Abstract
Early subclinical inflammation in kidney transplants is associated with later graft fibrosis and dysfunction. Regulatory T cells (Tregs) can reverse established inflammation in animal models. We conducted a pilot safety and feasibility trial of autologous Treg cell therapy in three kidney transplant recipients with subclinical inflammation noted on 6-month surveillance biopsies. Tregs were purified from peripheral blood and polyclonally expanded ex vivo using medium containing deuterated glucose to label the cells. All patients received a single infusion of ~320 × 106 (319, 321 and 363.8 × 106) expanded Tregs. Persistence of the infused Tregs was tracked. Graft inflammation was monitored with follow-up biopsies and urinary biomarkers. Nearly 1 × 109 (0.932, 0.956, 1.565 × 109) Tregs were successfully manufactured for each patient. There were no infusion reactions or serious therapy-related adverse events. The infused cells demonstrated patterns of persistence and stability similar to those observed in non-immunosuppressed subjects receiving the same dose of Tregs. Isolation and expansion of Tregs is feasible in kidney transplant patients on immunosuppression. Infusion of these cells was safe and well tolerated. Future trials will test the efficacy of polyclonal and donor alloantigen-reactive Tregs for the treatment of inflammation in kidney transplants.
INTRODUCTION
Despite advances in transplantation reducing early acute rejection, rate of long-term graft attrition remain high and are mostly attributed to chronic immune-mediated injury (1). Inflammation manifested by infiltration of mononuclear cells is noted in 11– 44% of surveillance biopsies within the first year in patients with stable graft function (2, 3). Although the overall prevalence of inflammation tends to decrease over time, it can persist after the first year in a significant proportion of patients (4). Such subclinical inflammation, even when insufficient to meet Banff criteria for acute rejection (5, 6), is thought to be the result of an incompletely suppressed alloimmune response (7) and has been associated with the progression of graft fibrosis and dysfunction in the long term (2, 3, 8, 9).
Tregs are a small subset of CD4+ T cells that depend on the FOXP3 transcription factor for their lineage differentiation and function (10). They function by preventing the initiation of unwanted immune activation and by suppressing ongoing immune responses to limit bystander tissue destruction (11). Tregs are long-lived and can function in a dominant and antigen specific manner. Human Tregs can be isolated and expanded in vitro while maintaining their immunoregulatory function. Clinical studies of Tregs have found them to be safe and possibly efficacious for graft-versus-host disease, type 1 diabetes mellitus, and liver transplantation (12–15).
Treg recruitment at the acute phase of the alloimmune response may diminish the interstitial inflammation and reverse kidney transplant rejection (16–19). Further, operationally tolerant kidney transplant recipients have been noted to have potent memory Tregs with a specific demethylation pattern of the FOXP3 Treg-specific demethylated region, which may contribute to the maintenance of graft tolerance (19, 20). Infusion of Tregs before extensive graft damage could therefore improve long-term graft outcomes (18, 21). Here we describe the results of a safety and feasibility trial of polyclonal Tregs in kidney transplant recipients with subclinical graft inflammation at 6 months. We selected this population for several reasons. They have already received maximal treatment with induction therapy and are maintained on dual or triple immunosuppression; more intense therapy has not been shown to control the inflammation (22) and can often be associated with drug-induced toxicities. Therefore, they are excellent candidates for immunomodulatory therapy that can alter the balance of the immune system towards a more regulatory phenotype and lead to better long-term function. Although it would be easier to detect an effect of therapy if patients had more intense inflammation at baseline, we chose not to enroll patients with Banff type ≥1A rejection due to the need for concomitant steroid therapy. Withholding or delaying steroid therapy due to the Treg manufacturing constraints could be considered unethical. Furthermore, steroid therapy could confound the interpretation of the follow up biopsies and of adverse events. The choice of introducing Tregs at 6 months offers multiple advantages over therapy at the time of surgery or soon after transplantation. By delaying the administration of Tregs, we avoid the effect of induction therapy (especially the clearly detrimental effect of the anti-IL-2 receptor antibodies) and the high exposure of calcineurin inhibitors that is required early post-transplant. Also, by enrolling clinically stable patients at a time point when no other interventions or manipulations are being performed routinely, we are better equipped to discern the effects of Treg therapy on the follow up biopsies.
MATERIALS AND METHODS
Study Design
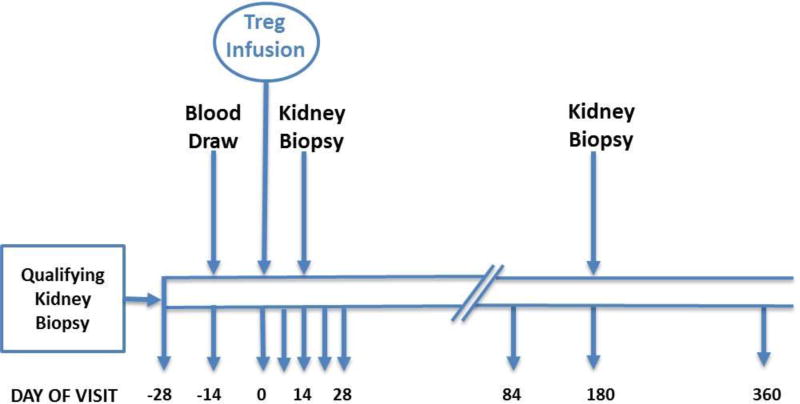
In this phase 1, single-center, open-label pilot study conducted at the University of California, San Francisco, kidney transplant recipients with inflammation on their 6-month post-transplant surveillance biopsy received a single infusion of 320 × 106 (allowable range 224–384 × 106) ex vivo–expanded autologous CD4+ CD127lo/−CD25+ polyclonal Tregs without any changein their immunosuppressive regimen at enrollment. Follow-up biopsies were performed at 2 weeks and 6 months post-infusion. Figure 1 depicts the schedule of events. This study was approved by the institutional review board at UCSF and conducted under IND 15711. It is registered with ClinicalTrials.gov (NCT02088931).
