Abstract
Collective cell migration is a process whereby cells move while keeping contact with other cells. The Xenopus Cranial Neural Crest (CNC) is a population of cells that emerge during early embryogenesis and undergo extensive migration from the dorsal to ventral part of the embryo’s head. These cells migrate collectively and require cadherin mediated cell-cell contact. In this review, we will describe the key features of Xenopus CNC migration including the key molecules driving their migration. We will also review the role of the various cadherins during Xenopus CNC emergence and migration. Lastly, we will discuss the recent and seemingly controversial findings showing that E-cadherin presence is essential for CNC migration.
Keywords: Cadherin, EMT, Xenopus, Cranial neural crest, Collective migration
1. Introduction
Cell migration is wide spread in the animal kingdom. During embryogenesis, cell migration is a critical part of morphogenesis (e.g. gastrulation, organogenesis), while in adults, cell migration plays a key role in immune defense through chemotactic responses of leukocytes and in tissue repair and regeneration. Surprisingly, many migrating cells start out as epithelial cells. These are immobile, highly polarized cells with strong cell-cell adhesions mediated by Adherens Junctions (i.e. intercellular junctions that join the actin cytoskeleton of each cell to the plasma membrane and form adhesive contacts between cells), and Tight Junctions (i.e. intercellular junctions that result in close juxtaposition of plasma membranes creating a permeability barrier). These adhesive properties, which result from interactions of cell-cell attachment proteins belonging to the cadherin family, give epithelia mechanical resilience and barrier function. Epithelial cells are ill equipped to perform cell migration, which typically requires a decrease in cell-cell adhesion, an increase in cell-extracellular matrix adhesions and activation of the actin-myosin based cytoskeleton (Ridley et al., 2003). While epithelium can undergo collective cell migration during embryogenesis (Montell et al., 2012), often the acquisition of cell motility is associated with an epithelium to mesenchyme transition (EMT). This is achieved notably by modulating the expression and activity of cadherins, which mediate intercellular junctions. A comprehensive review of all the cellular changes occurring during EMT has been published elsewhere (Nieto et al., 2016). Note that the full conversion from epithelial to mesenchyme does not have to be complete for cells to migrate and a couple of metastable states of EMT have now been described where cells undergo efficient migration while maintaining strong cell-cell contact (Nieto et al., 2016). The Xenopus laevis cranial neural crest (CNC) migrates in such a way (Alfandari et al., 2003). The goal of this article is to review and revisit the role of some of the cadherins (namely E-cadherin, N-cadherin and cadherin-11) during the migration of Xenopus CNC.
2. Xenopus Cranial Neural Crest
During early development of vertebrate embryos, the neural crest cells (NC) emerge from the sensory layer of the ectoderm, more specifically at the transition between the neural (future central nervous system) and non-neural (epidermis) ectoderm (Fig. 1A). Once induced, the CNC will stay stationary for a while, a phase called pre-migratory stage. Starting at the late neurula stage, these cells enter their migratory stage and acquire motility. The directionality of their migration depends on their antero/posterior origin: most cells will migrate ventrally but some, like the ones originating from the nuchal area or caudal area (called vagal and sacral crest), can also migrate antero/posteriorly. Once the cells reach their destination, they will differential into a variety of cell types and tissue (Le Douarin, 1980). The cranial neural crest (CNC) represents a subgroup of these cells that emerge at the most anterior part of the neural tissue. These cells distinguish themselves from the other NC in at least three ways. They are the first to emerge, segregating themselves from the neuroectoderm and emigrating well before the end of neurulation (Fig. 1A). Secondly, they undergo collective cell migration. Thirdly, they give rise to a wider array of derivatives than any other NC types, some of them specific of the cranial lineage (endothelial cells, chondrocytes and osteocytes of all of the viscerocranium and most of the neurocranium and odontoblasts).
Fig. 1.
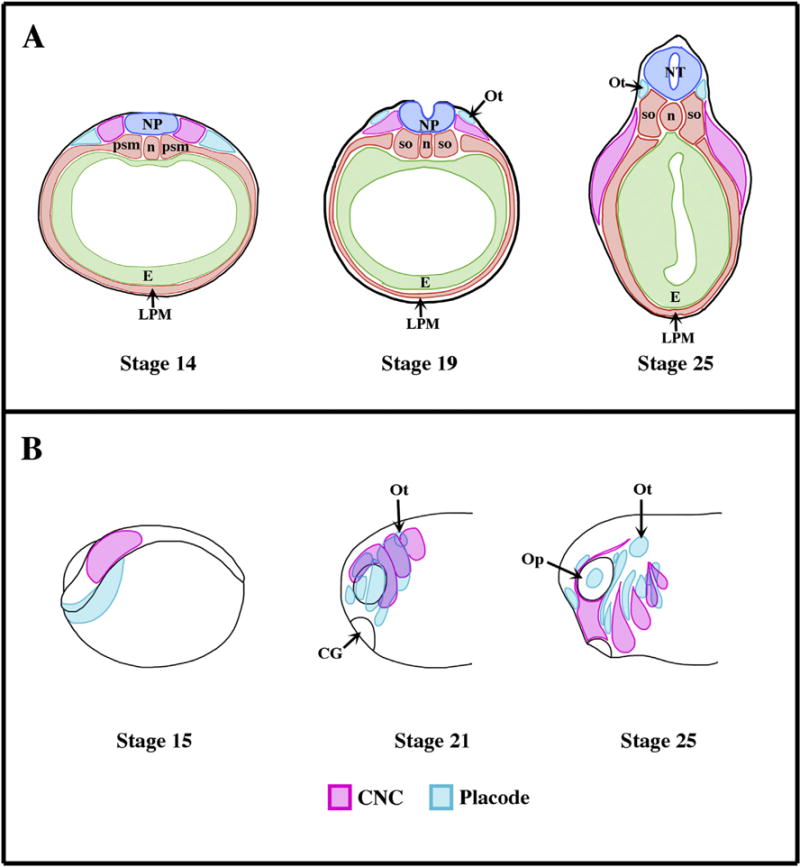
Origin and migration of the cranial neural crest cells in Xenopus laevis. (A) Schematics representing transverse sections though the prospective otic placode (Ot) of Xenopus embryos at the various stages of development (Nieuwkoop and Faber, 1967). Dorsal is up. The placodal ectoderm and neural crest prospective tissue emerge from the sensory layer of the ectoderm and are anatomically visible as soon as stage 14 (early neurulation). At stage 19 (late neurula stage) the cranial neural crest starts migrating ventrally (Sadaghiani and Thiebaud, 1987). (B) Schematic showing the relative positioning of CNC and the cranial placodes (Sadaghiani and Thiebaud, 1987; Schlosser, 2006). During the early neurula stage (stage 15) both placodal (blue) and CNC (pink) primordium appose one another. Starting with the early tailbud stage (stage 21), these territories are segmented into various subgroups that reflect their future differentiation. The placodal ectoderm secretes Sdf1, which attracts CNC. Once the CNC reaches a placode, CIL takes place and the placode migrates away (i.e. ventrally). Once the placodes and CNC are separated, the Sdf1-based chemoattraction reasserts itself. This “chase and run” contributes to the proper migration of the each of the CNC segments while the placodes lying in the path of the CNC (e.g. Epibranchial placodes) reach their proper dorsoventral location and are shaped into narrow strips of tissues. Ot: Placode; NT, neural tube; n: notochord; psm: presomitic mesoderm, so: somites; LPM: lateral plate mesoderm, CG: cement gland; Op: optic vesicle.
Wilhem His has discovered the neural crest in 1968 and their embryological origin and derivatives have been extensively studied in chicken and amphibian for the better part of the 20th century (Hörstadius, 1950; Le Douarin, 1980). The molecular underpinnings of NC development have been investigated more recently using a wider array of model systems including zebrafish and mouse. Xenopus laevis studies, while more recent, have made a sizable contribution to the CNC field. The development of classical embryological techniques (CNC grafts, explants and targeted injections), coupled to the well described strengths of the Xenopus model system (e.g. in vitro fertilization, ex-utero development, strong biochemical tools) and the emergence of better molecular biology tools were instrumental in the rise of Xenopus as model system for the study of collective cell migration in general and CNC migration in particular.
