduction for smokers. BMC Complementary and Alternative Medicine. 2007;7:2. doi: 10.1186/1472-6882-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Goldberg SB, Anderson MC, Manley AR, Smith SS, Baker TB. Randomized trial on mindfulness training for smokers targeted to a disadvantaged population. Substance Use and Misuse. 2014;49(5):571–585. doi: 10.3109/10826084.2013.770025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Manley AR, Goldberg SB, Smith SS, Jorenby DE. Randomized trial comparing mindfulness training for smokers to a matched control. Journal of Substance Abuse Treatment. 2014;47(3):213–221. doi: 10.1016/j.jsat.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Mills DM, Stankevitz KA, Manley AR, Majeskie MR, Smith SS. Pilot randomized trial on mindfulness training for smokers in young adult binge drinkers. BMC Complementary and Alternative Medicine. 2013;13:215. doi: 10.1186/1472-6882-13-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NJ, Curry SJ. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services (USDHHS), Public Health Service (PHS); 2008. [Google Scholar]
- Garland EL, Geschwind N, Peeters F, Wichers M. Mindfulness training promotes upward spirals of positive affect and cognition: multilevel and autoregressive latent trajectory modeling analyses. Front Psychol. 2015;6:15. doi: 10.3389/fpsyg.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Morrison A, Jazaieri H, Brozovich F, Heimberg R, Gross JJ. Group CBT Versus MBSR for Social Anxiety Disorder: A Randomized Controlled Trial. Journal of Consulting and Clinical Psychology. 2016 doi: 10.1037/ccp0000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotink RA, Chu P, Busschbach JJ, Benson H, Fricchione GL, Hunink MG. Standardised mindfulness-based interventions in healthcare: An overview of systematic reviews and meta-analyses of RCTs. PLoS One. 2015;10(4):e0124344. doi: 10.1371/journal.pone.0124344. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, Haythornthwaite JA. Meditation programs for psychological stress and well-being: A systematic review and meta-analysis. JAMA Intern Med. 2014 doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, Mermelstein RJ, Demirtas H. Analysis of binary outcomes with missing data: missing = smoking, last observation carried forward, and a little multiple imputation. Addiction. 2007;102(10):1564–1573. doi: 10.1111/j.1360-0443.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Delucchi KL, Hall SM. Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug Alcohol Depend. 2010;109(1–3):114–119. doi: 10.1016/j.drugalcdep.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CL, Updegraff JA. Mindfulness and its relationship to emotional regulation. Emotion. 2012;12(1):81–90. doi: 10.1037/a0026355. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gulliver SB, Fenwick JW, Valliere WA, Cruser K, Pepper S, Flynn BS. Smoking cessation among self-quitters. Health Psychology. 1992;11(5):331–334. doi: 10.1037//0278-6133.11.5.331. [DOI] [PubMed] [Google Scholar]
- Jahng S, Wood PK, Trull TJ. Analysis of affective instability in ecological momentary assessment: Indices using successive difference and group comparison via multilevel modeling. Psychological Methods. 2008;13(4):354–375. doi: 10.1037/a0014173. [DOI] [PubMed] [Google Scholar]
- Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. New England Journal of Medicine. 2013;368(4):341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Wherever you go, there you are: Mindfulness and meditation in everyday life. New York: Hyperion; 1994. [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter DW, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271(8):589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology. 2002;70(1):216–227. [PubMed] [Google Scholar]
- Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, Hofmann SG. Mindfulness-based therapy: A comprehensive meta-analysis. Clinical Psychology Review. 2013;33(6):763–771. doi: 10.1016/j.cpr.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug and Alcohol Dependence. 1994;34(3):211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Laird NM. Missing data in longitudinal studies. Statistics in Medicine. 1988;7(1–2):305–315. doi: 10.1002/sim.4780070131. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB. Gender differences in acute tobacco withdrawal: effects on subjective, cognitive, and physiological measures. Experimental and Clinical Psychopharmacology. 2007;15(1):21–36. doi: 10.1037/1064-1297.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addictive Behaviors. 2010;35(12):1120–1130. doi: 10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MD, Marcus MD, Kalarchian MA, Houck PR, Cheng Y. Weight concerns, mood, and postpartum smoking relapse. American Journal of Preventive Medicine. 2010;39(4):345–351. doi: 10.1016/j.amepre.