Figure 1. Study design and subject schedule of events.
Blood for Treg manufacturing was drawn at day −14, and Treg infusion was given on day 0. Subjects were seen for follow-up assessments on day 4, 7, 14 and 28, and at months 3, 6 and 12. Laboratory assessments were done weekly after the first week, and monthly after the first month.
Patients
This study enrolled recipients of primary renal transplants 18–65 years old with stable renal function (estimated glomerular filtration rate or eGFR ≥40 ml/min, proteinuria <500 mg/day) who were on maintenance immunosuppression consisting of tacrolimus and mycophenolate mofetil ± prednisone, had no history of acute rejection and had a surveillance renal allograft biopsy at 6 months post-transplant showing 5 – 25% inflammation (Banff i0 or i1) without evidence of rejection (Banff scores i<2, t<2)(5, 6). Adequate venous access was required to support a 400 ml whole blood draw and infusion of expanded Tregs. Other major inclusion criteria included current immunizations against tetanus, diphtheria, pertussis, hepatitis B, pneumococcus and influenza, and willingness to use a reliable and effective form of contraception for 2 years (women) or 3 months (men) after Treg dosing. Major exclusion criteria were: evidence of active infection [HIV-1/HIV-2, hepatitis B, hepatitis C, Epstein-Barr virus (EBV) or CMV or BK genomes, or positive purified protein derivative (PPD) skin test]; autoimmune disease; EBV seronegativity; CMV seronegativity with CMV seropositive donor; history of malignancy except adequately treated basal cell carcinoma; 6-antigen HLA match with their donor; history of transplant renal artery stenosis or wound healing complications following the transplant; any chronic illness or previous treatment that, in the opinion of the investigator, precluded participation in the trial. Laboratory exclusion criteria included hemoglobin <11 g/dl, leukocytes <3000/µl; neutrophils <1500/µl, lymphocytes <800/µl, platelets <100,000/µl, or Tregs <30/µl.
Study Endpoints
Primary outcome measures were the feasibility of isolating, expanding and infusing Tregs in kidney transplant recipients on immunosuppression, the incidence of adverse events, and patient and graft survivals. Secondary outcome measures included changes in circulating Tregs, changes in the inflammatory infiltrate in the allograft two weeks after the Treg infusion, and changes in urinary protein and mRNA markers reflecting immune mediated injury. Table 1 lists the specific objectives and endpoints for the study.
Table 1.
Study Objectives and Endpoints
| Objectives | Endpoints | |
|---|---|---|
| Primary | Assess the feasibility of isolating and expanding Tregs and of intravenous infusion of ex vivo–expanded autologous polyclonal Tregs in kidney transplant recipients with subclinical graft inflammation | Incidence of failure-to-treat |
| Assess the safety of intravenous infusion of ex vivo–expanded autologous polyclonal Tregs in kidney transplant recipients with subclinical graft inflammation | Incidence of infusion reactions Incidence of patient reported adverse events Incidence of laboratory abnormalities Incidence of infection Incidence of malignancy Incidence of acute rejection Incidence of graft dysfunction Patient and graft survival | |
| Secondary | Assess the fate of infused Tregs in circulation | Circulating Treg numbers Percentage of infused Tregs by deuterium tracking Intragraft Tregs in the kidney biopsy by IHC and deuterium tracking |
| Assess the impact of infused Tregs on graft inflammation | Inflammatory cell density in the renal allograft by histopathology and IHC Urine cytokines and inflammatory gene expression levels |
Treg manufacturing
Polyclonally expanded Tregs were produced from 400 ml of peripheral blood as previously described (23) Briefly, peripheral blood mononuclear cells were isolated via Ficoll density gradient (GE Healthcare Bio-Sciences) and Tregs were purified using flow activated cell sorting (FACS) based on cell surface phenotype of CD4+ CD127lo/−CD25+. Purified Tregs received two rounds of stimulation of anti-CD3 and anti-CD28 paramagnetic beads on day 0 and day 9 along with constant feeding of medium (X-Vivo 15, Lonza) containing interleukin-2 and deuterated glucose (Cambridge Isotopes) without the addition of rapamycin. Cells were harvested on day 14 after culture initiation and stimulating beads removed via magnetic separation. Products that met the preset release criteria were released for infusion.
Treg infusion
Results of blood chemistries and hematology were reviewed, a history of any recent illness or fever was obtained, and a physical exam performed before infusion of the cells. After premedication with acetaminophen and diphenhydramine, Tregs were infused via a peripheral intravenous line using syringe push over 10 minutes. The patients were monitored for 24 hours post-infusion in the clinical research unit.
Safety
Patients underwent follow-up assessments for 1 year post-infusion as noted in Figure 1 which included a safety questionnaire, medication review, physical exam and laboratory assessments. For each of the first two subjects, the study team met to review cumulative safety data after they had undergone their 2 week follow-up biopsies. If no grade 3 or higher adverse event had been observed, subsequent subjects could be treated. An independent data and safety monitoring board reviewed cumulative safety data at 6-month intervals.
