Abstract
Introduction: Oral lichen planus (OLP) is a mucocutaneous disease with uncertain etiology. As the etiology is unknown standard treatment modalities are not available. The traditional and common treatment relies on corticosteroids whether topical or systemic. In recent years, development of lasers made a proper path to use this instrument for treatment of the diseases which are refractory to conventional treatments. Previous studies in this field used CO2, ND:YAG, Excimer and some wavelength of diode lasers for the treatment of different types of lichen planus.
Case Report: In this study, we present an OLP case which is treated using 980 nm diode laser. The result was measured by visual analogue scale (VAS) and clinical assessment; as a result, symptoms including pain and soreness started to decrease within a week, and by the end of a month completely subsided; the lesion disappeared totally as well. No recurrence was observed after a month and no side-effect was reported.
Conclusion: 980 nm diode laser can be successfully used for treatment of patients with OLP
Keywords: Diode laser, Oral lichen planus, Laser therapy, Traditional treatment
Introduction
Lichen planus is considered as an autoimmune, chronic, mucocutaneous disease which can affect the skin, genital mucosa, nails, scalp and oral mucosa.1 In the oral cavity, it is located mostly on the buccal mucosa, tongue, and gingiva. Approximately half of the patients with oral lichen planus (OLP) have skin lesions as well.2 No definite etiology has been found but it may be due to viral infections, stress or collagen disease.3 Drug-related oral lesions are not common, yet if medically possible, adjusting probable related drugs like antihypertensives (e.g. ACE inhibitors), oral hypoglycaemics (e.g. tolbutamide), non-steroidal anti-inflammatory drugs, second line anti-arthritics (gold; penicillamine), xanthine oxidase inhibitors (allopurinol), psychoactive drugs (e.g. tricyclic antidepressants) is recommended.4
Notably, OLP is diagnosed in middle-aged women with 1.5 women/men ratio.5 OLP is defined with white striations (Wickham’s striae), erythema, erosions, and white papules. There are 2 methods for diagnosis; clinical examination alone or along with histopathological tests.1 Approximately 1%-2% of the population have OLP and besides the classic occurrence of lesions, other complications like increased incidence of Candida albicans infection and squamous cell carcinoma are probable.6 The chance of transformation to malignancy is less than 1% and the highest chance of occurrence concerns the tongue.7
Lichen planus may last for many years and even lifetime.8 There is no curative treatment for OLP and most treatments are symptomatic.9 The treatment plan for OLP can be surgical or non-surgical depending on patients and clinician choices.5 It is speculated that removing big amalgam restorations and dental plaque control may help reducing irritating symptoms of OLP, in particular with gingival involvement.10,11 Standard treatment line is not available for lichen planus but some therapeutic modalities have been recommended.
One of the non-surgical choices is corticosteroids; their effectiveness is proved in healing the erosive lesions but they do not have efficacy on reticular, popular or plaque-like lesions. Corticosteroids help reduce inflammatory signs but may promote adverse side-effect of Candida overgrowth as well. As lesions are diffuse, conventional surgeries do not give appropriate results, hence, cryotherapy, cauterization and recently laser surgeries are recommended as alternatives.12-14
Studies in recent years demonstrated controversies in use of lasers in dentistry, however, lichen planus lesions showed acceptable responses to laser therapy. Different types of lasers with different wavelengths have been utilized in the treatment of OLP. In a study by Agha-Hosseini et al, the efficacy of low-intensity and CO2 laser was evaluated; both lasers had a remarkable effect on pain, size of lesions and also clinical response.14 Another study which focused on oral mucosal lesions showed that eodymium-doped yttrium aluminium garnet (ND:YAG) and CO2 lasers both can have satisfactory results on treating benign intraoral lesions as both lasers could reduce pain, be site-specific, minimally invasive, and omit post-surgical sutures.15 Patients who were treated by low level laser therapy (LLLT) with wavelength of 970 nm reported acceptable results as well in a short follow-up study.16 Despite success of some lasers in treatment of mucocutaneous lesions, the efficacy of diode lasers is not fully evaluated yet; diode lasers have major advantages over others, such as easy to use design due to small size; this feature lets it come in handy in many dentistry fields, also new developments made it possible to transmit the beam through fiberoptics in this type of lasers so diode lasers have the capability to be applied in different sites.17 Among different wavelentghs, the 980 nm diode lasers have demonstrated acceptable hemostasis and coagulation features. Good wound healing, lack of swelling, bleeding, pain and scar tissue formation after soft tissue surgeries are other advantages named for diode lasers. The number of randomized clinical trials are rare up to this date and most studies are conducted as case presentations which seem to be mandatory prior to clinical studies with large number of samples. In this case study, 980 nm diode laser was used due to the aforementioned advantages for treatment of OLP refractory to conventional treatment in order to evaluate its efficacy.
