Abstract
Anticoagulation control has been associated with risk of thromboembolism and hemorrhage. Herein, we explore the relationship between anticoagulation control achieved in Left Ventricular Assist Device Patients (LVADs) and evaluate the association with risk of thromboembolism and hemorrhage. Patients (aged 19 or older) with a continuous flow LVAD placed from 2006–2012. Proportion of time spent in target range (PTTR) for INR was estimated with target range of 2.0–3.0. PTTR was categorized into PTTR>60%, PTTR≥50<60% and PTTR<50%. The relationship between PTTR and thromboembolism and hemorrhage was assessed. The 115 participants contributed 624.5 months of follow-up time. Only 20% of patients achieved anticoagulation control (PTTR >60% for INR range of 2–3). After adjusting for chronic kidney disease, history of diabetes, history of atrial fibrillation and age at implant, compared to patients with PTTR<50%, the relative risk of thromboembolism in patients with PTTR≥60% (HR 0.37, 95%CI 0.14–0.96, p=0.042) was significantly lower, but not for patients with a PTTR of ≥50<60% (HR 0.21, 95%CI 0.02–1.82, p=0.16). The relative risk for hemorrhage was also significantly lower among patients with a PTTR≥60 % (HR 0.45, 95%CI 0.21–0.98, p=0.045), but not among those with PTTR of ≥50<60% (HR 0.47, 95%CI 0.14–1.56, p=0.22). This current study demonstrates that LVAD patients remain in the INR target range an average of 42.9% of the time. To our knowledge, this is the first report with regard to anticoagulation control as assessed by PTTR and its association with thromboembolism, hemorrhage or death among patients with VADs.
Keywords: left ventricular assist devices, warfarin, percent time in target range, over-anticoagulation, hemorrhage
Introduction
Heart failure (HF) afflicts approximately 5.7 million Americans and is one of the leading causes of morbidity and mortality in the US.(1–3) Treatment of HF is aimed at decreasing symptoms, slowing disease progression and increasing patient survival and ranges from medication to surgical intervention.(3, 4) Although drug therapy is the mainstay of treatment for HF, ventricular assist devices (VAD) are increasingly utilized in advanced HF patients. VAD patients are at increased risk for thromboembolism due to blood flowing over non-biologic surfaces necessitating warfarin anticoagulation and antiplatelet therapy. While VAD use has increased HF patient survival and quality of life, thrombotic and bleeding events remain the most common complications. A recent report demonstrates a higher rate of device thrombosis even in newer continuous flow devices such as HeartMate II.(5)Anticoagulation management remains a critical challenge for LVAD therapy to yield a successful outcome for the patient.
Oral anticoagulation (OAC) with warfarin is typically initiated with a goal of achieving and maintaining an International Normalized Ratio (INR 2–3).(6) Antiplatelet therapy with aspirin or clopidogrel or both is initiated. Like patients with prosthetic valves and coronary stents, combined OAC and antiplatelet therapy may increase the risk of hemorrhagic complications in LVAD patients as well.(7–15) Despite routine initiation of these therapies, data remains limited on the anticoagulation control achieved in VAD patients. Anticoagulation control is commonly assessed by measuring percent time spent in target range (PTTR) with PTTR ≥60% being considered good anticoagulation control. Achieving PTTR≥60% is the goal of anticoagulation management as it has been shown to minimize the risk of hemorrhage and thromboembolism.(16–18) We present data on anticoagulation control achieved in 115 warfarin treated patients implanted with HeartMate II and HeartWare devices and evaluate its association with risk of thromboembolism and hemorrhage.
Methods
Study Setting
The study enrolled patients at the University of Alabama at Birmingham who received a ventricular assist device (VAD) from 2006–2012 under the approval of the Institutional Review Board.
Inclusion and exclusion
Patients aged 19 years and older who have had a HeartMate II (Thoratec Corporation, Pleasanton, CA) or HeartWare (HeartWare Inc, Framingham, MA) continuous flow VAD placed at UAB from 2006–2012 were included in this study. All patients received post-implant medical care through the faculty of the Advanced Heart Failure/ Mechanical Circulatory Support team. Patients typically receive inpatient care for 2–4 weeks post VAD implant, with some remaining hospitalized for longer duration. After discharge all patients receive care as outpatients and are seen in clinic at least once every month. Of the 127 patients who had a continuous flow VAD placed from 2006–2012, the medical records of 12 patients were unavailable preventing the collection of detailed clinical information at time of VAD implant. This resulted in the 115 patients included in this analysis.