Many groups have surgically manipulated amphibian embryo to study the origin, migration path and fate of the neural crest in both Xenopus (Macmillan, 1976; Sadaghiani and Thiebaud, 1987) and Axolotl (Hörstadius, 1950). They tracked the neural crest by using vital dye (Hörstadius, 1950), differentiation marker such as pigmentation (Macmillan, 1976) or histological markers when interspecies grafts were performed (Sadaghiani and Thiebaud, 1987). At the turn of the millennia, two groups set out to study the migration of CNC in live Xenopus laevis embryos by combining the grafting techniques described by their predecessor with the live lineage tracer GFP (Borchers et al., 2000; Carl et al., 1999). They performed their grafts at early neurula stages (stage 14 to 16 respectively), allowing them to fully exploit a key advantage of the Xenopus CNC; the ability to dissect an almost pure population. Indeed, the Xenopus CNC can be distinguished morphologically from the rest of ectodermal derivatives (neural plate, epidermal ectoderm) as soon as stage 14 (early neurula stage) but does not migrate until the end of neurulation (stage 19) (Sadaghiani and Thiebaud, 1987) (Fig. 1A). This allows the isolation of a population of CNC free of any neural, placodal, ectodermal or mesodermal contaminants hours before they migrate. The purity of these explants has been ascertained by in situ hybridization (Alfandari et al., 2003) and qRT-PCR (Huang et al., 2016). Incidentally, these explants can also be performed at the post-migratory stage (Huang et al., 2016) (H. Cousin, unpublished observations). Another advantage of the Xenopus system is that the geometry of their embryos makes them amenable to time-lapse imaging in vivo. Other model system such as mouse and zebrafish embryos has a pronounced ventral curvature and a relatively isometric distribution of their tissues along the mediolateral and dorsoventral axis, making the visualization of the migration of the entirety of CNC challenging. In the case of some model systems, like fish, the localization of the yolk on the ventral side of the embryo further complicates visualization of the late events of CNC migration and development. The Xenopus embryos however, have flattened sides, giving an un-impeded view of the CNC migration from stage 20 onward.
Alfandari and colleagues developed the first in vitro CNC migration assays (Alfandari et al., 2003). While explants have been developed in other species and particularly chicken embryos, Xenopus offered many advantages over them. For example, as mentioned above, the CNC explant can be obtained pure with no contaminant that may complicate data analysis (e.g. the neural tube). The presence of yolk in the cells also frees the experimenters from adding any growth factors that could introduce experimental bias. The morphological analysis of these Xenopus CNC explant experiments yielded a few surprising findings. Firstly, fibronectin is both necessary and sufficient for Xenopus CNC migration. While the CNC express several fibronectin binding integrins, they only seem to use integrin α5β1 for their migration (Alfandari et al., 2003). Secondly, the behavior of these CNC during their migration in vitro is unique, including the formation of segments in a tissue autonomous manner (Fig. 2A). They also migrate in two clearly distinct phases. During the first phase of migration, the CNC cells adopt a pseudoepithelial morphology and keep strong contact with one another while migrating (Fig. 2A). This first phase lasts 4–5 h at 18 °C and was named at the time, sheet migration. During the second phase, the cells adopt a more mesenchymal morphology and undergo single cell migration (Fig. 2A). The sheet migration terminology has been renamed “collective cell migration” by Dr. Roberto Mayor, a historical term coined by cancer biologists used to described the migration pattern of metastatic cells. However, this renaming may, in retrospect, be unfortunate as mesenchymal CNC cells still requires extensive and transient cell-cell contacts with fellow CNC cells to migrate appropriately (Theveneau et al., 2010). Nonetheless, for the sake of continuity, we will refer CNC cells in their “sheet migration” phase as cell undergoing “collective migration”. The collective migration is also observed in vivo and this collectiveness proved to be essential for multiple aspects of CNC migration (see below). One example from the original publication showed that while intact CNC explants could not migrate on laminin, dissociated cells from the same stage explant could, demonstrating the importance of intercellular contacts to restrict the migration potential (Alfandari et al., 2003). The second phase of migration has yet to be confirmed in vivo but could be at the origin of the thorough invasion of cephalic tissues by the CNC, which is essential for their proper differentiation. This collective migration was met with skepticism at the time, as it seemed to be unique to the Xenopus CNC model. However it was not the first time these observations were made in the neural crest community and it has since been confirmed that avian cephalic NC keep short range contacts with one another throughout their migration (Erickson, 1985; Kulesa and Fraser, 1998; Teddy and Kulesa, 2004). The main difference is that the CNC of amniotes tend to migrate in chains while those of Xenopus migrate in sheets. Even more interesting is that collective migration is also observed in pathological types of cell migration including cancer metastasis (Bidard et al., 2008; Christiansen and Rajasekaran, 2006; Friedl and Wolf, 2003). In any case, the ability to study CNC migration both in vitro and in vivo has allowed investigators to perform extensive investigations of the molecular mechanisms of CNC migration, uncovering new roles for certain molecules such as ADAM metalloproteases and refining the role of others such as N-cadherin and Sdf1 (Alfandari et al., 2010; Cousin et al., 2011; Theveneau et al., 2010).
Fig. 2.
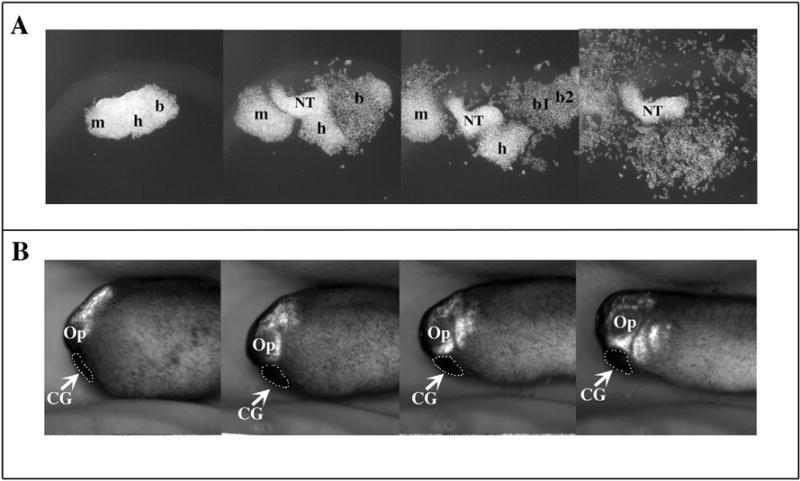
Migration of CNC cells in vitro and in vivo. GFP expressing CNC are explanted at stage 15 and either grown on a substrate of fibronectin in a saline media (A) or grafted into a wild type embryo (B) (anterior left, dorsal top). The migration of the CNC is followed by time-lapse photography (3 minute intervals, 10 h of total migration) and still pictures from these movies were extracted at regular time points throughout the movies. The migration of CNC in vitro (A) recapitulates the migration of the CNC observed in vivo (B) including the formation of segments (mandibular, hyoid, branchial), and the collective migration which is observed in the first 3 frames and the single cell migration which is observed in the last frame. Note that the explant in A was cut purposely large to demonstrate the formation of segments and therefore contains some neural tissue contaminant (NT). m: mandibular; h: hyoid segment b: branchial segments (b, b1, b2); CG: cement gland.
3. The neural crest as a model of physiological EMT and cell migration
Since 1999, CNC migration studies in the Xenopus model have greatly helped our understanding of the molecular underpinnings of neural crest induction and migration and many excellent reviews detail these discoveries (Scarpa et al., 2016; Simoes-Costa and Bronner, 2015; Theveneau and Mayor, 2012). For migration to occur, CNC need to separate from the neuroectodermal epithelium and then respond to various environmental cues to migrate ventrally. Both events rely on the ability of CNC to undergo at least partial EMT, which is achieved by changing the level of expression of various cadherins including E-cadherin, N-cadherin and cadherin-11 (Borchers et al., 2001; Scarpa et al., 2015; Theveneau et al., 2010). Then the cells respond to the chemoattractant Stromal-cell derived factor 1 Sdf1 (aka CXCL12), which is secreted by the cranial placodes and to which the CNC respond via their cognate receptor C-X-C Motif Chemokine Receptor 4 (CXCR4) (Theveneau et al., 2010). The requirement of Sdf1/CXCR4 for CNC migration appears also critical in other species (Duband et al., 2016; Escot et al., 2013; Olesnicky Killian et al., 2009; Rehimi et al., 2008; Yusuf et al., 2005). The response to Sdf1 is optimal when the CNC cells have physical contact with each other. CNC cells must stay together and while this appears at first counter intuitive, it is a major driver of CNC migration. As the leader cells (defined as the cells at front of the explant and therefore have a free edge) respond to the Sdf1 gradient, all CNC cells secrete the complement component 3a (C3a), which at this stage is used as a chemoattractant. CNC cells express the receptor C3aR and therefore respond to C3a by migrating toward one another (Carmona-Fontaine et al., 2011). This system allows the cells to stay together which is essential for the Sdf1/CXCR4 to function properly (Theveneau and Mayor, 2012). In order to keep cells moving once they contact one another, a system must be in place to reverse the effect of this C3a based mutual attraction. The Contact Inhibition of Locomotion (CIL) fulfills this function and many more (Carmona-Fontaine et al., 2008b).