Case Presentation
A 46 years old female patient with complaint of pain and soreness on the corner of the left buccal mucosa and anterior maxillary gingiva referred to the dental office. The patient reported no remarkable medical history. In the examination of the oral cavity, plaque-like lesion with keratotic striae (Wickham’s striae) was found on the left buccal mucosa, also mild to severe gingivitis and erythematous lesion was seen on the maxillary gingiva (Figures 1 and 2); xerostomia due to lack of sufficient saliva was obvious.18 These signs and symptoms raised suspension that the lesion might be OLP.
Figure 1.
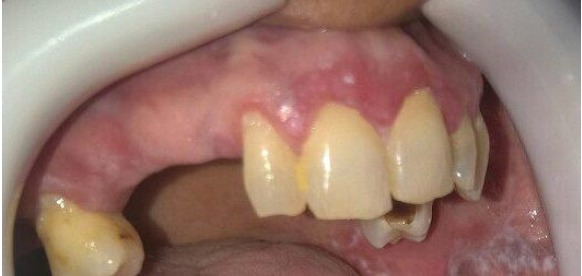
Mild Gingivitis Accompanied by Erosive Lesion on the Maxillary Gingiva.
Figure 2.
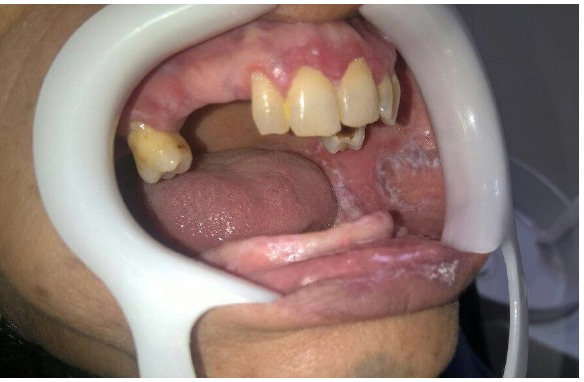
Wickham’s Striae one Left Buccal Mucosa.
Nothing special was found in the conventional hematological and biochemistry tests, therefore incisional biopsy was performed under local anesthesia from the left buccal mucosa and two samples with an approximate size of 5×3×3 mm were obtained. The histopathological investigation confirmed the diagnosis of OLP.
The patient was prescribed Nystatin drops, chlorhexidine, and triamcinolone ointment. But after 2 months of treatment, no recovery was seen in signs and symptoms. After this period, the patient was referred to a private office to begin laser therapy. The patient was asked to stop taking drugs for three months so that the effect of laser therapy could be evaluated. Firstly the 980 nm diode laser (Wiser, Doctor Smile, Italy) was used for photobiomodulation (PBM), ( 0.3 W, 6 J, 20 seconds, continuous wavelength (CW) mode, energy density of 6 J/cm2), after 4 sessions and with slight decrease of pain and soreness the setting changed to 0.2 W, 4 J, 20 seconds, CW, energy density of 4 J/cm2; then the treatment continued up to 10 sessions (3 sessions in a week); as a result, the patient was satisfied and declared no pain and soreness, also, the saliva showed significant increase (Figure 3).
Figure 3.
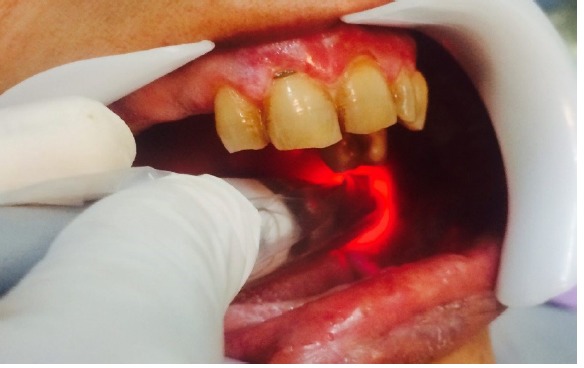
Applying 980 nm diode laser on the buccal mucosa lesion.
The surgical phase was again performed using 980 nm diode laser (1 W, CW), the tip was initiated and was in contact with soft tissue. The field of study was subjected to inferior alveolar nerve block anesthesia and all personnel and the patient wore protective glasses.