Data collection
For all patients a detailed baseline (pre-VAD) clinical phenotype including demographic variables (age at implant, self-reported race, etc.), medical history prior to VAD (comorbid conditions, heart failure etiology etc.), medications, and laboratory assessments was collected. Information on Post-VAD demographic and clinical data (including medications, laboratory assessments and outcomes) was collected using definitions from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) Registry.(19) Information was updated during monthly clinic visits for up to a 1-year follow-up. Information on anticoagulant management, including INR and change in warfarin dose, and current therapy with antiplatelet agents was ascertained for all patients during their clinic and hospital visits. Detailed information, including reason for hospital stay, laboratory tests, medications, and surgical interventions was collected anytime a patient was hospitalized during the 1-year follow-up.
Percent Time in Target INR Range
Proportion of time spent in target range (PTTR) for INR was estimated for each patient using the Rosendaal linear interpolation method.(20) This method assumes a linear relationship exists between two consecutively measured INR values and allows one to allocate a specific INR value to each day for each patient. Time in target range for each patient was assessed by the percentage of interpolated INR values within the target range of 2.0–3.0 after attainment of first INR in target range. PTTR was categorized into PTTR>60%, PTTR≥50<60% and PTTR<50% based on the current categories from anticoagulation control studies in other populations.
Definition of Outcomes
All thromboembolic and hemorrhagic events were documented during the one-year follow-up. As patient with VADs receive their sole care at UAB, the study ascertainment of complications was robust. Thromboembolic events included ischemic stroke, transient ischemic attack, pulmonary embolus, deep vein thrombosis, pump thrombosis requiring hospitalization, and mediastinal clot requiring surgical removal. Hemorrhagic events included intracranial hemorrhage, gastrointestinal bleeding, mediastinal bleeding requiring surgical intervention, or an episode requiring transfusion of greater than four units of packed red blood cells. All patients were followed from VAD implant for up to 1 year or until the time of thromboembolism or hemorrhage event. Multiple events per subject were not considered as the occurrence of thromboembolism while on warfarin resulted in an increase in anticoagulation intensity (INR goal 2.5 to 3.5) whereas occurrence of hemorrhage while on warfarin resulted in a decrease in INR range or discontinuation of warfarin. For patients who did not experience a thromboembolic or hemorrhagic event, follow-up time was censored at end of the study (1 year) or explantation/transplantation/death (if earlier than 1 year).
Statistical Analysis
The χ2 test was used to assess differences for categorical variables and Student’s t-test or Wilcoxon Rank Sum where appropriate for continuous variables. Cox proportional hazards models were used to assess the influence of PTTR and thromboembolic and hemorrhagic events. Multivariable analyses for thromboembolism and hemorrhage account for the influence of age at implant, history of diabetes, history of atrial fibrillation and kidney function (based on estimated glomerular filtration rate (eGFR) and categorized into three groups: >60ml/min/1.73m2, 30–59 ml/min/1.73m2, and <30ml/min/1.73m2).(21) Multivariable analyses for death account for the influence of age at implant, and kidney function at implant. All tests were performed using SAS version 9.2(SAS Institute, Cary, NC) at a non-directional alpha level of 0.05.
Results
Overall Population
Of the 127 patients who were treated at UAB between 2006 and 2012 with a continuous flow VAD, 115 patients were included in this study. Patients who were implanted at an outside hospital (N=12) were excluded due missing information on anticoagulation after implantation. The median age at implant for the cohort is 56 with the majority of patients being male (78.3%), white (67.8%) and implanted with a VAD as a bridge to transplant (56.6%). Baseline characteristics for the entire cohort as well as stratified by percent time in target range (PTTR) are presented in Table 1. Demographic and clinical characteristics such as concomitant antiplatelet therapy did not differ according to PTTR.
Table 1.