Tracking of infused Tregs
Expanded Tregs were labeled with deuterium using deuterated glucose in the expansion medium (12) which provides a means to track the autologous Tregs after infusion. In addition, by measuring deuterium in non-Tregs (cells outside the CD4+ CD127lo/−CD25+ gate), stability of the infused Tregs was monitored. CD4+ CD127lo/−CD25+ Tregs were purified via FACS from peripheral blood collected at specified time points post-infusion. CD4+ cells outside the CD25+CD127lo/− Treg gate were further sorted into three subsets: CD45RO+CD62L+, CD45RO+CD62L−, and CD45RO−CD62L+. For tracking infused Tregs in kidneys, biopsies obtained after Treg infusion were enzymatically digested into single cells and CD4+ cells were FACS purified. At least 1,000 cells were collected from each cell subset. DNA was extracted from all purified cells and subjected to gas chromatography and mass spectrometry (GC-MS) analysis to measure deuterium enrichment in circulating Tregs (12).
Graft pathology
Kidney transplant biopsies were performed per protocol at 2 weeks and 6 months following the Treg infusion. Clinically indicated biopsies were performed for elevated serum creatinine, proteinuria or rising donor specific antibody. Two cores of renal allograft tissue were obtained during the biopsy- one used for histopathology and molecular analysis, and the second for Treg deuterium tracking. All biopsies were scored by a single pathologist using the Banff classification of kidney allograft pathology (5, 6). Multiparameter immunohistochemical and/or immunofluorescence (IF) staining were performed for the following cell markers: CD45 (leukocyte common antigen), CD4, CD8, FOXP3, CD20, and CD68.
RESULTS
Patient characteristics
Three kidney transplant recipients with inflammation on their 6-month surveillance kidney transplant biopsy were enrolled in the study. All patients had received transplants from ABO and human leukocyte antigen (HLA)-compatible donors and had stable graft function at the time of the biopsy. Table 2 lists the demographic and clinical characteristics of the enrolled patients as well as the findings on the qualifying biopsy. Patients 1 and 2 were maintained on the same immunosuppressive regimen without any changes for the duration of the study. The immunosuppressive regimen of patient 3 was modified after the follow up biopsy at 6 months post-infusion.
Table 2.
Patient characteristics
| Subject 1 | Subject 2 | Subject 3 | |
|---|---|---|---|
| Age at enrollment (years) | 62 | 58 | 46 |
| Sex | Male | Male | Male |
| Race/ Ethnicity | Caucasian | Hispanic | Hispanic |
| Cause of renal failure | Membranous glomerulonephritis | Hypertension | Focal segmental glomerulosclerosis, likely secondary to hypertension |
| Duration of dialysis (months) | 5 | 61 | 0 (pre-emptive transplant) |
| Type of transplant | Living donor | Deceased donor | Living donor |
| Calculated panel reactive antibody (%) | 0% | 17% | 0% |
| Induction immunosuppression | Basiliximab | Basiliximab | Basiliximab |
| Maintenance immunosuppression | |||
| Tacrolimus (mg/day) | 4 | 4 | 3 |
| Mycophenolate mofetil (mg/day) | 2000 | 1000 | 1000 |
| Prednisone (mg/day) | 5 | 5 | 5 |
| 6-month surveillance biopsy scores by the Banff classification* (5) | i1, t1 | i0, t1 | i0, t1 |
Under the Banff classification of renal allograft pathology, i- and t-scores reflect the amount of mononuclear cell infiltration in the interstitium and tubules respectively.
Treg manufacturing
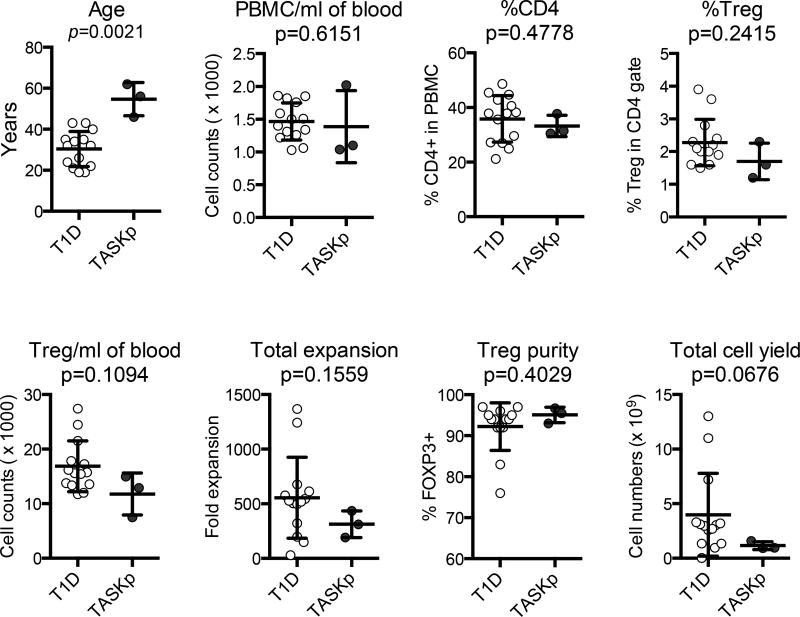
Immunosuppressive drugs, particularly calcineurin inhibitors, can impact Treg number in peripheral blood and their ability to expand. Figure 2 compares Treg manufacturing data from the patients in this trial who were receiving maintenance immunosuppression (solid symbols) to all patients in a previous type 1 diabetes trial who were infused products (open symbols) (12). Despite their more advanced age, immunosuppressive therapy, a trend toward lower number of Tregs in the peripheral blood and lower expansion in culture, we were able to produce close to 1×109 Tregs from one unit of blood for all subjects in this study. More importantly, the purity of Tregs assessed by flow cytometric analysis of percentage of FOXP3+ cells in the products was 93, 95.5, and 96.7%, far exceeding the 60% release criterion. Altogether, three expansions were attempted and all successfully met the release criteria and dose requirement for infusion (Table 3).