The visual analogue scale (VAS) was used to measure the efficieny of the treatment and severity of the symptoms; the score of this scale varies from 0 (no pain) to 10 (maximum pain) (Table 1).19
Table 1. Assessment of Pain Before and After Treatment by VAS Score .
| Elapsed-time | Before treatment | 48 houras | First week | First month |
| VAS score | 80% | 70% | 50% | 0% |
As a result of the surgery, the lesion began to disappear in a week. The 1-month follow-up showed no sign of recurrence (Figure 4).
Figure 4.
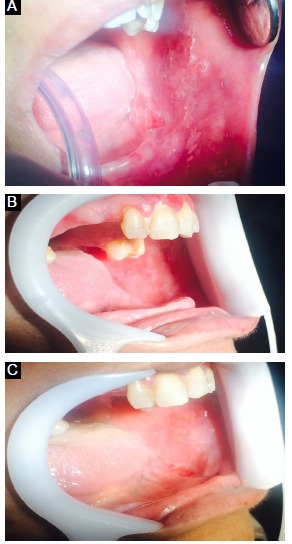
Appearance of the Lesion Just After the Surgery (A), After 48 Hours of the Surgery (B) and 1 Week After the Surgery (C).
Discussion
The definitive etiology for OLP is still unknown, but some studies related the occurrence to T-cell mediated immune response and other considered psychosomatic disorders triggered by anxiety.20,21 There is no standard treatment protocol for OLP and different types of OLP, need different treatment. The traditional method relies on using steroids drugs either topical or systemic22; selective corticosteroids treatments help to manage lymphocyte. Non-steroidal inflammatory drugs can be used as alternatives but with less efficacy.22 OLP does not seem to associate with systemic disease, drugs, smoking or heredity; the prevalence of C. albicans is rare as well, however, some useful effects of antifungal drugs have been reported in controlling the symptoms.23
When OLP is refractory to conventional treatment and long-lasting lesions have been formed, surgery can be considered as a treatment option. The new modalities of surgeries are based on laser therapy.
The early and traditional use of laser belongs to CO2 which had acceptable clinical results, could relieve symptoms immediately and long-term follow-ups showed remarkable results as well.24 Further investigation showed that low-intensity laser therapy (LILT) and CO2 both can be beneficial in relieving symptoms; LILT even showed better results in short-term observations.14
Another study focused on ND:YAG and CO2 laser simultaneously, and clarified that both lasers can be effective in the treatment of mucocutaneous intraoral lesions with minimal post-operative pain. The advantages were being conservative, site-specific, minimally invasive and omitting sutures after surgery.15 Other studies which used low-dose 308 nm Excimer laser for treatment of the symptomatic and erosive OLP had acceptable results as well, even with poor response of some patients during 18 months follow-up. The outcome of treatment suggested this type of laser in treatment of erosive OLP.25
In this case study, diode laser was used due to unique advantages in comparison with other devices; the wavelengths of diode lasers are the 810-980 nm range, where they can be better absorbed by soft tissue rather than hard tissues like bone.26 Based on wavelength, penetration depth can be 2 mm to 3 mm (this depth can cover subepithelial tissue as well). With the use of fiberoptics to transmit radiations, it can be applied to different sites, and is equipped with small heads that enhance maneuvers of clinicians. The radiation can be set to pulse or continuous modes.27 Deeper incisions can be achieved by diode laser in comparison with CO2 and ND:YAG lasers even with fewer movements. The clean cut and perfect coagulation ability alongside with hemostasis are remarkable in diode lasers, this type of lasers also bring bloodless surgeries with minimal swelling, scarring, and pain.28,29
Besides these features, diode lasers can destroy the epithelium alongside underlying connective tissue, (other lasers can denature proteins as well but do not affect connective tissue); because of denaturation of proteins, white spots are seen in surgical sites which play a role as a layer that can control pain and decrease chance of infection; this feature leads to a better healing.30,31
In a study by Jajarm et al, 630 nm diode laser was used for LILT, the result showed LILT was as effective as topical corticosteroids with fewer side-effects. Pain score, lesion severity, and appearance were reduced in both groups. Based on the findings mentioned, LILT could be an appropriate alternative for treatment of erosive-atrophic OLP.32
With all these advantages some precautions must be considered in using diode lasers. In a retrospective in vivo study by Angiero, 808 nm diode laser did not achieve acceptable results for excisions of lesions under 3 mm due to serious thermal damages and biopsies could not be made, but the laser proved to be a valid therapeutic instrument for lesions larger than 5 mm.33
Soleiman et al evaluated the efficacy of 980 nm diode laser in the treatment of OLP, the results were remarkable, no serious side-effect was reported and recurrence was recorded in 3 out of 25 patients only. So the method was suggested as an appropriate alternative for local and systemic treatment using drugs.30
In this study, a 980 nm diode laser was applied on buccal mucosa, firstly to improve pain and soreness using low-intensity setting; then after this phase the laser was used to do the surgery, the result was satisfying. At 48 hours’ follow-up, reduction in size was obvious, after one week the lesion disappeared totally, at one month follow-up no sign of recurrence was seen. A case-series study in which, 970 nm diode laser was used to perform LLLT almost presented the same results but the initial improvement was seen after 2 weeks,16 whereas in the present study significant changes were obvious within a week.