Baseline Characteristics for the Entire Cohort and Stratified by Percent time in INR (2–3) Target Range (PTTR)
| All Patients (N=115) | PTTR <50 (N=31) | PTTR 50–60 (N=13) | PTTR≥60 (N=70) | p-value | |
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
| |||||
| Age at Implant | 53.6 (14.3) | 55 (13.9) | 52 (15) | 51 (14.0) | 0.29 |
|
| |||||
| Body Mass Index at VAD Implantation | 29 (6.3) | 31 (5.9) | 30 (6.8) | 28 (5.5) | 0.42 |
|
| |||||
| N (%) | N (%) | N (%) | N (%) | ||
|
| |||||
| Male | 90 (78.3%) | 23 (74.2%) | 10 (76.9%) | 56 (80.0%) | 0.80 |
|
| |||||
| Black Race | 37 (32.2%) | 11 (35.5%) | 2 (15.4%) | 24 (34.3%) | 0.37 |
|
| |||||
| Status in the first year** | 0.51 | ||||
|
| |||||
| Deceased | 19 (16.5%) | 7 (22.6%) | 2 (15.4%) | 9 (12.9%) | |
|
| |||||
| Transplanted | 23 (20.0%) | 9 (29.0%) | 2 (15.4%) | 12 (17.1%) | |
|
| |||||
| Recovered | 4 (3.5%) | 1 (3.2%) | 0 | 3 (4.3%) | |
| Etiology of Heart Failure | 0.70 | ||||
|
| |||||
| Ischemic | 60 (52.2%) | 13 (41.9%) | 9 (69.2%) | 37 (54.4%) | |
|
| |||||
| Idiopathic | 45 (39.1%) | 15 (48.4%) | 3 (23.1%) | 27 (39.7%) | |
|
| |||||
| Other | 10 (8.7%) | 3 (9.6%) | 1 (7.7%) | 4 (5.9%) | |
|
| |||||
| VAD Implantation Strategy | 0.32 | ||||
|
| |||||
| Bridge to transplant | 64 (56.6%) | 19 (61.3%) | 8 (61.5%) | 36 (52.9%) | |
|
| |||||
| Bridge to candidacy | 2 (1.8%) | 0 | 1 (7.7%) | 1 (1.5%) | |
|
| |||||
| Bridge to recovery | 1 (0.9%) | 1 (3.2%) | 0 | 0 | |
|
| |||||
| Destination | 46 (40.7%) | 11 (35.5%) | 4 (30.8%) | 31 (45.6%) | |
|
| |||||
| Comorbid Conditions*** | |||||
|
| |||||
| Diabetes | 45 (39.1%) | 10 (32.3%) | 9 (69.2%) | 26 (37.1%) | 0.05 |
|
| |||||
| Right Ventricular Dysfunction | 28 (24.4%) | 6 (19.4%) | 3 (23.1%) | 19 (27.1%) | 0.70 |
|
| |||||
| Coronary Artery Disease | 46 (40.0%) | 10 (32.3%) | 5 (38.5%) | 31 (44.3%) | 0.52 |
|
| |||||
| History of Myocardial Infarction | 23 (20.0%) | 5 (16.1%) | 2 (15.4%) | 15 (21.4%) | 0.77 |
|
| |||||
| Hypertension | 71 (61.7%) | 17 (54.8%) | 9 (69.2%) | 44 (62.9%) | 0.62 |
|
| |||||
| Hyperlipidemia | 52 (45.2%) | 13 (41.9%) | 7 (53.9%) | 31 (44.3%) | 0.76 |
|
| |||||
| Atrial Fibrillation | 43 (37.4%) | 10 (32.3%) | 7 (53.9%) | 26 (37.1%) | 0.39 |
|
| |||||
| Ventricular Tachycardia | 55 (47.8%) | 14 (45.2%) | 6 (46.2%) | 34 (48.6%) | 0.95 |
|
| |||||
| Concurrent Medications at VAD Implantation | |||||
|
| |||||
| Warfarin | 115 (100%) | 31 (100%) | 13 (100%) | 70 (100%) | - |
|
| |||||
| Aspirin | 113 (98.2%) | 31 (100%) | 13 (100%) | 69 (98.6%) | 0.73 |
|
| |||||
| Clopidogrel | 67 (58.3%) | 18 (58.1%) | 7 (53.8%) | 42 (60%) | 0.91 |
|
| |||||
| Colchicine | 6 (5.2%) | 1 (3.2%) | 0 | 5 (7.1%) | 0.48 |
|
| |||||
| Amiodarone | 70 (60.9%) | 15 (48.4%) | 8 (61.5%) | 47 (67.1%) | 0.20 |
|
| |||||
| Kidney Function prior to VAD implantation | 0.60 | ||||
|
| |||||
| eGFR at least 60 ml/min/1.73m2 | 47 (42.3%) | 9 (32.1%) | 5 (38.5%) | 33 (47.8%) | |
|
| |||||
| eGFR from 30 to 59 ml/min/1.73m2 | 54 (48.7%) | 16 (57.1%) | 6 (46.1%) | 31 (44.9%) | |
|
| |||||
| eGFR less than 30 ml/min/1.73m2 | 10 (9.0%) | 3 (10.7%) | 2 (15.4%) | 5 (7.3%) | |
INR target range of 2–3
These numbers do not include patients who are currently on VAD therapy
Patients can have concurrent comorbidities
Percent Time in Target Range for the VAD population
The 115 participants contributed 624.5 months of follow-up time with the average duration 5.4 months (±4.8months). Patients were seen at least once monthly with an average of 1.4 visits per month (Table 2). Patients’ spent 42.9% (±22.5) of their treatment time within the range INR range of 2–3. Over the duration of therapy only 20% of patients achieved good anticoagulation control (defined as PTTR >60% for INR range of 2–3).
Table 2.