CIL is a process whereby the migration of mesenchymal cells is affected by their interaction with other cells (Abercrombie and Heaysman, 1953). This process affects the migration of mesenchymal cells, whether they are involved in a pathological process, like cancer metastasis, or a physiological process, like CNC migration. The molecular mechanisms responsible for CIL vary from cell type and model system but they all depend on the cell-cell contact signaling machinery. In Xenopus, CIL requires the activation of the Wnt-Planar Cell Polarity (PCP) pathway. This activation requires N-cadherin and cadherin-11 and modulates the activity of small GTPases RhoA and Rac1 at a specific location within cells (Becker et al., 2013; Carmona-Fontaine et al., 2008a, 2008b; Matthews et al., 2008a, 2008b; Theveneau et al., 2010; Theveneau and Mayor, 2013). RhoA activates the cell’s cytoskeleton contraction, which is essential for retraction of the migratory cell’s trailing edge. Rac1 mediates new actin polymerization at the cells leading edge and therefore keeps the cell moving forward (Ridley and Hall, 1992; Ridley et al., 1992, 2003). The activation of Rac1 and RhoA by the PCP pathway results in the separation of cells at their point of contact (i.e. the trailing edge) and the development of a new leading edge at the opposite site ultimately leading the cells to migrate in opposite directions. The self-attraction of neural crest combined with the CIL is likely to account for the ability of CNC to migrate in vitro in the absence of chemoattractant. The transition from “sheet” migration to single cell migration observed in vitro could be the result of a loss of C3a responsiveness or the emergence of a putative scattering factor (Fig. 2A and B, last panel). Alternatively, the single cell migration could be the result of the down regulation of cadherins. In this regard, the inhibition of metalloproteases by a broad range inhibitor (Marimastat) decreases the number of single cells during the second phase of CNC migration in vitro but has no effect on the initial phase (McCusker et al., 2009). These metalloproteases are known to cleave various cell adhesion molecules, including cadherins (Maretzky et al., 2005; McCusker et al., 2009; Najy et al., 2008; Reiss et al., 2005; Uemura et al., 2006). Last but not least, many other extrinsic contributors to CNC migration, such as chemorepellents, also contribute to the directed migration and the proper homing of the CNC (Theveneau and Mayor, 2012). In summary, two opposite intrinsic forces provide a balance that stimulates migration away from the CNC mass (CIL) while maintaining cohesion between cells (co-attraction). Extrinsic factors provide directional cues to this dynamic system to move the entire cell population collectively.
4. Overview of cadherin function during CNC migration
Cadherins are members of a superfamily of single pass transmembrane proteins, originally described to mediate homophilic calcium-dependent cell-cell adhesion (Sano et al., 1993; Sotomayor et al., 2014). Members of this family contain a unique extracellular domain composed of between 5 and 34 repeats of cadherin motifs (called EC domains), the majority containing only 5 or 6 (Sotomayor et al., 2014). They are the primary molecules responsible for the differential adhesion principle observed by Townes and Holtfreter (1955) and defined by Steinberg (1963, 1964) which provides a simple yet elegant way to segregate cells into various tissues and organs based on quantitative differences in cell-cell adhesion (Gumbiner, 2005; Nelson, 2008; Stepniak et al., 2009). Other functions have been described since then and many new members have been identified based on their molecular structure and function. Based on the sequence of their cytoplasmic domain and function, cadherins are classified into classical (type I or type II), desmosomal (desmocolin and desmoglein), protocadherins (alpha, beta and gamma) and unclassified (aka epsilon) (Sotomayor et al., 2014).
4.1. The type I cadherins
The classical type I cadherins typically mediate homotypic cell-cell adhesion while anchoring to the cytoskeleton by binding to catenins. N-cadherin and E-cadherin are grouped in that category. As described above, N-Cadherin has to be expressed for CNC to migrate. Conversely, E-Cadherin expression is downregulated at the beginning of CNC migration. This downregulation has been observed in many EMT and migration events, including cancer cell metastasis and mesoderm ingression during amniotes gastrulation (Cano et al., 2000; Ciruna et al., 1997; Damjanov et al., 1986; Edelman et al., 1983). While the downregulation of E-cadherin and the up-regulation of N-cadherin is often used as an indicator of EMT, data are accumulating showing that this downregulation may not be strictly required for the acquisition of cell motility, at least in some instances such as mesoderm ingression or the metastasis of certain types of cancer (discussed below). New findings by Huang and colleagues show that in CNC, E-cadherin protein function may in fact be required for cell migration and we will discuss their results in the last section of this review (Huang et al., 2016).
4.2. The type II cadherins
The type II cadherins are traditionally expressed in mesenchymal cells. They usually display weaker homophilic binding properties. In chickens, Cadherin 6, 6B, 7 and 11 play an important role during NC migration (Chalpe et al., 2010; Clay and Halloran, 2014; Coles et al., 2007; Inoue et al., 1997; Nakagawa and Takeichi, 1995; Park and Gumbiner, 2010, 2012; Simoes-Costa and Bronner, 2015) In Xenopus, cadherin-11 seems to be the main type II cadherin (Hadeball et al., 1998; Vallin et al., 1998). In Xenopus, cadherin-11 is quite specifically expressed in the neural crest and is essential for CNC migration. Its function requires both the intracellular and the extracellular domains. The cadherin-11 extracellular domain is cleaved by the metalloproteases ADAM9 and 13 generating a fragment EC 1–3 containing the first 3 cadherin repeats, that is necessary for CNC migration (McCusker et al., 2009). This fragment can only be detected at the onset of migration and the replacement of endogenous cadherin-11 by an engineered uncleavable form of the protein does not support CNC migration, demonstrating that cadherin-11 cleavage is essential for neural crest migration (Abbruzzese et al., 2016; McCusker et al., 2009). Since the homophilic binding domain of EC1–3 is dispensable for CNC migration, it is unlikely that EC1–3 acts as an antagonist of homophilic interactions between CNC cells (Abbruzzese et al., 2016). It is interesting to note that ADAM metalloproteases can also shed N- and E-cadherin extracellular domains in vitro (Najy et al., 2008; Reiss et al., 2005) and that these N-terminal fragments also have biological properties such as activating Receptor Tyrosine Kinases (Cifuentes-Diaz et al., 1994; Najy et al., 2008; Paradies and Grunwald, 1993). It is therefore possible that the cadherin-11 EC1–3 fragment may do the same. In addition, the depletion of the extracellular domain of cadherin-11 or the overexpression of the EC1–3 fragment in Xenopus leads to loss of CIL and directionality of the CNC (Abbruzzese et al., 2016; Becker et al., 2013). The intracellular domain of cadherin-11 has also a crucial function for CNC migration. It binds to the guanidine exchange factor (GEF) trio and modulates small Rho-GTPases, which in turn, are essential for the formation of filopodia and lamelipodia during cell migration (Kashef et al., 2009). Recently, cadherin-11 was shown to localize to nascent focal adhesion and to interact with Syndecan-4 suggesting that this cadherin may regulate cell ECM interaction in the neural crest (Langhe et al., 2016).
4.3. The protocadherins
Protocadherins are non-classical cadherins that contain 5 EC repeats or more in their extracellular domain and lack a docking site for β-catenin in the cytoplasmic domain (Angst et al., 2001; Sotomayor et al., 2014). Like many classical cadherins, protocadherins play a role in the differential adhesion events during embryogenesis (Chen and Gumbiner, 2006). While protocadherins are capable of homophilic adhesion, they are weak (Chen and Gumbiner, 2006; Sotomayor et al., 2014). They can also mediate differential cell adhesion by modulating classical cadherin based interactions (Chen and Gumbiner, 2006; Rangarajan et al., 2006; Schneider et al., 2014). For example, AXPC (Axial protocadherin) and PAPC (paraxial protocadherin) modulate C-cadherin activity during gastrulation to separate the axial mesoderm (future notochord) from the paraxial mesoderm (future somites) (Kuroda et al., 2002; Yamamoto et al., 1998). In the case of PAPC, it has been shown that it does so in a Wnt11/Fz7 dependent manner (Kim et al., 1998; Kraft et al., 2012). In Xenopus CNC, only PCNS and PAPC have been shown to have a function during their migration. PCNS (Protocadherin expressed in Neural Crest and Somites) is the ho-molog of human PCDH 8.1 and as the name suggests, is expressed in the CNC (both premigratory and migratory) as well as two pairs of somites undergoing or having recently undergone segmentation (Rangarajan et al., 2006). The loss of PCNS in the CNC prevents their migration in vivo and in vitro. PAPC (paraxial protocadherin), the homolog of human PCDH 8.2, is also expressed in the CNC but at levels barely detectable by in situ hybridization (Schneider et al., 2014). While its knock down does not affect CNC migration, its misexpression rescues the loss of PCNS effect on CNC migration (Schneider et al., 2014). Considering that PCNS and PAPC share 65% amino acid identity, this indicate that the PCNS domain involved in CNC migration is also present in PAPC. Interestingly, while PAPC is able to mediate cell sorting in animal cap ex-plants, PCNS cannot, indicating that PCNS function is unlikely to be related to its ability to modulate homophilic cadherin interactions (Rangarajan et al., 2006).