Figure 2. Treg manufacturing.
Parameters of Treg manufacturing for the three kidney transplant patients in this study - TASKp (pilot Treg adoptive therapy for subclinical kidney transplant inflammation, n=3) (solid symbols) are compared with those obtained from type 1 diabetes patients (T1D) enrolled in a separate trial (n=14) (12)(open symbols). Identical standard operating procedures were followed in the two trials. Mann Whitney test was used to calculate p values and determine the statistical significance between the two groups.
Table 3.
Purity and Viability of Expanded Tregs
| Parameters | FOXP3 % | CD4% | CD8% | Viability |
|---|---|---|---|---|
| Release criteria | >60% | >95% | <5% | >85% |
| Subject 1 | 93.1 | 97.9 | 0.16 | 99.5 |
| Subject 2 | 95.5 | 97.1 | 0.37 | 99.6 |
| Subject 3 | 96.7 | 97.6 | 0.25 | 99.4 |
Safety
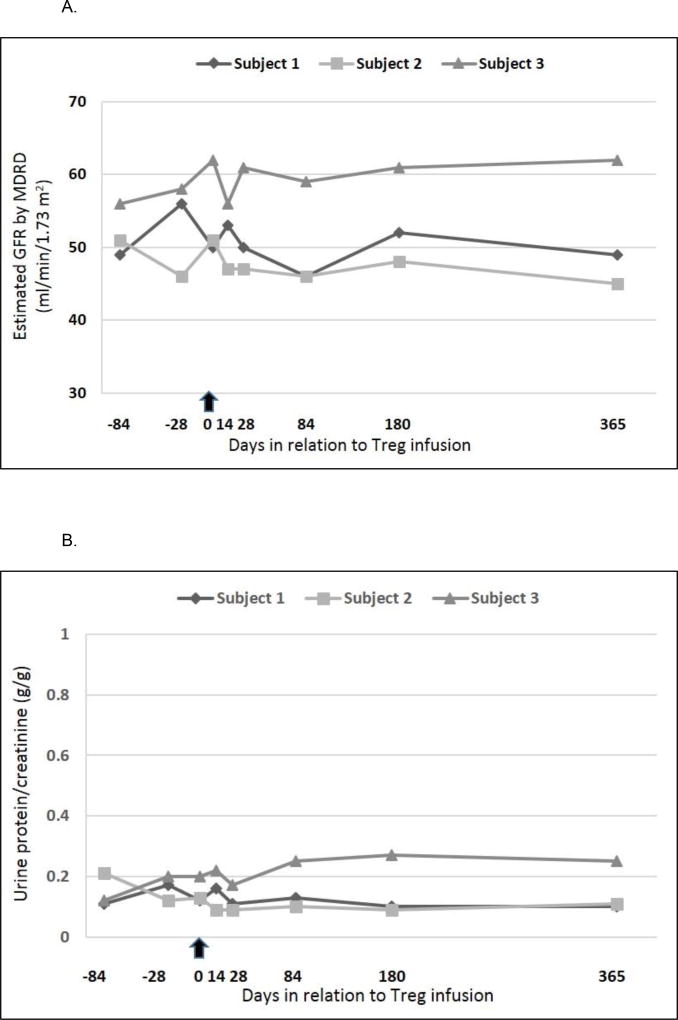
All patients received the target dose. There were no infusion reactions. No infections or malignancies were observed during the 1 year follow-up period. Leukopenia was the only reported adverse event possibly related to the study therapy (only in subject 1), with highest severity being grade 3 (neutropenia, absolute neutrophil count 0.96 × 109/L). The leukopenia resolved spontaneously without any special interventions or any change in the immunosuppression regimen. There were no therapy-related serious adverse events. Patient and graft survival were 100% at one year and there were no episodes of graft dysfunction or malignancy. Renal function parameters before and during the course of the study are depicted in Figure 3.
Figure 3. Graft function before and after Treg infusion.
The arrow indicates the day of infusion (day 0)
3A. Estimated GFR by MDRD equation for all subjects
3B. Urinary protein excretion for all subjects.