Anticoagulation Control for the Entire Cohort using an INR range of 2–3 (PTTR)
| Mean(SD) | |
|---|---|
| Number of Patients | 115 |
| Number of Visits | 4902 |
| Total Follow Up (months)* | 624.5 |
| Follow Up months/patient | 5.4 ± 4.8 |
| Number of Visits/patient/month | 1.4 ± 0.96 |
|
| |
| Anticoagulation control for INR Range of 2–3 | |
| Percent Time Below Range | 41.6 ± 28.4 |
| Percent In Range | 42.9 ± 22.5 |
| Percent Time Above Range | 15.8 ± 13.9 |
|
| |
| Number of patients based on PTTR achieved for INR range 2–3 | N (%) |
| PTTR <50% in range | 67 (58.3%) |
| PTTR ≥50–60% in range | 25 (21.7%) |
| PTTR ≥60% in range | 23 (20.0%) |
Follow up time is designated as time to thromboembolism, hemorrhage, death, transplant, withdrawal or end of study (1-year)
Absolute Risk and Relative Risk of Percent Time in Target Range with Thromboembolism
Over the 51.3 person years of follow up 23 thromboembolic events occurred (6 ischemic strokes, 2 transient ischemic attacks, 2 pulmonary emboli, 5 deep vein thrombi, 6 pump thrombi, and 2 mediastinal clots) with an incidence rate (IR) of 4.5 per 10 person years (95%CI 2.9–6.6). The incidence rate of thromboembolism decreased as percent time in target range increases (Table 3). The absolute risk (measured as incidence rate ratio (IRR)) for thromboembolic events was significantly lower for patients with a PTTR of ≥50<60% (IRR 0.13, 95%CI 0.006–0.79) and PTTR≥60% (IRR 0.33, 95%CI 0.14–0.82) when compared to patients with a PTTR<50%.
Table 3.
Association of Percent Time in Target Range with Absolute (incidence rate per 10 person-years) and Relative (Hazard ratio (HR) and 95% Confidence interval (95%CI)) Risk of Thromboembolism, Hemorrhage and Death
| N | Number of Events | Follow-up* | Incidence Rate (95%CI) per 10 person years | Incidence Rate Ratio (95% CI) | |
|---|---|---|---|---|---|
| Thromboembolism | |||||
| Entire Sample | 115 | 23 | 51.3 years | 4.5 (2.9–6.6) | - |
| PTTR <50% | 31 | 9 | 8.3 years | 10.8 (5.3–19.9) | ref |
| PTTR≥50–60% | 13 | 1 | 7.1 years | 1.4 (0.07–6.9) | 0.13 (0.006–0.79) |
| PTTR≥60% | 70 | 13 | 35.9 years | 3.6 (2.0–6.0) | 0.33 (0.14–0.82) |
|
| |||||
| Hemorrhage | |||||
| Entire Sample | 115 | 36 | 51.3 years | 7.0 (5.0–9.6) | - |
| PTTR <50% | 31 | 11 | 8.3 years | 13.2 (6.9–23.0) | ref |
| PTTR≥50–60% | 13 | 4 | 7.1 years | 5.6 (1.8–13.6) | 0.43 (0.12–1.29) |
| PTTR≥60% | 70 | 21 | 35.9 years | 5.8 (3.7–8.9) | 0.44 (0.21–0.95) |
|
| |||||
| Death | |||||
| Entire Sample | 115 | 19 | 86 years | 2.2 (1.4–3.4) | - |
| PTTR <50% | 31 | 8 | 21 years | 3.8 (1.8–7.2) | ref |
| PTTR≥50–60% | 13 | 2 | 9.9 years | 2.0 (0.34–6.7) | 0.53 (0.08–2.29) |
| PTTR≥60% | 70 | 9 | 55.2 years | 1.6 (0.79–2.9) | 0.43 (0.16–1.15) |
|
| |||||
| Crude Analyses | |||||
|
| |||||