5. E-cadherin versus N-cadherin: switch or change in function?
5.1. Dynamic cadherin expression during the various stages of NC development
For the neural crest to undergo migration, the NC needs to first separate themselves from the neuroectoderm and ectoderm sensory layer (Fig. 2A). The molecular underpinnings of these transitions have been extensively studied in the trunk neural crest cells. To achieve tissue segregation, the NC decrease the expression of type I cadherins like N-cadherin and increase the expression of type II cadherins like cadherin-11, 6B) (Akitaya and Bronner-Fraser, 1992; Coles et al., 2007; Dady et al., 2012; Duband et al., 1988; Nakagawa and Takeichi, 1995; Nieto et al., 2016; Taneyhill et al., 2007). To initiate NC migration, the cells need to undergo either partial or complete EMT, which entails the down regulation of E-cadherin, and the activation of the migration machinery. It is important to note that in the trunk, the neural crest cells are part of the dorsal neural tube within an epithelium surrounded by a basement membrane prior to their emigration. The change of expression in cadherins, called cadherin switch, is pivotal in both instances (Duband et al., 1988; Nieto et al., 2016). However, in the CNC, which are not part of the closing neural tube, persistent expression of E-cadherin has been observed in pre-migratory and early migratory stages (Dady et al., 2012; Lee et al., 2013). While these observations need to be further explored in other species, they suggest that the CNC, at least in two animal species, may have slightly different mechanism to achieve their migration. The discovery of a weak but physiologically relevant expression of E-cadherin in Xenopus CNC leads credence to that line of thought.
In Xenopus CNC, a complete description of the junction proteins during development is lacking and therefore the exact status of the EMT prior to and during migration is not clear. The collective behavior of CNC suggests that the EMT may be partial with the cells keeping significant cell-cell adhesions for the first few hours of their migration. This behavior could be correlated with a robust expression of the N-cadherin (Dady et al., 2012; Duband et al., 1988; Lee et al., 2013; Nieto et al., 2016; Scarpa et al., 2015; Theveneau et al., 2010; Theveneau and Mayor, 2013). In other species, the expression of N-cadherin in the NC has also been observed (Duband et al., 1988; Monier-Gavelle and Duband, 1995; Piloto and Schilling, 2010; Powell et al., 2015; Xu et al., 2001). The cadherin switch seen during EMT events such as cancer metastasis, can be described to happen in either two ways. The first is the “swap” of expression between two cadherins (e.g. E-cadherin off and N-cadherin on). The second the “turning on” of the expression of one (or more) cadherin necessary for cell motility (e.g. N cadherin and cadherin-11 need to be expressed in CNC) (Wheelock et al., 2008). The neural crest studies done so far, performed in majority on trunk neural crest inferred that the “swap” between E and N-cadherin is employed to initiate their migration. However, the data recently accumulated on the CNC in chick, mouse and now Xenopus seems to indicate that a remnant of E-cadherin is still present in these CNC and may have a physiological role to play during their migration (Dady et al., 2012; Huang et al., 2016; Lee et al., 2013). It implies that the cadherin switch in that case fit with the model of a rheostat.
The initiation of the cadherin switch usually happens through the expression of transcription factors. Slug (Snail1) and Snail (Snail2) negatively regulate the expression of type I cadherins in both the Neural Crest and cancer cells (Chaffer et al., 2016; Simoes-Costa and Bronner, 2015). During embryo development, these transcription factors are expressed strongly and specifically in the neural crest across the phylum (Nieto et al., 2016). In addition to the transcriptional regulation, other factors have been shown to contribute to the cadherin switch including, proteolytic cleavage, increased turn-over of E and N-cadherin as well as a change in surface localization of N-cadherin in the premigratory and migratory CNC (Nieto et al., 2016; Wheelock et al., 2008).
5.2. N-cadherin presence is essential for CNC migration
N-cadherin has been implicated in different aspects of NC migration. In Xenopus, it plays at least 2 critical roles. First, it mediates the CIL between CNC cells, which is, as we have seen above, one of the main engines behind their migration (Theveneau et al., 2010). Second, it mediates the repulsion between CNC cells and placodes, which plays a critical role during CNC migration (Theveneau et al., 2013). As mentioned above, cranial placodal ectoderm is the source of the chemoattactant Sdf1. However, these placodes are localized close to the neural tube at the onset of CNC migration, which begs the question of how the CNC are able to migrate past the placodes toward the ventral-most part of the embryo. Theveneau and colleagues elegantly addressed the issue when they uncovered the “chase and run” behavior between the placodes and the CNC (Theveneau et al., 2010). Once the CNC reach the placodes, they form transitory homophilic N-cadherin based adhesions, resulting in Rac1 inhibition in these cells and therefore leading to CIL. In response to CIL, some of the placodes move ventrally, away from the CNC (the “run” part of the process). As they move, so does the Sdf1 source, attracting the CNC to the placode once more (the “chase” part of the process). This system is truly ingenious as it allows the CNC, attracted by the placodes, to migrate ventrally and the placodes to be pushed toward their final destination. It is important to notice that as the epibranchial placodes still express E-cadherin, these cells stay together while achieving a collective migration on their own. It is possible that, thanks to CIL, the emigrating CNC may even be responsible for the elongated form of some of these placodes such as the epibranchials (Fig. 1B). These events imply dynamic subcellular locali-zation of N-cadherin and the molecular underpinings of this dynamic are currently under investigation (Hardy et al., 2011; Powell et al., 2015).
5.3. E-cadherin downregulation is essential for CNC migration
The importance of E-cadherin down regulation at the onset of CNC migration has been documented in many model systems but it has been recently revisited in Xenopus (Scarpa et al., 2015). Xenopus CNC CIL is drastically increased at the onset of the migratory stage, which correlates with the loss of E-cadherin expression and the re-expression the N-cadherin. Overexpression of E-cadherin is sufficient to block the emergence of CIL behavior in migratory stage CNC and prevent their migration. If dissociated, single CNC cell can migrate, indicating that the basal cell machinery for migration is intact. The CNC migration defects were observed upon overexpression of E-cadherin but not N-cadherin. Lastly, the effect of E-cadherin misexpression depends exclusively on the p120-catenin docking site present in the cytoplasmic domain. Migration of E-cadherin overexpressing CNC could be rescued by decreasing p120 catenin expression. Importantly, decreasing p120 catenin did not affect E-cadherin and N-cadherin levels in the cells. The authors concluded that the presence of E-cadherin in CNC explants controls the localization of active rac1 in a p120 catenin dependent manner that ultimately results in the inhibition of protrusions formed at the free edge of pre-migratory CNC. This mechanism would be used to prevent precautious migration of CNC until E-cadherin level drop below a threshold. In support of this model, reducing E-cadherin levels by morpholino knock down induced early CNC migration and CIL.
5.4. E-cadherin plays a role during CNC migration
Scarpa and colleagues were the first to report the effect of E-cadherin loss on CNC. The reduction of E-cadherin in pre-migratory neural crest cells resulted in a polarity reversal with protrusion pointing toward the cell free space, as expected for migratory CNC cells subject to CIL. Consistent with this apparent precocious activation of CIL, they also observed an inhibition of CNC cell intermixing, another property of migratory CNC. Interestingly, the authors have performed the loss of function experiments using multiple techniques, including a function-blocking antibody that prevents E-cadherin homophilic binding (Choi and Gumbiner, 1989). While most of their work focuses on the role of the intracellular domain, this particular experiment suggests that the extracellular domain of E-cadherin may also have a function during CNC development. This is consistent with the idea that E-cadherin might aid CNC migration. While these results are consistent with a key role of E-Cadherin in pre-migratory CNC cells, they do not address a potential role of E-cadherin during CNC migration. Unfortunately, Scarpa and colleague did not investigate or report the loss of function experiments in migratory stage CNC in vitro or in vivo. This is understandable as the authors did not expect to have any expression of E-cadherin in migrating CNC and were therefore using the gain of function approach in the migrating CNC stages. Huang and colleagues have furthered the investigation of E-cadherin role during CNC migration (Huang et al., 2016).
At first glance, Huang and colleagues’ results seem at odds with the concept that E-cadherin needs to be turned off in order for cells to achieve EMT and migrate. These authors showed that the transcription of E-cadherin (and many other type I cadherins) is actually increased in vivo throughout CNC migration and that the E-cadherin protein is present in migrating CNC cell at least at stage 25. Using morpholinos, they showed that E-cadherin knock down inhibits CNC migration and that this inhibition can only be rescued by the expression of a morpholino-resistant E-cadherin. No other cadherin tested was able to rescue the defect strongly arguing for the specificity of the phenotype (XB-cadherin, N-cadherin and cadherin-11). Rescue experiments performed with mutant forms of E-cadherin showed that the cytoplasmic domain was dispensable. However, the extracellular domain and more particularly the homophilic binding site is essential suggesting that E-cadherin homophilic adhesion is required for CNC migration. In vitro, CNC lacking E-cadherin display a rounded morphology, and had a decreased protrusion activity.