Tracking of infused Tregs
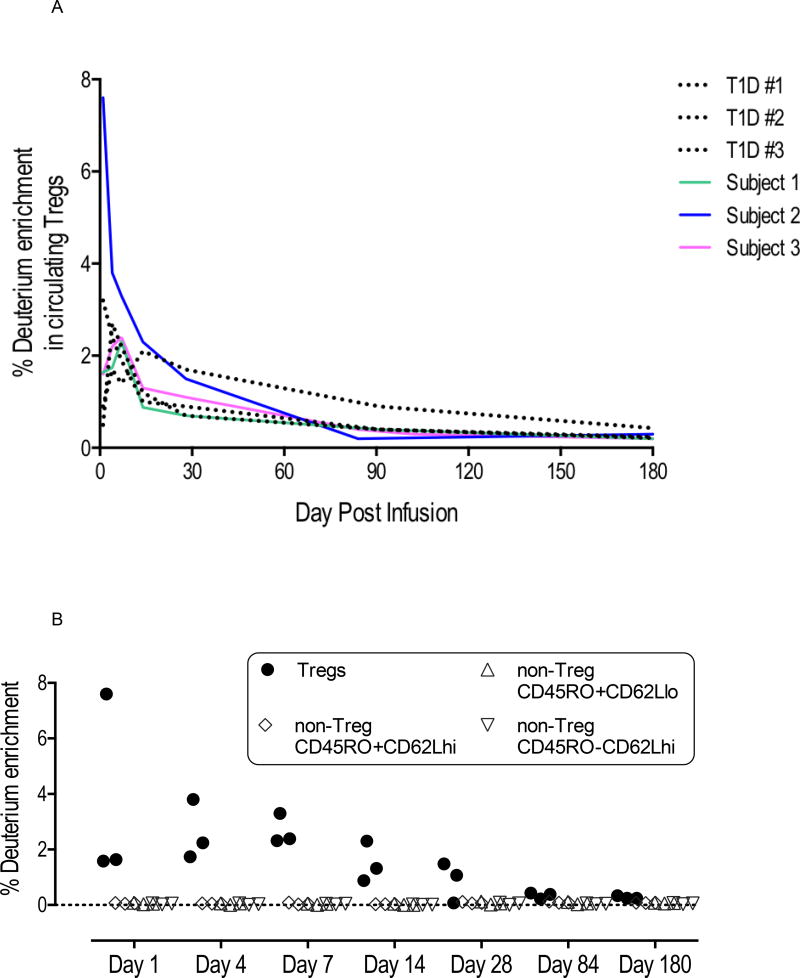
Figure 4A compares the results of deuterium enrichment in Tregs in the three patients in this trial (solid colored lines) with those from three type 1 diabetes patients (dotted black lines) in another trial conducted at UCSF who had also received 320 × 106 polyclonal Tregs (12). The purpose of this comparison was to identify any impact of immunosuppressive medications, particularly calcineurin inhibitors, on the persistence of Tregs. Infused Tregs peaked in circulation in the first week, reaching 2 to 8% of circulating Tregs. Deuterium signals remain detectable in the first month after infusion in all subjects and fell near the detection limit of 0.2% by 3 months after infusion. The pattern of Treg persistence was not different between the type 1 diabetes subjects and the transplant patients on immunosuppression. In addition, we did not detect deuterium among various subsets of non-Tregs at any time point after infusion, suggesting that most Tregs maintained their phenotype after infusion (Figure 4B).
Figure 4. Treg tracking in vivo.
4A. Persistence of infused Tregs. Tregs were expanded ex vivo in the presence of deuterated glucose, which achieves ~60% enrichment of deuterium in the DNA of expanded Tregs and allows tracking of the infused Tregs after infusion. On the indicated days after Treg infusion, Tregs were isolated using FACS on indicated days and deuterium enrichment in the genomic DNA of purified Tregs were determined using gas chromatography and mass spectrometry. The enrichment values from the 3 kidney patients in this study (solid lines) are compared with those obtained from type 1 diabetes patients (dotted lines) who received similar dose of Tregs in a separate trial.
4B. Stability of infused Tregs. Peripheral blood CD4 cells outside The CD25+CD127lo/− Treg gate (non-Tregs) are sorted into three fractions based CD45RO and CD62L expression. Deuterium levels in these cell subsets were monitored for potential destabilization of Tregs into non-Tregs.
Graft pathology
All subjects underwent kidney biopsies per study protocol at 2 weeks and 6 months post-infusion. Tables 4 and 5 and Figure 5 demonstrate the clinical and histologic data and the corresponding images of renal pathology.
Table 4. Renal function and renal pathology at baseline and post-Treg infusion.
The Banff i-scores and t-scores represent the degree of mononuclear cell infiltration in the interstitium and tubules respectively seen on light microscopy. Quantitative image analysis of immunohistochemical staining for CD45 was used to calculate the density of overall inflammation.
| Kidney biopsy time point |
Kidney transplant function | Histopathology | Immunohistochemistry | ||||
|---|---|---|---|---|---|---|---|
| Serum creatinine (mg/dl) |
Estimated GFR (ml/min/1.73m2) |
Urine protein/ creatinine (mg/g) |
Banff i-score |
Banff t-score |
LCA+ cells/mm2 | ||
| Subject 1 | Baseline | 1.30 | 56 | 170 | 1 | 1 | 337.2 |
| 2 weeks | 1.37 | 53 | 160 | 0 | 0 | 18.9 | |
| 6 months | 1.38 | 54 | NA* | 0 | 0 | 126.1 | |
| Subject 2 | Baseline | 1.55 | 49 | 120 | 0 | 1 | 469.1 |
| 2 weeks | 1.53 | 49 | 90 | 0 | 0 | 303.5 | |
| 6 months | 1.49 | 51 | 90 | 0 | 1 | 74.9 | |
| Subject 3 | Baseline | 1.32 | 64 | 200 | 0 | 1 | 278.0 |
| 2 weeks | 1.37 | 61 | 220 | 1 | 1 | 454.4 | |
| 6 months | 1.27 | 67 | 270 | 2 | 2 | 514.0 | |
Lab unable to calculate urine protein/creatinine as urinary protein concentration was <6 mg/dl.
Table 5.
Inflammatory cell subsets on biopsies at baseline and post-Treg infusion by quantitative image analysis of multiparameter immunofluorescence for cell-specific markers.