| Hazard Ratio | 95% Confidence Interval | p-value | |||
|
| |||||
| Thromboembolism | |||||
| PTTR <50% | ref | ref | ref | ||
| PTTR≥50–60% | 0.15 | 0.02–1.20 | 0.07 | ||
| PTTR≥60% | 0.37 | 0.16–0.87 | 0.023 | ||
|
| |||||
| Hemorrhage | |||||
| PTTR <50% | ref | ref | ref | ||
| PTTR≥50–60% | 0.50 | 0.16–1.59 | 0.24 | ||
| PTTR≥60% | 0.48 | 0.23–1.01 | 0.05 | ||
|
| |||||
| Death | |||||
| PTTR <50% | ref | ref | ref | ||
| PTTR≥50–60% | 0.54 | 0.11–2.54 | 0.43 | ||
| PTTR≥60% | 0.44 | 0.17–1.14 | 0.09 | ||
|
| |||||
| Adjusted Analyses | |||||
|
| |||||
| Thromboembolism* | |||||
| PTTR <50% | ref | ref | ref | ||
| PTTR≥50–60% | 0.21 | 0.02–1.82 | 0.16 | ||
| PTTR≥60% | 0.37 | 0.14–0.96 | 0.042 | ||
| Hemorrhage* | |||||
| PTTR <50% | ref | ref | ref | ||
| PTTR≥50–60% | 0.47 | 0.14–1.56 | 0.22 | ||
| PTTR≥60% | 0.45 | 0.21–0.98 | 0.045 | ||
| Death** | |||||
| PTTR <50% | ref | ref | ref | ||
| PTTR≥50–60% | 0.59 | 0.12–2.93 | 0.52 | ||
| PTTR≥60% | 0.34 | 0.12–0.95 | 0.04 | ||
adjusting for chronic kidney disease stage prior to VAD, age at implant, history of diabetes and history of atrial fibrillation
adjusting for chronic kidney disease stage prior to VAD, and age at implant
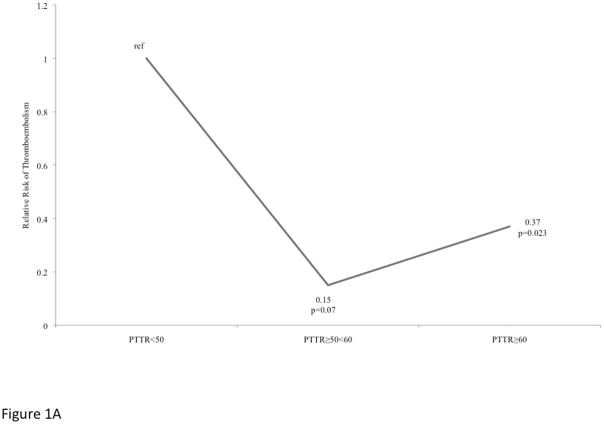
After adjusting for clinical factors, the relative risk of thromboembolism (Figure 1A) compared to patients with PTTR<50% remained lower; patients with a PTTR of ≥50<60% had lower risk of thromboembolism (hazard ratio (HR) 0.15, 95%CI 0.02–1.20), a marginally statistically significant (p=0.07) finding. Compared to patients with PTTR<50%; patients with PTTR≥60% were at significantly lower relative risk for thromboembolism (HR 0.37, 95%CI 0.16–0.87, p=0.023) with the association remaining after adjusting for chronic kidney disease stage prior to VAD, history of diabetes, history of atrial fibrillation and age at implant (HR 0.37, 95%CI 0.14–0.96, p=0.042).
Figure 1.
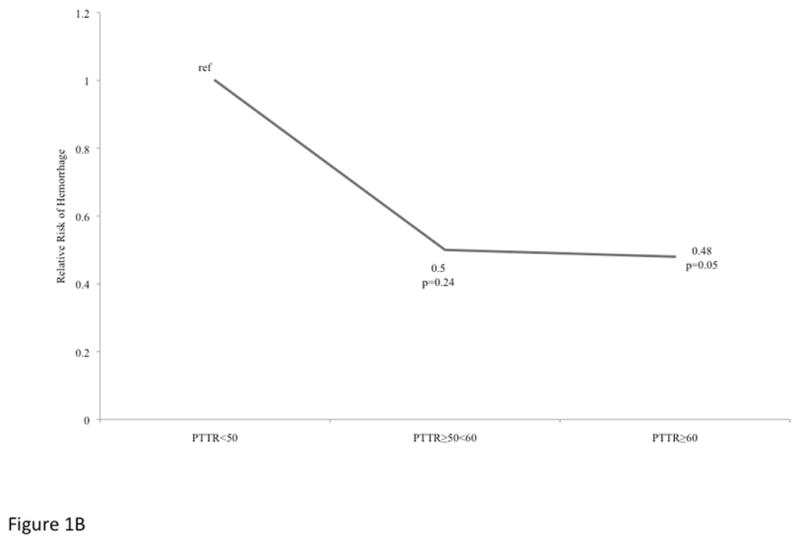
Figure 1A. Relative Risk of Thromboembolism among VAD patients stratified by Percent Time in Target Range.
Figure 1B: Relative Risk of Hemorrhage among VAD patients stratified by Percent Time in Target Range.
Absolute Risk and Relative Risk of Percent Time in Target Range with Hemorrhage
Over the 51.3 person years of follow- up 36 hemorrhages occurred (3 intracranial hemorrhages 25 gastrointestinal bleeds, 6 mediastinal bleeds, 2 requiring greater than 4 units of packed red blood cells without clinical site of bleeding) with an incidence rate for hemorrhagic events 7.0 per 10 person years (95%CI 5.0–9.6). Compared to patients with PTTR<50%; the absolute risk for hemorrhagic events was lower in patients with a PTTR of ≥50<60% (IRR 0.43, 95%CI 0.12–1.29) and significantly lower among patients with PTTR≥60% (IRR 0.44, 95%CI 0.21–0.95).