Some of these findings conflict with previous observations. Huang and colleagues were the first to report a clear and dramatic increase of E-cadherin transcripts in the CNC during their migration using purified isolated CNC (E-cadherin transcript number per cell goes from 4.2 to 16.7) (Huang et al., 2016). However, the presence of E-cadherin protein in the CNC seems to conflict directly with a previous report (Theveneau et al., 2013). Both groups performed immunofluorescence on stage 25 embryos sections using two different but well characterized monoclonal antibodies raised against the extracellular domain. The resolution of this apparent conflict came from the work of Scarpa et al. (2015). Immunofluorescence performed on CNC explants at both pre-migratory and migratory stages showed that while there is a clear reduction of E-cadherin between premigratory and migratory stages, E-cadherin can still be detected at the migratory stage. While there is admittedly very little E-cadherin present, the profound effect of E-cadherin knockdown reported by Huang and colleagues coupled to all of the controls they performed suggest that this small amount of E-cadherin has an important role during CNC migration. Immunoprecipitation from labeled cell surface CNC or western blot of isolated CNC explants will have to be done to confirm the presence of E-cadherin at the cell surface as well as the presence of potential cleavage fragments. Using the E-cadherin function-blocking antibody during CNC migration would also confirm the loss of function finding. It would also be interesting to see if the amount of E-cadherin found in the CNC correlates or not with the increase of E-cadherin transcripts and if not, investigate the turnover of E-cadherin at the surface of the CNC. If it does not, it would mean that these transcripts accumulating in the CNC are actually not translated at the time of their migration and would open up another line of investigation as to why CNC would accumulate these untranslated transcripts.
The strongest case for the presence and function of E-cadherin during CNC migration comes from the loss of function experiments performed by the Kashef group (Huang et al., 2016). This line of evidence is well controlled, including the rescue of the defects by the re-expression of physiological levels of E-cadherin. They also showed that the knock down did not affect the expression of cadherin-11, XB cadherin or even more importantly N-cadherin. E-cadherin knock down does not appear to affect neural crest specification as the expression of neural crest markers AP2, c-Myc and Twist remain unchanged. This indicates that the effect of E-cadherin loss is truly specific to E-cadherin and involves translation of these mRNAs. The presence or lack of E-cadherin in the CNC at the time of their migration in other species is poorly described. The reduction of E-cadherin expression in chicken heads at the time of CNC has been reported (Rogers et al., 2013) but the head is hardly a pure population of neural crest. More recently, a comprehensive and systematic analysis of E- and N-cadherin expression in chicken embryo that revealed that cranial neural crest expressed E-cadherin protein throughout delamination and at least the initial stages of migration (Dady et al., 2012). This proved to be true of mouse CNC as well (Lee et al., 2013). To our knowledge, nobody has shown whether E-cadherin altogether disappears in CNC in other species. There is however a consensus that E-cadherin needs to be downregulated in order for the CNC to form and migrate, an observation that was confirmed by the gain of function experiments (Schafer et al., 2014). The findings by Huang and colleagues suggest that, while downregulation is important for the Neural Crest development, the presence of some E-cadherin is required for CNC migration. The presence of E-cadherin protein in mouse and chick CNC at premigratory and early migratory stages suggest that this role of E-cadherin is conserved, at least in the tetrapod lineage. Whether the critical evolutionary conserved role of E-cadherin is to maintain the pre-migratory status or to actually promote CNC migration or both remains to be determined.
Another discrepancy that can be noticed between the Scarpa and Huang manuscripts is the behavior of CNC cells lacking E-cadherin in vitro. Huang and colleagues reported that E-cadherin knock down cells display cell blebbing and a decrease in protrusive activity in vitro. Looking closely at the movies, the cell blebbing appears to correlate with a decreased cell adhesion on the substrate, which would correlate well with the decrease in cell protrusion the author reported. On the other hand, the experiment performed by Scarpa and colleagues at pre-migratory stages shows no gross defect in cell spreading and the authors do not report any defects other than the reversal of the protrusive activity mentioned earlier. The migration of CNC in vitro is sensitive to multiple factors including the stage of the embryos at the time of the dissection, the quality of the substrate and the temperature at which the migration is performed. It is our observation that when the explants are dissected early, they sometime partially heal and attach to the substrates less efficiently. Of course one of the key aspects is the concentration of coated fibronectin and to a lesser extent the stiffness of the substrate. We originally described a range of acceptable FN coating concentrations on non-tissue culture treated plastic dishes (Alfandari et al., 2003). The lower concentration (1 to 5 μg/ml) tends to lead to more variability while 10 to 20 μg/ml is optimum. To obtain the same coating concentration on glass, we have had to use up to 50 μg FN/ml with variable results or alternatively coat gelatin first (1 mg/ml) so that the fi-bronectin (10 to 20 μg/ml) binds efficiently to the gelatin coat. Based on the methods of both papers, a low coating concentration by Huang and colleagues may have helped them visualize a subtle difference in adhesion that may have been absent at higher FN concentrations (Scarpa et al., 2015). Lastly, the temperature at which the explants are incubated can affect the migration. While Xenopus CNC can migrate in a wide range of temperature (15 °C to 20 °C), higher temperatures (above 18 °C) tend to amplify subtle defect in CNC migration and could account for the difference in phenotype between the 2 studies. The possibility of morpholino usage discrepancy between the two groups is remote as both groups used the same morpholino (Nandadasa et al., 2009).
One needs to be careful when interpreting experiments where cadherin levels are manipulated. The manipulation of cadherin levels can have important repercussions on the biogenesis of other cadherins and on the activity of the Wnt signaling pathway. Both have important repercussions in the context of CNC development. The overexpression of classical cadherin has been shown to affect the pool of available β-catenin and therefore affect canonical Wnt signaling pathway (Koehler et al., 2013). While the Wnt/β-catenin pathway is critical for the specification of the neural crest (Simoes-Costa and Bronner, 2015). More recently, it has been reported that the Wnt/β-catenin signaling pathway need to be carefully regulated during CNC migration in frogs (Maj et al., 2016). Cadherin-11 overexpression reduces the pool of β-catenin available for Wnt signaling pathway. This was demonstrated by rescuing the expression of key markers as well as CNC migration by overexpressing β-catenin (Borchers et al., 2001). For the E-cadherin study, Q-PCR and in situ hybridization showed that there is no change in AP2 and cMyc expression in morphant embryos (Huang et al., 2016). While these tests have not been performed in the case of gain of function experiments, the isolated CNC cells appeared to migrate properly in vitro, arguing against an overall defect in CNC specification (Scarpa et al., 2015). Similarly, changing the expression of one cadherin has been reported to affect the expression of another (Rogers et al., 2013) although not always (Coles et al., 2007). Considering that N-cadherin expression is essential for CNC migration and that the misregulation of one cadherin can alter the expression of others, Scarpa and colleagues have evaluated the expression of N-cadherin in embryos overexpressing E-cadherin by western blot (Scarpa et al., 2015). The quantification of their blot shows a small but detectable decrease in N-cadherin. A careful statistical analysis of the quantification they performed should determine whether or not this decrease is in fact statistically significant and if it is, whether is it enough to contribute to the lack of CNC migration. However, the inability of N-cadherin to rescue the E-cadherin knock down suggests that the observed phenotype is not due to a decrease in N-cadherin.
6. Is E-cadherin presence and requirement during CNC migration so surprising?
The role of E-cadherin in CNC migration is counter-intuitive for two reasons. First because the classical model of EMT based on the trunk neural crest cell emigration from the neural tube requires a downregulation of E-cadherin. Second, because E-cadherin is a critical component of the adherens junction, a structure that maintains stability of epithelium and keep cells anchored to one another. Our questions should be, are there other evidence of EMT in which E-cadherin is maintained? And is there evidence of E-cadherin actively participating in cellular migrations?
The report of E-cadherin expression in a tissue undergoing EMT is not the first one. In cancer, many tumors have been shown to downregulate E-cadherin and upregulate N-cadherin at the onset of metastasis. This switch is directly correlated to their ability to metastasize and is used as a prognosis indicator (Halbleib and Nelson, 2006; Islam et al., 1996; Johnson et al., 2004; Shimazui et al., 1996). However there are a few instances of tumors such as breast and ovarian tumors that does not downregulate E-cadherin or even upregulate it (Halbleib and Nelson, 2006; Kleer et al., 2001; Kowalski et al., 2003; Nieman et al., 1999; Orlichenko et al., 2010; Wheelock et al., 2008). During embryo-genesis, similar observations have been made. During fruit fly gastrulation, and more particularly the mesoderm invagination, the overall level of E-cadherin is similar between the ectoderm and the mesoderm (Schafer et al., 2014). This suggests that the presence of E-cadherin in the tissue is not detrimental for gastrulation. While these results indicate that E-cadherin presence is not detrimental to cell movements, other results shows that E-cadherin is actually necessary for these movements. For example, E-cadherin is critical for epiboly during zebrafish gastrulation, (Babb and Marrs, 2004; Kane et al., 2005; Shimizu et al., 2005). This E-cadherin mediates cell intercalations of the blastomeres during epiboly (Bruce, 2016). Interestingly, E-cadherin activity is tightly regulated by various factors. The transcription factor Oct4/Pou5f1 regulates the endosomal trafficking of E-cadherin and the dynamic re-localization of E-cadherin throughout epiboly processes is critical for gastrulation (Song et al., 2013). Interestingly, EGF signaling also affects E-cadherin mediated cell movements, presumably by modulating p120 catenin (Song et al., 2013). E-cadherin has also been shown to be essential for other collective cell migrations. During drosophila oogenenesis, a group of cells called border cells migrate collectively from one end of the egg chamber toward the oocyte through many layers of nurse cells (Montell et al., 2012). The inhibition of E-cadherin in these cells leads to severe migration defects. The authors showed that cluster cells achieve this migration by forming homotypic E-cadherin based adhesions with the nurse cells (Cai et al., 2014). In light of these data, it would be interesting to investigate whether CNC could also use the E-cadherin-based homophilic interactions between the CNC and surrounding tissues (e.g. ectoderm).