| Kidney biopsy time point |
Immunofluorescence | |||||
|---|---|---|---|---|---|---|
| CD4+ cells/mm2 |
CD8+ cells/mm2 |
CD20+ cells/mm2 |
FOXP3+ cells/mm2 (% of CD4+ cells) |
CD68+ cells/mm2 |
||
| Subject 1 | Baseline | 239.6 | 112.5 | 104 | 11.4 (4.7) | 44.6 |
| 2 weeks | 116.2 | 35.9 | 1.25 | 0.4 (0.3) | 0.9 | |
| 6 months | 229.3 | 40 | 32.8 | 5.6 (2.4) | 12.2 | |
| Subject 2 | Baseline | 224 | 45 | 520.3 | 8.3 (3.7) | 14.6 |
| 2 weeks | 158.7 | 21.5 | 66.2 | 3.1 (1.9) | 14 | |
| 6 months | 78.7 | 27.1 | 29 | 0.4 (0.5) | 3.7 | |
| Subject 3 | Baseline | 139.6 | 48.4 | 44 | 20.1 (14.4) | 4.6 |
| 2 weeks | 158.1 | 33.7 | 5.3 | 15.1 (9.6) | 3.7 | |
| 6 months | 631.2 | 222.1 | 158.1 | 38.1 (5.9) | 21.2 | |
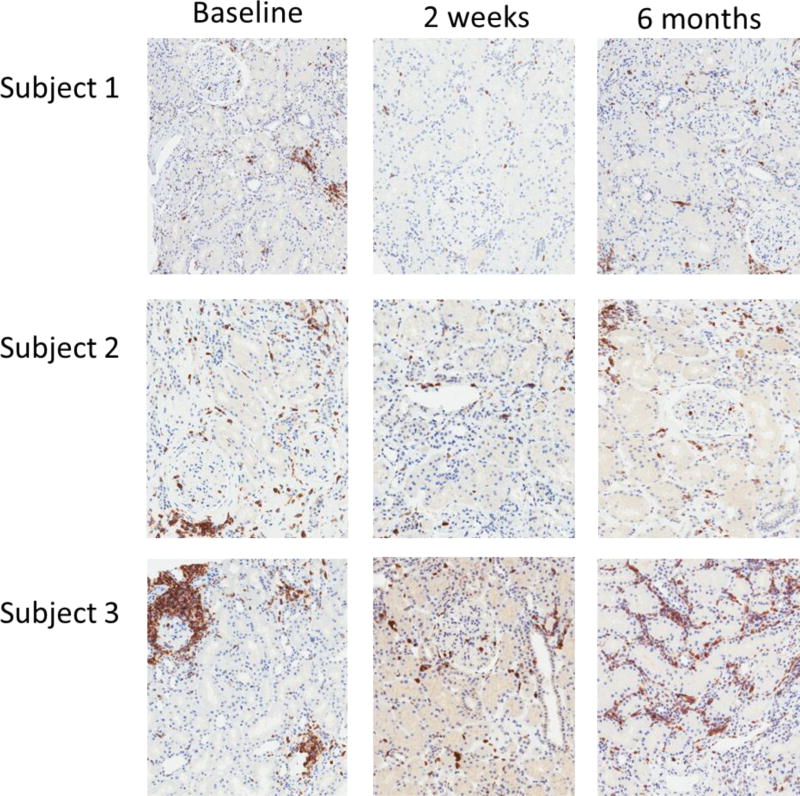
Figure 5. Graft inflammation on kidney biopsies at baseline and post-Treg infusion.
CD45 (leukocyte common antigen) immunohistochemical staining was done on kidney biopsy sections at baseline and at 2 weeks and 6 months post-Treg infusion. Representative slides from each subject are shown. The positive cells stain brown.
Subjects 1 and 2 had lower LCA+ cell density on their follow-up biopsies compared to the screening biopsy.
Subject 3 underwent three biopsies: study biopsies at 2 weeks and 6 months post-infusion plus a clinically indicated biopsy at 3 months post-infusion for a rising level of de novo donor-specific anti-HLA antibody. The 2-week biopsy, similar to the baseline biopsy, showed subclinical inflammation which was insufficient to meet criteria for acute cellular rejection. The 6-month follow up study biopsy showed worsened inflammation now sufficient to meet criteria for Banff type 1a acute cellular rejection. C4d staining was negative on both biopsies and there was no evidence of acute antibody mediated rejection. Of note, graft function had remained stable during the 6 months post-infusion. He was treated with steroids for subclinical acute cellular rejection on his 6-month follow up biopsy. His maintenance immunosuppression was also modified: the total dose of mycophenolate was increased to 2000 mg/ daily and tacrolimus dose was adjusted to maintain a level of 8–10 mcg/L
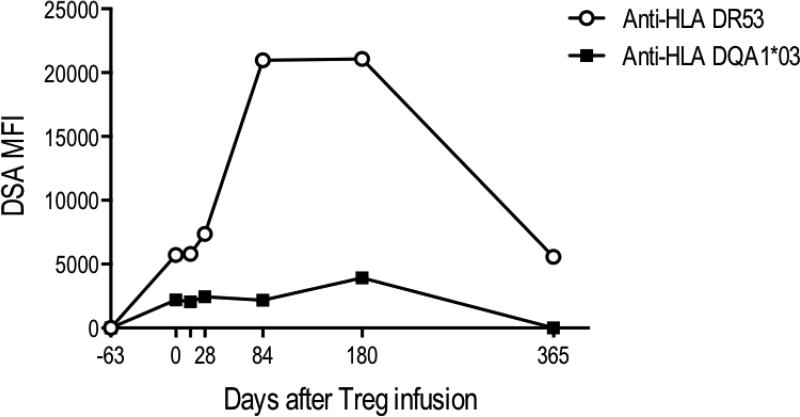
Interestingly, subject 3 was also noted to have detectable donor specific antibodies to HLA DR53 and HLA DQA1*03 at the time of his 2-week follow-up biopsy. Retrospective analysis of stored sera showed that he had no detectable HLA antibodies at the time of his initial 6-month surveillance biopsy prior to study enrollment (day −63) but these became detectable on serum from the day of infusion of Tregs prior to receiving Tregs. Continued monitoring after his 2-week study biopsy showed a rising level of these antibodies to the donor (Figure 6). Although serum creatinine remained stable, a biopsy was performed at 3 months (day 103) post-infusion to rule out antibody-mediated rejection as per the local standard of care. This biopsy showed no inflammation or rejection (Banff scores: i0, t0; C4d stain negative) and he received no specific treatment. Antibody levels showed a substantial decline after treatment for subclinical acute cellular rejection on his 6-month post-infusion follow-up biopsy Of note, subject 1 did not have sufficient T cell infiltrate in the biopsy samples collected at 2 weeks or 6 months post infusion for deuterium analyses. For subjects 2 and 3, 2,781 to 10,000 CD4+ cells were isolated from the 2-wk and 6-month biopsies and deuterium signals were near the 0.2% threshold of detection.