After adjusting for adjusting for chronic kidney disease stage prior to VAD, history of diabetes, history of atrial fibrillation and age at implant, compared to patients with PTTR<50%, the relative risk for hemorrhage was significantly lower among patients with a PTTR≥60 % (HR 0.45, 95%CI 0.21–0.98, p=0.045; Figure 1B), but not among those with PTTR of ≥50<60% (HR 0.47, 95%CI 0.14–1.56, p=0.22).
Absolute Risk and Relative Risk of Percent Time in Target Range with Death
Through the 86 person years of follow-up 19 deaths occurred (11 due to multisystem organ failure, 5 due to sepsis, 2 to intracranial hemorrhage and 1 due to ischemic stroke) with an incidence rate of 2.2 per 10 person years (95%CI 1.4–3.4). The absolute risk for death decreases as the percent time in range increases. Patients who are PTTR of ≥50<60% (IRR 0.53, 95%CI 0.08–2.29) and PTTR ≥60% (IRR 0.43, 95%CI 0.16–1.15) have a lower absolute risk of death compared to PTTR<50%, however these associations were not statistically significant. The unadjusted relative risk for death was lower in patients with PTTR of ≥50<60% (HR 0.54, 95%CI 0.11–2.54, p=0.43) and in patients with PTTR ≥60% (HR 0.44, 95%CI 0.17–1.14, p=0.09) compared to patients with PTTR <50%. After adjustment for chronic kidney disease and age, the lowered risk for death for the patients who are PTTR ≥60% compared to patients with PTTR <50% was statistically significantly lower (HR 0.34, 95%CI 0.12–0.95, p=0.04).
Discussion
To our knowledge, this is the first report with regard to anticoagulation control as assessed by PTTR and the association with clinically relevant outcomes such as thromboembolism, hemorrhage or death among patients with LVADs. The influence of anticoagulation control on thromboembolism and hemorrhage are well documented in other chronically anticoagulated populations, which have shown that greater time spent in INR target range decreases the risk of adverse events.(18) This current study demonstrates that LVAD patients remain in the INR target range an average of 42.9% of the time. This is consistent with previous findings from a small case series with 16 VAD patients who also demonstrated low rates of anticoagulation control.(22) Despite the rigorous anticoagulation and VAD management patients undergo, the percent time in range is less than what has been shown in previous reports of other chronically anticoagulated populations such as nonvalvular atrial fibrillation (PTTR 68%).(17, 23, 24) (25)
Patients in highly specialized anticoagulation clinics, particularly randomized clinical trial settings with intensive monitoring protocols, spend 68% of their time in target range compared to those not in anticoagulation specific clinic care such as general medical care (40%–60%).(17, 24–26) The PTTR in this cohort is lower than other strictly monitored populations despite the intensive LVAD patient care program.(23) Target range maintenance is difficult due to individual variation in the effects of warfarin therapy, and it is further compounded by the complexities of VAD management issues such as driveline infections and concomitant antithrombotic therapies. Blood-flow over non-biologic surfaces increasing platelet activation and high local shear stresses lead to acquired type 2A von Willebrand functional deficiency syndrome, particularly in those with continuous flow devices.(3, 20, 27) Significant reductions in the concentration of the functional intermediate and high molecular weight multimers of von Willebrand Factor (vWF) have been documented in LVAD patients with continuous flow devices. Impairment of the critical role of vWF high molecular weight multimers and their regulation through ADAMTS-13 in endothelial, platelet, and coagulation factor interaction increases bleeding risk. Furthermore, genetic factors could influence anticoagulation control and subsequently impact outcomes.CYP2C9 and VKOR variants influence anticoagulation control, and patient with these variants could have more difficulty with anticoagulation control. These VAD specific factors influence anticoagulation control, which in turn influences percent time in target range and can contribute to the higher INR variability seen in this strictly monitored population.