In summary, CNC cells need to down regulate their E-cadherin expression in order to initiate directed migration. The negative effect of E-cadherin on CNC EMT resides within its cytoplasmic domain and particularly its ability to bind p120 catenin, a master regulator of cell-cell adhesion and motility (Kourtidis et al., 2013). However, in Xenopus at least, CNC need to maintain some E-cadherin expression in order for the cells to migrate properly, and that function relies on the extracellular domain and in particular the residues responsible for homophilic cell interaction. As shown for cadherin-11, it would be interesting to determine if the separation of the two domain function (intracellular vs extracellular) could involve proteolytic cleavage and could explain the difficulty to detect the intact E-cadherin in CNC using either extracellular or intracellular antibodies.
7. What role might the E-cadherin remnant have on CNC migration?
E-cadherin could affect CNC migration in different ways. It could complex or otherwise interact with other transmembrane proteins and modulate signaling pathways necessary for CNC migration. This behavior has been described for other cadherins such as N-cadherin interacting with the FGF receptor (Cavallaro and Christofori, 2004; Williams et al., 2001). Another possibility is that the E-cadherin ectodomain is cleaved by a metalloprotease such as an ADAM (Maretzky et al., 2005; Najy et al., 2008; Reiss et al., 2005) to produce independent N- and C-terminal fragments (NTF and CTF) with distinct functions. The resulting extracellular domain fragment (NTF) could participate in loosening cell-cell contact by competing with cadherin mediated homophilic adhesion (Wheelock et al., 1987). While E-cadherin cleavage has yet to be demonstrated in Xenopus CNC, the E-cadherin homophilic binding domain requirement for CNC migration seem consistent with that interpretation (Huang et al., 2016). However, cadherin extracellular fragments are also capable of interfering with signaling pathways. In CNC, cadherin-11 is cleaved by ADAM13 and the released EC1–3 is required for CNC migration (McCusker et al., 2009). Since the EC1–3 activity does not rely on the homophilic binding site, it is unlikely to play a role by competing with cadherin mediated cell-cell adhesion (Abbruzzese et al., 2016). Instead, it may be required to activate a de-fined signaling pathway necessary for CNC migration (Methavan et al., in preparation). Similarly, the N-cadherin NTF has been shown to activate the FGFR-Akt-PI3K pathway while a E-cadherin NTF has been shown to activate the EGF signaling pathway (Her2 and Her3), resulting in increased cell migration in vitro (Brouxhon et al., 2014; Kohutek et al., 2009; Lyon et al., 2009; Najy et al., 2008). Cadherin cytoplasmic domain cleavage by gamma secretase has been reported for both E and N cadherin in vitro (Marambaud et al., 2002; Marambaud et al., 2003; Uemura et al., 2006). If it were shown to happen for E-cadherin in the context of CNC, it would provide an elegant way to remove any lingering negative impact of E-Cadherin cytoplasmic domain on CNC migration and possibly protect β-catenin from degradation during the transit toward the nucleus. Recently, the cleaved CTF of cadherin-6B was shown to associate with β-catenin, translocate into the nucleus and regulate the expression of multiple genes associated with EMT in Chick neural crest (Schiffmacher et al., 2016).
In conclusion, the current view of the cadherin switch is more complex than the turn off/on mechanism used currently. The recent literature points out that E-cadherin has various roles during the CNC formation and migration. In premigratory CNC, E-cadherin is likely necessary to keep the CNC together and, by inhibiting CIL though its cytoplasmic domain could prevent them from migrating too early. At the onset of migration, the CNC activate CIL by upregulating N-cadherin and down regulating E-Cadherin. However, CNC cells need to keep a certain amount of E-cadherin expressed for their migration to occur properly. This function depends in the extracellular domain in general, and the homotypic-binding domain in particular.
Acknowledgments
The author would like to thank Drs. Dominique Alfandari, Samuel Black and Craig Albertson for their comments on the manuscript. H.C. is supported by NIH/NIDCR DE025692.
References
- Abbruzzese G, Becker SF, Kashef J, Alfandari D. ADAM13 cleavage of cadherin-11 promotes CNC migration independently of the homophilic binding site. Dev Biol. 2016;415:383–390. doi: 10.1016/j.ydbio.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res. 1953;5:111–131. doi: 10.1016/0014-4827(53)90098-6. [DOI] [PubMed] [Google Scholar]
- Akitaya T, Bronner-Fraser M. Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev Dyn. 1992;194:12–20. doi: 10.1002/aja.1001940103. [DOI] [PubMed] [Google Scholar]
- Alfandari D, Cousin H, Gaultier A, Hoffstrom BG, DeSimone DW. Integrin alpha5beta1 supports the migration of Xenopus cranial neural crest on fibronectin. Dev Biol. 2003;260:449–464. doi: 10.1016/s0012-1606(03)00277-x. [DOI] [PubMed] [Google Scholar]
- Alfandari D, Cousin H, Marsden M. Mechanism of Xenopus cranial neural crest cell migration. Cell Adhes Migr. 2010;4:553–560. doi: 10.4161/cam.4.4.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–641. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- Babb SG, Marrs JA. E-cadherin regulates cell movements and tissue formation in early zebrafish embryos. Dev Dyn. 2004;230:263–277. doi: 10.1002/dvdy.20057. [DOI] [PubMed] [Google Scholar]
- Becker SF, Mayor R, Kashef J. Cadherin-11 mediates contact inhibition of locomotion during Xenopus neural crest cell migration. PLoS One. 2013;8:e85717. doi: 10.1371/journal.pone.0085717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidard FC, Pierga JY, Vincent-Salomon A, Poupon MF. A “class action” against the microenvironment: do cancer cells cooperate in metastasis? Cancer Metastasis Rev. 2008;27:5–10. doi: 10.1007/s10555-007-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers A, Epperlein HH, Wedlich D. An assay system to study migratory be-havior of cranial neural crest cells in Xenopus. Dev Genes Evol. 2000;210:217–222. doi: 10.1007/s004270050307. [DOI] [PubMed] [Google Scholar]
- Borchers A, David R, Wedlich D. Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development. 2001;128:3049–3060. doi: 10.1242/dev.128.16.3049. [DOI] [PubMed] [Google Scholar]
- Brouxhon SM, Kyrkanides S, Teng X, O’Banion MK, Clarke R, Byers S, Ma L. Soluble-E-cadherin activates HER and IAP family members in HER2+ and TNBC human breast cancers. Mol Carcinog. 2014;53:893–906. doi: 10.1002/mc.22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AE. Zebrafish epiboly: spreading thin over the yolk. Dev Dyn. 2016;245:244–258. doi: 10.1002/dvdy.24353. [DOI] [PubMed] [Google Scholar]
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell. 2014;157:1146–1159. doi: 10.1016/j.cell.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Carl TF, Dufton C, Hanken J, Klymkowsky MW. Inhibition of neural crest migration in Xenopus using antisense slug RNA. Dev Biol. 1999;213:101–115. doi: 10.1006/dbio.1999.9320. [DOI] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Matthews H, Mayor R. Directional cell migration in vivo: Wnt at the crest. Cell Adhes Migr. 2008a;2:240–242. doi: 10.4161/cam.2.4.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, Stern CD, Mayor R. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008b;456:957–961. doi: 10.1038/nature07441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Theveneau E, Tzekou A, Tada M, Woods M, Page KM, Parsons M, Lambris JD, Mayor R. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev Cell. 2011;21:1026–1037. doi: 10.1016/j.devcel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–654. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- Chalpe AJ, Prasad M, Henke AJ, Paulson AF. Regulation of cadherin expression in the chicken neural crest by the Wnt/beta-catenin signaling pathway. Cell Adhes Migr. 2010;4:431–438. doi: 10.4161/cam.4.3.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol. 2006;174:301–313. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Gumbiner B. Expression of cell adhesion molecule E-cadherin in Xenopus embryos begins at gastrulation and predominates in the ectoderm. J Cell Biol. 1989;108:2449–2458. doi: 10.1083/jcb.108.6.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- Cifuentes-Diaz C, Nicolet M, Goudou D, Rieger F, Mege RM. N-cadherin expression in developing, adult and denervated chicken neuromuscular system: accumulations at both the neuromuscular junction and the node of Ranvier. Development. 1994;120:1–11. doi: 10.1242/dev.120.1.1. [DOI] [PubMed] [Google Scholar]
- Ciruna BG, Schwartz L, Harpal K, Yamaguchi TP, Rossant J. Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: a role for FGFR1 in morphoge-netic movement through the primitive streak. Development. 1997;124:2829–2841. doi: 10.1242/dev.124.14.2829. [DOI] [PubMed] [Google Scholar]