Figure 6. Donor specific antibody (DSA) levels in subject 3 pre- and post-Treg infusion.
Day 0 refers to the day of Treg infusion. Antibodies were already present prior to the infusion of Tregs and continued rising post-infusion. Biopsies were done on day 14, day 103 and day 180 post-infusion. MFI=mean fluorescent intensity
DISCUSSION
Interventions that modulate the immune response have the potential to resolve graft inflammation and improve long-term graft survival. Tregs are known to traffic to sites of inflammation and their presence in the urine and renal allograft of patients with rejection has been correlated with improved outcomes (16, 24).
Here, we have expanded polyclonal Tregs and tested their safety after reinfusion in kidney transplant recipients on tacrolimus, mycophenolate mofetil and corticosteroids. While Tregs have been previously isolated from uremic patients (25, 26), this is the first report showing that isolation and expansion of Tregs is not only possible from kidney transplant recipients on immunosuppression but also feasible in sufficient numbers for in vivo therapy. The Treg infusions were well tolerated without cytokine release, infusion reactions, or infectious complications. A subset of infused Tregs remained detectable in circulation for at least a month post infusion with pharmacokinetics similar to that seen in non-immunosuppressed type 1 diabetes patients. In addition, we did not detect Treg destabilization in these patients on tacrolimus-based immunosuppression. Our findings on Treg persistence in human subjects are distinct from a previous study in non-human primates that showed expanded Tregs peak within minutes after infusion and fall sharply afterwards and that there was a negative impact of tacrolimus on Treg persistence and stability (27). This study differs from the previous study in terms of species of study subjects, cell manufacturing protocol, and dose of immunosuppression. Tregs manufactured using our established approach persisted and were stable in immunosuppressed patients. These results are reassuring given previously noted concerns in literature about the phenotypic and functional stability of infused Tregs (28–30).
Graft inflammation, as assessed by the density of LCA+ cells, showed improvement on follow-up biopsies in the first 2 patients but not in the third patient. The third patient continued to have inflammation on his follow biopsies at 2 weeks and 6 months post-infusion. Unlike the first two patients, the third patient had started developing de novo donor-specific antibodies prior to the infusion and it is possible that his alloimmune response was stronger or more resistant to modulation by Tregs.
As a phase 1 study with a limited number of participants and no controls, this study was not powered to detect improvement in graft inflammation or derive any correlation between changes in graft pathology and the infusion of Tregs. Therefore, we are unable to ascertain whether the persistence of infused Tregs in circulation had any impact on graft inflammation and whether the changes in the graft infiltrate observed in patients 1 and 2 were related to the infusion. The strength of the study design is the relative uniformity and stability of enrolled patients. We kept factors such as the immunosuppressive regimen constant before and after the infusion to maximize our confidence in associating changes with Treg infusion. Selecting patients with pre-existing inflammation in the graft provides us an opportunity to observe the therapeutic impact of Tregs on the inflammation. Although we cannot draw any conclusions regarding the efficacy of Tregs for graft inflammation based on this study, the positive safety and feasibility results have allowed us to devise and conduct a full-scale protocol of Clinical Trials in Organ Transplantation-21 (CTOT-21, NCT02088931) utilizing the same design backbone to study efficacy of infused polyclonal Tregs versus donor alloantigen-reactive Tregs in a randomized controlled trial.
In summary, this study reports the successful isolation, expansion, and reinfusion of polyclonal Tregs derived from kidney transplant recipients on immunosuppression with subclinical graft inflammation, therefore providing the impetus and support for future clinical trials of immunotherapy with Tregs in transplantation.
Acknowledgments
We thank Angela Lares, Michael Lee, Weihong Liu, PhD, and Florinna Dekovic for their assistance with Treg manufacturing and Lisa Masiello, PhD and Peggy Millar for their support of trial management. This work was supported by the Sally Klingbeil Foundation, the Sean N. Parker Autoimmunity Laboratory and by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U01-AI113362 (to F.V.).
Abbreviations
- DSA
Donor specific antibody
- eGFR
Estimated glomerular filtration rate
- FACS
Flow activated cell sorting
- HLA
Human leukocyte antigen
- IF
Immunofluorescence
- Treg
Regulatory T cell.
Footnotes
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. F.V. has received research grants from Novartis, Immucor and Genentech. J.A. B. is an unpaid adviser in a phase 2 trial of Treg therapy for type 1 diabetes sponsored by Caladrius Biosciences. J.A. B and Q.T. have a patent pending on polyTregs. The other authors have no conflicts of interest to disclose.