Balancing the risk of thromboembolism with the risk of hemorrhage is particularly challenging in VAD patients, particularly with continuous flow devices, since VAD patients have higher mortality compared to advanced heart failure patients only treated with medical therapy.(28) The effectiveness and safety of chronic warfarin management is tightly linked to PTTR with a reduction in thromboembolism and hemorrhage risk with better anticoagulation control.(16, 18, 27) Our study illustrates that VAD patients with a higher proportion of PTTR have a lower incidence of thromboembolism, hemorrhage and death, and is comparable to other populations. (29, 30)
Anticoagulation therapy with a low target INR (1.5–2.5) has been previously advocated to reduce the risk of bleeding complications in VAD patients.(31, 32) Suggesting caution with a low target INR strategy, low PTTR (PTTR<50%) due to sub-therapeutic INRs with the 2.0–3.0 target was associated with substantially higher risk of thromboembolism in our cohort. This suggests tighter anticoagulation control may in general be a better strategy than lowering INR targets. The observed association between sub-therapeutic anticoagulation and thrombotic events is biologically plausible. Interestingly, we observe that patients with good anticoagulation control reflected by higher PTTR, have a lower risk of bleeding events. We hypothesize that even sub-therapeutic anticoagulation unmasks patients that despite the factors adjusted for are “less healthy” and at intrinsically higher risk of bleeding. We speculate that the biological relationships between PTTR and bleeding versus thrombotic risks are not similar. The relationship between PTTR and thrombotic risk appears more linear and predictable above a certain minimum threshold. The relationship between PTTR and bleeding risk is likely more patient specific with bleeding being highly likely in some patients well before the therapeutic range is achieved. Despite the majority of deaths in this sample resulting from multisystem organ failure, PTTR ≥60% is associated with a reduced risk of mortality. Out of the 19 patients who died in this sample, 14 had a thromboembolism or hemorrhage prior to death.
The association between PTTR and outcomes observed supports the role for randomized trials to prospectively test whether strategies to improve PTTR in LVAD patients improve both PTTR and outcomes. These strategies should potentially include genomic guidance, more frequent point of care monitoring, and target personalization or adjustment based on predictive models for thrombosis and bleeding risk. These individualized strategies likely need to consider INR lability, vWF function, and other measures of platelet reactivity. Key strengths are the 1-year follow-up and minimal loss to follow-up with complete capture of clinically relevant events in a racially diverse population. Detailed clinical information is available including medications, labs, and INRs with minimal missing information. While there is uniformity of care from a single institution experience, sample size and therefore power are necessarily limited. While most tertiary LVAD centers likely follow similar anticoagulation and monitoring protocols, generalizability is uncertain. As with any observational study, observed associations between factors such as PTTR and outcomes cannot be proven to be causal.
The significance of these findings is highlighted by the increasing rate of thromboembolism with the newer continuous flow VAD devices, in addition to the relatively high risks of hemorrhage and death with LVAD therapy.(5) The number of LVADs implanted should increase with the FDA approval for the use of VADs as destination therapy. This increases the number of patients with a LVAD implanted and thereby increases the number of patients at risk for thromboembolism or hemorrhage. These results suggest PTTR is a useful measure of INR control in VAD patients for research and perhaps clinical practice with more time in target range being associated with lower risk of thromboembolism and hemorrhage. Further research is needed to assess the generalizability of these findings. The role of platelet and vWF function in thrombotic and bleeding risks require further study in LVAD patients. Predictive models incorporating multiple clinical, laboratory, and genomic factors might help guide individualized therapy to optimize antithrombotic therapy and outcomes.
Acknowledgments
Funding/Support:
This work was supported in part by grants from the National Heart Lung and Blood Institute (RO1HL092173; 1K24HL133373), the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program (UL1 TR000165) and the American Heart Association (13PRE13830003).
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessup M, Albert NM, Lanfear DE, Lindenfeld J, Massie BM, Walsh MN, et al. ACCF/AHA/HFSA 2011 survey results: current staffing profile of heart failure programs, including programs that perform heart transplant and mechanical circulatory support device implantation: a report of the ACCF Heart Failure and Transplant Committee, AHA Heart Failure and Transplantation Committee, and Heart Failure Society of America. Circ Heart Fail. 2011;4(3):378–87. doi: 10.1161/HHF.0b013e3182186210. [DOI] [PubMed] [Google Scholar]