- Clay MR, Halloran MC. Cadherin 6 promotes neural crest cell detachment via F-actin regulation and influences active rho distribution during epithelial-to-mesenchymal transition. Development. 2014;141:2506–2515. doi: 10.1242/dev.105551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles EG, Taneyhill LA, Bronner-Fraser M. A critical role for Cadherin6B in regulating avian neural crest emigration. Dev Biol. 2007;312:533–544. doi: 10.1016/j.ydbio.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin H, Abbruzzese G, Kerdavid E, Gaultier A, Alfandari D. Translocation of the cytoplasmic domain of ADAM13 to the nucleus is essential for Calpain8-a expression and cranial neural crest cell migration. Dev Cell. 2011;20:256–263. doi: 10.1016/j.devcel.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dady A, Blavet C, Duband JL. Timing and kinetics of E- to N-cadherin switch during neurulation in the avian embryo. Dev Dyn. 2012;241:1333–1349. doi: 10.1002/dvdy.23813. [DOI] [PubMed] [Google Scholar]
- Damjanov I, Damjanov A, Damsky CH. Developmentally regulated expression of the cell-cell adhesion glycoprotein cell-CAM 120/80 in peri-implantation mouse embryos and extraembryonic membranes. Dev Biol. 1986;116:194–202. doi: 10.1016/0012-1606(86)90056-4. [DOI] [PubMed] [Google Scholar]
- Duband JL, Volberg T, Sabanay I, Thiery JP, Geiger B. Spatial and temporal distribution of the adherens-junction-associated adhesion molecule A-CAM during avian embryogenesis. Development. 1988;103:325–344. doi: 10.1242/dev.103.2.325. [DOI] [PubMed] [Google Scholar]
- Duband JL, Escot S, Fournier-Thibault C. SDF1-CXCR4 signaling: a new player involved in DiGeorge/22q11-deletion syndrome. Rare Dis. 2016;4:e1195050. doi: 10.1080/21675511.2016.1195050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM, Gallin WJ, Delouvee A, Cunningham BA, Thiery JP. Early epochal maps of two different cell adhesion molecules. Proc Natl Acad Sci U S A. 1983;80:4384–4388. doi: 10.1073/pnas.80.14.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson CA. Control of neural crest cell dispersion in the trunk of the avian embryo. Dev Biol. 1985;111:138–157. doi: 10.1016/0012-1606(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Escot S, Blavet C, Hartle S, Duband JL, Fournier-Thibault C. Misregulation of SDF1-CXCR4 signaling impairs early cardiac neural crest cell migration leading to conotruncal defects. Circ Res. 2013;113:505–516. doi: 10.1161/CIRCRESAHA.113.301333. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Hadeball B, Borchers A, Wedlich D. Xenopus cadherin-11 (Xcadherin-11) expression requires the Wg/Wnt signal. Mech Dev. 1998;72:101–113. doi: 10.1016/s0925-4773(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- Hardy KM, Yatskievych TA, Konieczka J, Bobbs AS, Antin PB. FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression. BMC Dev Biol. 2011;11:20. doi: 10.1186/1471-213X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörstadius S. The Neural Crest. Oxford University Press; Oxford: 1950. [Google Scholar]
- Huang C, Kratzer MC, Wedlich D, Kashef J. E-cadherin is required for cranial neural crest migration in Xenopus laevis. Dev Biol. 2016;411:159–171. doi: 10.1016/j.ydbio.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Inoue T, Chisaka O, Matsunami H, Takeichi M. Cadherin-6 expression transiently delineates specific rhombomeres, other neural tube subdivisions, and neural crest subpopulations in mouse embryos. Dev Biol. 1997;183:183–194. doi: 10.1006/dbio.1996.8501. [DOI] [PubMed] [Google Scholar]
- Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol. 1996;135:1643–1654. doi: 10.1083/jcb.135.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E, Theisen CS, Johnson KR, Wheelock MJ. R-cadherin influences cell motility via Rho family GTPases. J Biol Chem. 2004;279:31041–31049. doi: 10.1074/jbc.M400024200. [DOI] [PubMed] [Google Scholar]
- Kane DA, McFarland KN, Warga RM. Mutations in half-baked/E-cadherin block cell behaviors that are necessary for teleost epiboly. Development. 2005;132:1105–1116. doi: 10.1242/dev.01668. [DOI] [PubMed] [Google Scholar]
- Kashef J, Kohler A, Kuriyama S, Alfandari D, Mayor R, Wedlich D. Cadherin-11 regulates protrusive activity in Xenopus cranial neural crest cells upstream of Trio and the small GTPases. Genes Dev. 2009;23:1393–1398. doi: 10.1101/gad.519409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Yamamoto A, Bouwmeester T, Agius E, Robertis EM. The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development. 1998;125:4681–4690. doi: 10.1242/dev.125.23.4681. [DOI] [PubMed] [Google Scholar]
- Kleer CG, van Golen KL, Braun T, Merajver SD. Persistent E-cadherin expression in inflammatory breast cancer. Mod Pathol. 2001;14:458–464. doi: 10.1038/modpathol.3880334. [DOI] [PubMed] [Google Scholar]
- Koehler A, Schlupf J, Schneider M, Kraft B, Winter C, Kashef J. Loss of Xenopus cadherin-11 leads to increased Wnt/beta-catenin signaling and up-regulation of target genes c-myc and cyclin D1 in neural crest. Dev Biol. 2013;383:132–145. doi: 10.1016/j.ydbio.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Kohutek ZA, diPierro CG, Redpath GT, Hussaini IM. ADAM-10-mediated N-cadherin cleavage is protein kinase C-alpha dependent and promotes glioblastoma cell migration. J Neurosci. 2009;29:4605–4615. doi: 10.1523/JNEUROSCI.5126-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtidis A, Ngok SP, Anastasiadis PZ. p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Prog Mol Biol Transl Sci. 2013;116:409–432. doi: 10.1016/B978-0-12-394311-8.00018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5:R217–R222. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft B, Berger CD, Wallkamm V, Steinbeisser H, Wedlich D. Wnt-11 and Fz7 reduce cell adhesion in convergent extension by sequestration of PAPC and C-cadherin. J Cell Biol. 2012;198:695–709. doi: 10.1083/jcb.201110076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa PM, Fraser SE. Neural crest cell dynamics revealed by time-lapse video microscopy of whole embryo chick explant cultures. Dev Biol. 1998;204:327–344. doi: 10.1006/dbio.1998.9082. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Inui M, Sugimoto K, Hayata T, Asashima M. Axial protocadherin is a mediator of prenotochord cell sorting in Xenopus. Dev Biol. 2002;244:267–277. doi: 10.1006/dbio.2002.0589. [DOI] [PubMed] [Google Scholar]
- Langhe RP, Gudzenko T, Bachmann M, Becker SF, Gonnermann C, Winter C, Abbruzzese G, Alfandari D, Kratzer MC, Franz CM, Kashef J. Cadherin-11 localizes to focal adhesions and promotes cell-substrate adhesion. Nat Commun. 2016;7:10909. doi: 10.1038/ncomms10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. Migration and differentiation of neural crest cells. Curr Top Dev Biol. 1980;16:31–85. doi: 10.1016/s0070-2153(08)60153-2. [DOI] [PubMed] [Google Scholar]
- Lee RT, Nagai H, Nakaya Y, Sheng G, Trainor PA, Weston JA, Thiery JP. Cell delamination in the mesencephalic neural fold and its implication for the origin of ectomesenchyme. Development. 2013;140:4890–4902. doi: 10.1242/dev.094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon CA, Johnson JL, Williams H, Sala-Newby GB, George SJ. Soluble N-cadherin overexpression reduces features of atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2009;29:195–201. doi: 10.1161/ATVBAHA.108.178087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan GJ. Melanoblast-tissue interactions and the development of pigment pattern in Xenopus larvae. J Embryol Exp Morpholog. 1976;35:463–484. [PubMed] [Google Scholar]
- Maj E, Kunneke L, Loresch E, Grund A, Melchert J, Pieler T, Aspelmeier T, Borchers A. Controlled levels of canonical Wnt signaling are required for neural crest migration. Dev Biol. 2016;417:77–90. doi: 10.1016/j.ydbio.2016.06.022. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathavan K, Khedgikar V, Bartolo V, Alfandari D. Cadherin-11 ectodomains activate Akt signaling in neural crest. doi: 10.1371/journal.pone.0188963. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HK, Broders-Bondon F, Thiery JP, Mayor R. Wnt11r is required for cranial neural crest migration. Dev Dyn. 2008a;237:3404–3409. doi: 10.1002/dvdy.21758. [DOI] [PubMed] [Google Scholar]
- Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, Parsons M, Mayor R. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008b;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- McCusker C, Cousin H, Neuner R, Alfandari D. Extracellular cleavage of cadherin-11 by ADAM metalloproteases is essential for Xenopus cranial neural crest cell migration. Mol Biol Cell. 2009;20:78–89. doi: 10.1091/mbc.E08-05-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier-Gavelle F, Duband JL. Control of N-cadherin-mediated intercellular adhesion in migrating neural crest cells in vitro. J Cell Sci. 1995;108(Pt 12):3839–3853. doi: 10.1242/jcs.108.12.3839. [DOI] [PubMed] [Google Scholar]