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon DP, Fairchild AJ. Current Directions in Mediation Analysis. Curr Dir Psychol Sci. 2009;18(1):16. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York: Guilford Press; 1985. [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: I. Abstinence distress in lapsers and abstainers. Journal of Abnormal Psychology. 2003a;112(1):3–13. [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. Journal of Abnormal Psychology. 2003b;112(1):14–27. [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72(2):139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Raja M, Saha S, Mohd S, Narang R, Reddy LV, Kumari M. Cognitive Behavioural Therapy versus Basic Health Education for Tobacco Cessation among Tobacco Users: A Randomized Clinical Trail. J Clin Diagn Res. 2014;8(4):ZC47–49. doi: 10.7860/JCDR/2014/8015.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio AC, Muench C, Brede E, Waters AJ. Effect of Brief Mindfulness Practice on Self-Reported Affect, Craving, and Smoking: A Pilot Randomized Controlled Trial Using Ecological Momentary Assessment. Nicotine Tob Res. 2015 doi: 10.1093/ntr/ntv074. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression: A new approach to preventing relapse. New York: Guilford; 2002. [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Siddique J, Harel O, Crespi CM, Hedeker D. Binary variable multiple-model multiple imputation to address missing data mechanism uncertainty: application to a smoking cessation trial. Statistics in Medicine. 2014;33(17):3013–3028. doi: 10.1002/sim.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist J, Lilja A, Palmer K, Memon AA, Wang X, Johansson LM, Sundquist K. Mindfulness group therapy in primary care patients with depression, anxiety and stress and adjustment disorders: randomised controlled trial. Br J Psychiatry. 2015;206(2):128–135. doi: 10.1192/bjp.bp.114.150243. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Moore RG, Hayhurst H, Pope M, Williams S, Segal ZV. Metacognitive awareness and prevention of relapse in depression: empirical evidence. Journal of Consulting and Clinical Psychology. 2002;70(2):275–287. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological Review. 1990;97(2):147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tovote KA, Fleer J, Snippe E, Peeters AC, Emmelkamp PM, Sanderman R, Schroevers MJ. Individual mindfulness-based cognitive therapy and cognitive behavior therapy for treating depressive symptoms in patients with diabetes: results of a randomized controlled trial. Diabetes Care. 2014;37(9):2427–2434. doi: 10.2337/dc13-2918. [DOI] [PubMed] [Google Scholar]
- USDHHS. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. [PubMed] [Google Scholar]
- Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addictive Behaviors. 1990;15(3):271–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- Vidrine JI, Businelle MS, Cinciripini P, Li Y, Marcus MT, Waters AJ, Wetter DW. Associations of mindfulness with nicotine dependence, withdrawal, and agency. Substance Abuse. 2009;30:318–327. doi: 10.1080/08897070903252973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, Wetter DW. Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: A randomized clinical trial. J Consult Clin Psychol. 2016;84(9):824–838. doi: 10.1037/ccp0000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warttig SL, Forshaw MJ, South J, White AK. New, normative, English-sample data for the Short Form Perceived Stress Scale (PSS-4) Journal of Health Psychology. 2013;18(12):1617–1628. doi: 10.1177/1359105313508346. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology. 2003;22(4):378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Brandon TH, Baker TB. The relation of affective processing measures and smoking motivation indices among college-age smokers. Advances in Behaviour Research and Therapy. 1992;14(3):169–193. [Google Scholar]
- Wetter DW, Vidrine JI, Fine M, Rowan PJ, Reitzel LR, Tindle H. Mindfulness-Based Addiction Treatment (MBAT) Manual 2009 [Google Scholar]
- Witkiewitz K, Bowen S, Harrop EN, Douglas H, Enkema M, Sedgwick C. Mindfulness-based treatment to prevent addictive behavior relapse: theoretical models and hypothesized mechanisms of change. Subst Use Misuse. 2014;49(5):513–524. doi: 10.3109/10826084.2014.891845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Why and how do substance abuse treatments work? Investigating mediated change. Addiction. 2008;103(4):649–650. doi: 10.1111/j.1360-0443.2008.02193.x. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Warner K, Sully B, Barricks A, Stauffer C, Thompson BL, Luoma JB. Randomized trial comparing mindfulness-based relapse prevention with relapse prevention for women offenders at a residential addiction treatment center. Substand Use and Misuse. 2014;49(5):536–546. doi: 10.3109/10826084.2013.856922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