- 3.Wilson SR, Givertz MM, Stewart GC, Mudge GH., Jr Ventricular assist devices the challenges of outpatient management. J Am Coll Cardiol. 2009;54(18):1647–59. doi: 10.1016/j.jacc.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 4.Haeck ML, Hoogslag GE, Rodrigo SF, Atsma DE, Klautz RJ, van der Wall EE, et al. Treatment options in end-stage heart failure: where to go from here? Neth Heart J. 2011 doi: 10.1007/s12471-011-0211-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano CA, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 6.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 7.Dale J, Myhre E, Loew D. Bleeding during acetylsalicylic acid and anticoagulant therapy in patients with reduced platelet reactivity after aortic valve replacement. Am Heart J. 1980;99(6):746–52. doi: 10.1016/0002-8703(80)90625-0. [DOI] [PubMed] [Google Scholar]
- 8.Chesebro JH, Fuster V, Elveback LR, McGoon DC, Pluth JR, Puga FJ, et al. Trial of combined warfarin plus dipyridamole or aspirin therapy in prosthetic heart valve replacement: danger of aspirin compared with dipyridamole. Am J Cardiol. 1983;51(9):1537–41. doi: 10.1016/0002-9149(83)90673-2. [DOI] [PubMed] [Google Scholar]
- 9.Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331(8):496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 10.Rogers JG, Butler J, Lansman SL, Gass A, Portner PM, Pasque MK, et al. Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. J Am Coll Cardiol. 2007;50(8):741–7. doi: 10.1016/j.jacc.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 11.Lazar RM, Shapiro PA, Jaski BE, Parides MK, Bourge RC, Watson JT, et al. Neurological events during long-term mechanical circulatory support for heart failure: the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) experience. Circulation. 2004;109(20):2423–7. doi: 10.1161/01.CIR.0000129414.95137.CD. [DOI] [PubMed] [Google Scholar]
- 12.Pae WE, Connell JM, Boehmer JP, Korfer R, El-Banayosy A, Hetzer R, et al. Neurologic events with a totally implantable left ventricular assist device: European LionHeart Clinical Utility Baseline Study (CUBS) J Heart Lung Transplant. 2007;26(1):1–8. doi: 10.1016/j.healun.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Slaughter MS, Sobieski MA, Gallagher C, Dia M, Silver MA. Low incidence of neurologic events during long-term support with the HeartMate XVE left ventricular assist device. Tex Heart Inst J. 2008;35(3):245–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas CE, Jichici D, Petrucci R, Urrutia VC, Schwartzman RJ. Neurologic complications of the Novacor left ventricular assist device. Ann Thorac Surg. 2001;72(4):1311–5. doi: 10.1016/s0003-4975(01)03004-1. [DOI] [PubMed] [Google Scholar]
- 15.Tsukui H, Abla A, Teuteberg JJ, McNamara DM, Mathier MA, Cadaret LM, et al. Cerebrovascular accidents in patients with a ventricular assist device. J Thorac Cardiovasc Surg. 2007;134(1):114–23. doi: 10.1016/j.jtcvs.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 16.Ansell J, Hirsh J, Dalen J, Bussey H, Anderson D, Poller L, et al. Managing oral anticoagulant therapy. Chest. 2001;119(1 Suppl):22S–38S. doi: 10.1378/chest.119.1_suppl.22s. [DOI] [PubMed] [Google Scholar]
- 17.Ansell J, Hollowell J, Pengo V, Martinez-Brotons F, Caro J, Drouet L. Descriptive analysis of the process and quality of oral anticoagulation management in real-life practice in patients with chronic non-valvular atrial fibrillation: the international study of anticoagulation management (ISAM) J Thromb Thrombolysis. 2007;23(2):83–91. doi: 10.1007/s11239-006-9022-7. [DOI] [PubMed] [Google Scholar]
- 18.Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335(8):540–6. doi: 10.1056/NEJM199608223350802. [DOI] [PubMed] [Google Scholar]
- 19.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33(6):555–64. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–9. [PubMed] [Google Scholar]
- 21.Levey A, Coresh J, Greene T, Stevens L, Zhang Y, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Jennings D, McDonnell J, Schillig J. Assessment of long-term anticoagulation in patients with a continuous-flow left-ventricular assist device: a pilot study. J Thorac Cardiovasc Surg. 2011;142(1):e1–2. doi: 10.1016/j.jtcvs.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 23.van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006;129(5):1155–66. doi: 10.1378/chest.129.5.1155. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP, et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116(22):2563–70. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 25.Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costs. Arch Intern Med. 1998;158(15):1641–7. doi: 10.1001/archinte.158.15.1641. [DOI] [PubMed] [Google Scholar]
- 26.Chamberlain MA, Sageser NA, Ruiz D. Comparison of anticoagulation clinic patient outcomes with outcomes from traditional care in a family medicine clinic. J Am Board Fam Pract. 2001;14(1):16–21. [PubMed] [Google Scholar]
- 27.Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation. 2003;107(12):1692–711. doi: 10.1161/01.CIR.0000063575.17904.4E. [DOI] [PubMed] [Google Scholar]
- 28.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 29.Wan Y, Heneghan C, Perera R, Roberts N, Hollowell J, Glasziou P, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1(2):84–91. doi: 10.1161/CIRCOUTCOMES.108.796185. [DOI] [PubMed] [Google Scholar]
- 30.Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009;15(3):244–52. doi: 10.18553/jmcp.2009.15.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menon AK, Gotzenich A, Sassmannshausen H, Haushofer M, Autschbach R, Spillner JW. Low stroke rate and few thrombo-embolic events after HeartMate II implantation under mild anticoagulation. Eur J Cardiothorac Surg. 2012;42(2):319–23. doi: 10.1093/ejcts/ezr312. discussion 23. [DOI] [PubMed] [Google Scholar]
- 32.Boyle AJ, Russell SD, Teuteberg JJ, Slaughter MS, Moazami N, Pagani FD, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant. 2009;28(9):881–7. doi: 10.1016/j.healun.2009.05.018. [DOI] [PubMed] [Google Scholar]