- Montell DJ, Yoon WH, Starz-Gaiano M. Group choreography: mechanisms orchestrating the collective movement of border cells. Nat Rev Mol Cell Biol. 2012;13:631–645. doi: 10.1038/nrm3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najy AJ, Day KC, Day ML. The ectodomain shedding of E-cadherin by ADAM15 supports ErbB receptor activation. J Biol Chem. 2008;283:18393–18401. doi: 10.1074/jbc.M801329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development. 1995;121:1321–1332. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- Nandadasa S, Tao Q, Menon NR, Heasman J, Wylie C. N- and E-cadherins in Xenopus are specifically required in the neural and non-neural ectoderm, respectively, for F-actin assembly and morphogenetic movements. Development. 2009;136:1327–1338. doi: 10.1242/dev.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) second. Amsterdam: North-Holland: 1967. [Google Scholar]
- Olesnicky Killian EC, Birkholz DA, Artinger KB. A role for chemokine signaling in neural crest cell migration and craniofacial development. Dev Biol. 2009;333:161–172. doi: 10.1016/j.ydbio.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlichenko L, Geyer R, Yanagisawa M, Khauv D, Radisky ES, Anastasiadis PZ, Radisky DC. The 19-amino acid insertion in the tumor-associated splice iso-form Rac1b confers specific binding to p120 catenin. J Biol Chem. 2010;285:19153–19161. doi: 10.1074/jbc.M109.099382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies NE, Grunwald GB. Purification and characterization of NCAD90, a soluble endogenous form of N-cadherin, which is generated by proteolysis during retinal development and retains adhesive and neurite-promoting function. J Neurosci Res. 1993;36:33–45. doi: 10.1002/jnr.490360105. [DOI] [PubMed] [Google Scholar]
- Park KS, Gumbiner BM. Cadherin 6B induces BMP signaling and de-epithelialization during the epithelial mesenchymal transition of the neural crest. Development. 2010;137:2691–2701. doi: 10.1242/dev.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Gumbiner BM. Cadherin-6B stimulates an epithelial mesenchymal transition and the delamination of cells from the neural ectoderm via LIMK/cofilin mediated non-canonical BMP receptor signaling. Dev Biol. 2012;366:232–243. doi: 10.1016/j.ydbio.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piloto S, Schilling TF. Ovo1 links Wnt signaling with N-cadherin localization during neural crest migration. Development. 2010;137:1981–1990. doi: 10.1242/dev.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DR, Williams JS, Hernandez-Lagunas L, Salcedo E, O’Brien JH, Artinger KB. Cdon promotes neural crest migration by regulating N-cadherin localization. Dev Biol. 2015;407:289–299. doi: 10.1016/j.ydbio.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan J, Luo T, Sargent TD. PCNS: a novel protocadherin required for cranial neural crest migration and somite morphogenesis in Xenopus. Dev Biol. 2006;295:206–218. doi: 10.1016/j.ydbio.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Rehimi R, Khalida N, Yusuf F, Dai F, Morosan-Puopolo G, Brand-Saberi B. Stromal-derived factor-1 (SDF-1) expression during early chick development. Int J Dev Biol. 2008;52:87–92. doi: 10.1387/ijdb.072374rr. [DOI] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rogers CD, Saxena A, Bronner ME. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. J Cell Biol. 2013;203:835–847. doi: 10.1083/jcb.201305050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani B, Thiebaud CH. Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Dev Biol. 1987;124:91–110. doi: 10.1016/0012-1606(87)90463-5. [DOI] [PubMed] [Google Scholar]
- Sano K, Tanihara H, Heimark RL, Obata S, Davidson M, St John T, Taketani S, Suzuki S. Protocadherins: a large family of cadherin-related molecules in central nervous system. EMBO J. 1993;12:2249–2256. doi: 10.1002/j.1460-2075.1993.tb05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa E, Szabo A, Bibonne A, Theveneau E, Parsons M, Mayor R. Cadherin switch during EMT in neural crest cells leads to contact inhibition of locomotion via repolarization of forces. Dev Cell. 2015;34:421–434. doi: 10.1016/j.devcel.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa E, Roycroft A, Theveneau E, Terriac E, Piel M, Mayor R. A novel method to study contact inhibition of locomotion using micropatterned substrates. Biol Open. 2016;5:1553. doi: 10.1242/bio.020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer G, Narasimha M, Vogelsang E, Leptin M. Cadherin switching during the formation and differentiation of the Drosophila mesoderm—implications for epithelial-to-mesenchymal transitions. J Cell Sci. 2014;127:1511–1522. doi: 10.1242/jcs.139485. [DOI] [PubMed] [Google Scholar]
- Schiffmacher AT, Xie V, Taneyhill LA. Cadherin-6B proteolysis promotes the neural crest cell epithelial-to-mesenchymal transition through transcriptional regulation. J Cell Biol. 2016;215:735–747. doi: 10.1083/jcb.201604006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schneider M, Huang C, Becker SF, Gradl D, Wedlich D. Protocadherin PAPC is expressed in the CNC and can compensate for the loss of PCNS. Genesis. 2014;52:120–126. doi: 10.1002/dvg.22736. [DOI] [PubMed] [Google Scholar]
- Shimazui T, Giroldi LA, Bringuier PP, Oosterwijk E, Schalken JA. Complex cadherin expression in renal cell carcinoma. Cancer Res. 1996;56:3234–3237. [PubMed] [Google Scholar]
- Shimizu T, Yabe T, Muraoka O, Yonemura S, Aramaki S, Hatta K, Bae YK, Nojima H, Hibi M. E-cadherin is required for gastrulation cell movements in zebrafish. Mech Dev. 2005;122:747–763. doi: 10.1016/j.mod.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Simoes-Costa M, Bronner ME. Establishing neural crest identity: a gene regulatory recipe. Development. 2015;142:242–257. doi: 10.1242/dev.105445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Eckerle S, Onichtchouk D, Marrs JA, Nitschke R, Driever W. Pou5f1-dependent EGF expression controls E-cadherin endocytosis, cell adhesion, and zebrafish epiboly movements. Dev Cell. 2013;24:486–501. doi: 10.1016/j.devcel.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor M, Gaudet R, Corey DP. Sorting out a promiscuous superfamily: towards cadherin connectomics. Trends Cell Biol. 2014;24:524–536. doi: 10.1016/j.tcb.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MS. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science. 1963;141:401–408. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- Steinberg MS. The Problem of Adhesive Selectivity in Cellular Interactions 1964 [Google Scholar]
- Stepniak E, Radice GL, Vasioukhin V. Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol. 2009;1:a002949. doi: 10.1101/cshperspect.a002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneyhill LA, Coles EG, Bronner-Fraser M. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development. 2007;134:1481–1490. doi: 10.1242/dev.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol. 2012;366:34–54. doi: 10.1016/j.ydbio.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Theveneau E, Mayor R. Collective cell migration of epithelial and mesenchymal cells. Cell Mol Life Sci. 2013;70:3481–3492. doi: 10.1007/s00018-012-1251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, Steventon B, Scarpa E, Garcia S, Trepat X, Streit A, Mayor R. Chase-and-run between adjacent cell populations promotes directional collective migration. Nat Cell Biol. 2013;15:763–772. doi: 10.1038/ncb2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes PL, Holtfreter J. Directed movements and selective adhesion of embryonic amphibian cells. J Exp Zool. 1955;128:53–120. doi: 10.1002/jez.a.114. [DOI] [PubMed] [Google Scholar]
- Uemura K, Kihara T, Kuzuya A, Okawa K, Nishimoto T, Ninomiya H, Sugimoto H, Kinoshita A, Shimohama S. Characterization of sequential N-cadherin cleavage by ADAM10 and PS1. Neurosci Lett. 2006;402:278–283. doi: 10.1016/j.neulet.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Vallin J, Girault JM, Thiery JP, Broders F. Xenopus cadherin-11 is expressed in different populations of migrating neural crest cells. Mech Dev. 1998;75:171–174. doi: 10.1016/s0925-4773(98)00099-9. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Buck CA, Bechtol KB, Damsky CH. Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. J Cell Biochem. 1987;34:187–202. doi: 10.1002/jcb.240340305. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Williams G, Howell FV, Skaper SD, Walsh FS, Doherty P. Identification of an N-cadherin motif that can interact with the fibroblast growth factor receptor and is required for axonal growth. J Biol Chem. 2001;276:43879–43886. doi: 10.1074/jbc.M105876200. [DOI] [PubMed] [Google Scholar]
- Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, Radice GL, Lo CW. Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J Cell Biol. 2001;154:217–230. doi: 10.1083/jcb.200105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Amacher SL, Kim SH, Geissert D, Kimmel CB, De Robertis EM. Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm. Development. 1998;125:3389–3397. doi: 10.1242/dev.125.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf F, Rehimi R, Dai F, Brand-Saberi B. Expression of chemokine receptor CXCR4 during chick embryo development. Anat Embryol. 2005;210:35–41. doi: 10.1007/s00429-005-0013-9. Berl. [DOI] [PubMed] [Google Scholar]