References
- 1.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(3):527–35. doi: 10.1111/j.1600-6143.2008.02519.x. [DOI] [PubMed] [Google Scholar]
- 2.Heilman RL, Devarapalli Y, Chakkera HA, Mekeel KL, Moss AA, Mulligan DC, et al. Impact of subclinical inflammation on the development of interstitial fibrosis and tubular atrophy in kidney transplant recipients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(3):563–70. doi: 10.1111/j.1600-6143.2009.02966.x. [DOI] [PubMed] [Google Scholar]
- 3.Thierry A, Thervet E, Vuiblet V, Goujon JM, Machet MC, Noel LH, et al. Long-term impact of subclinical inflammation diagnosed by protocol biopsy one year after renal transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(10):2153–61. doi: 10.1111/j.1600-6143.2011.03695.x. [DOI] [PubMed] [Google Scholar]
- 4.Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation. 2004;78(2):242–9. doi: 10.1097/01.tp.0000128167.60172.cc. [DOI] [PubMed] [Google Scholar]
- 5.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney international. 1999;55(2):713–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 6.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(4):753–60. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann SC, Hale DA, Kleiner DE, Mannon RB, Kampen RL, Jacobson LM, et al. Functionally significant renal allograft rejection is defined by transcriptional criteria. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(3):573–81. doi: 10.1111/j.1600-6143.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 8.Moreso F, Ibernon M, Goma M, Carrera M, Fulladosa X, Hueso M, et al. Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(4):747–52. doi: 10.1111/j.1600-6143.2005.01230.x. [DOI] [PubMed] [Google Scholar]
- 9.Park WD, Griffin MD, Cornell LD, Cosio FG, Stegall MD. Fibrosis with inflammation at one year predicts transplant functional decline. Journal of the American Society of Nephrology : JASN. 2010;21(11):1987–97. doi: 10.1681/ASN.2010010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y, van der Veeken J, Shugay M, Putintseva EV, Osmanbeyoglu HU, Dikiy S, et al. A mechanism for expansion of regulatory T-cell repertoire and its role in self-tolerance. Nature. 2015;528(7580):132–6. doi: 10.1038/nature16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nature immunology. 2008;9(3):239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Science translational medicine. 2015;7(315):315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 14.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juscinska J, et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol. 2014;153(1):23–30. doi: 10.1016/j.clim.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Todo S, Yamashita K, Goto R, Zaitsu M, Nagatsu A, Oura T, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64(2):632–43. doi: 10.1002/hep.28459. [DOI] [PubMed] [Google Scholar]
- 16.Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. The New England journal of medicine. 2005;353(22):2342–51. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 17.Taflin C, Nochy D, Hill G, Frouget T, Rioux N, Verine J, et al. Regulatory T cells in kidney allograft infiltrates correlate with initial inflammation and graft function. Transplantation. 2010;89(2):194–9. doi: 10.1097/TP.0b013e3181c3ca11. [DOI] [PubMed] [Google Scholar]
- 18.Tang Q, Bluestone JA. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harbor perspectives in medicine. 2013;3(11) doi: 10.1101/cshperspect.a015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chesneau M, Michel L, Dugast E, Chenouard A, Baron D, Pallier A, et al. Tolerant Kidney Transplant Patients Produce B Cells with Regulatory Properties. Journal of the American Society of Nephrology : JASN. 2015;26(10):2588–98. doi: 10.1681/ASN.2014040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braza F, Dugast E, Panov I, Paul C, Vogt K, Pallier A, et al. Central Role of CD45RA- Foxp3hi Memory Regulatory T Cells in Clinical Kidney Transplantation Tolerance. Journal of the American Society of Nephrology : JASN. 2015;26(8):1795–805. doi: 10.1681/ASN.2014050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(6):1457–63. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 22.Naesens M, Salvatierra O, Benfield M, Ettenger RB, Dharnidharka V, Harmon W, et al. Subclinical inflammation and chronic renal allograft injury in a randomized trial on steroid avoidance in pediatric kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(10):2730–43. doi: 10.1111/j.1600-6143.2012.04144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58(3):652–62. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bestard O, Cruzado JM, Rama I, Torras J, Goma M, Seron D, et al. Presence of FoxP3+ regulatory T Cells predicts outcome of subclinical rejection of renal allografts. Journal of the American Society of Nephrology : JASN. 2008;19(10):2020–6. doi: 10.1681/ASN.2007111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berglund D, Korsgren O, Lorant T, Schneider K, Tufveson G, Carlsson B. Isolation, expansion and functional assessment of CD4+CD25+FoxP3+ regulatory T cells and Tr1 cells from uremic patients awaiting kidney transplantation. Transplant immunology. 2012;26(1):27–33. doi: 10.1016/j.trim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Guinan EC, Cole GA, Wylie WH, Kelner RH, Janec KJ, Yuan H, et al. Ex Vivo Costimulatory Blockade to Generate Regulatory T Cells From Patients Awaiting Kidney Transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(7):2187–95. doi: 10.1111/ajt.13725. [DOI] [PubMed] [Google Scholar]
- 27.Singh K, Stempora L, Harvey RD, Kirk AD, Larsen CP, Blazar BR, et al. Superiority of rapamycin over tacrolimus in preserving nonhuman primate Treg half-life and phenotype after adoptive transfer. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014;14(12):2691–703. doi: 10.1111/ajt.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu M, Wang YM, Wang Y, Zhang GY, Zheng G, Yi S, et al. Regulatory T cells in kidney disease and transplantation. Kidney international. 2016;90(3):502–14. doi: 10.1016/j.kint.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nature medicine. 2014;20(1):62–8. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 30.Rossetti M, Spreafico R, Saidin S, Chua C, Moshref M, Leong JY, et al. Ex vivo-expanded but not in vitro-induced human regulatory T cells are candidates for cell therapy in autoimmune diseases thanks to stable demethylation of the FOXP3 regulatory T cell-specific demethylated region. J Immunol. 2015;194(1):113–24. doi: 10.4049/jimmunol.1401145. [DOI] [PMC free article] [PubMed] [Google Scholar]