This study showed successful use of 980 nm diode laser for treating an OLP patient; further investigations with large number of patients and longer follow-ups are needed to approve this method as a standard clinical treatment.
Ethical Considerations
This study was approved by ethical committee of Lorestan University of Medical Sciences and the patient signed the consent form before begining treatment.
Conflict of Interests
The authors declare that there is no conflict of interest.
Please cite this article as follows: Derikvand N, Ghasemi SS, Moharami M, Shafiei E, Chiniforush N. Management of oral lichen planus by 980 nm diode laser. J Lasers Med Sci. 2017;8(3):143-147. doi:10.15171/jlms.2017.26.
References
- 1.Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation . J Oral Sci . 2007;49(2):89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg E. Oral lichen planus: a benign lesion. J Oral Maxillofac Surg. 2000;58(11):1278–1285. doi: 10.1053/joms.2000.16629. [DOI] [PubMed] [Google Scholar]
- 3.McCartan B. McCartan BPsychological factors associated with oral lichen planus . J Oral Pathol Med . 1995;24(6):273–275. doi: 10.1111/j.1600-0714.1995.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 4.van der Waal I. Oral lichen planus: diagnosis and management. J Dent Indonesia. 2015;22(3):89–92. [Google Scholar]
- 5.Mollaoglu N. Mollaoglu NOral lichen planus: a review . Br J Oral Maxillofac Surg . 2000;38(4):370–377. doi: 10.1054/bjom.2000.0335. [DOI] [PubMed] [Google Scholar]
- 6.Sugerman P, Savage N, Walsh L. et al. The pathogenesis of oral lichen planus . Crit Rev Oral Biol Med . 2002;13(4):350–365. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 7.Bombeccari GP, Guzzi G, Tettamanti M. et al. Oral lichen planus and malignant transformation: a longitudinal cohort study . Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol . 2011;112(3):328–334. doi: 10.1016/j.tripleo.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Thorn J, Holmstrup P, Rindum J, Pindborg J. Course of various clinical forms of oral lichen planusA prospective follow‐up study of 611 patients. J Oral Pathol Med . 1988;17(5):213–218. doi: 10.1111/j.1600-0714.1988.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 9. Bhattacharyya I, Cohen D, Silverman S Jr. Red and white lesions of the oral mucosa. In: Greenberg Ms, Gllick M, eds. Burket’s Oral Medicine: Diagnosis and Treatment. 10th ed. Hamilton, Ontario: Bc Decker; 2003:85-125.
- 10.Mårell L, Tillberg A, Widman L, Bergdahl J, Berglund A. Regression of oral lichenoid lesions after replacement of dental restorations . J Oral Rehabil . 2014;41(5):381–391. doi: 10.1111/joor.12151. [DOI] [PubMed] [Google Scholar]
- 11.Salgado DS, Jeremias F, Capela MV, Onofre MA, Massucato EMS, Orrico SR. Plaque control improves the painful symptoms of oral lichen planus gingival lesionsA short‐term study. J Oral Pathol Med. 2013;42(10):728–732. doi: 10.1111/jop.12093. [DOI] [PubMed] [Google Scholar]
- 12.Koch P, Bahmer FA. Oral lesions and symptoms related to metals used in dental restorations: a clinical, allergological, and histologic study. J Am Acad Dermatol. 1999;41(3):422–430. doi: 10.1016/s0190-9622(99)70116-7. [DOI] [PubMed] [Google Scholar]
- 13.Salah-El-Din M, Salah-El-Din M, Ezz El-Arab A. Comparative study between CO2 laser and systemic steroid therapy in the management of oral lichen planus: a clinical and histopathological study . Egyptian Dent J . 1997;43:242–247. [Google Scholar]
- 14.Agha-Hosseini F, Moslemi E, Mirzaii-Dizgah I. Comparative evaluation of low-level laser and CO 2 laser in treatment of patients with oral lichen planus . Int J Oral Maxillofac Surg . 2012;41(10):1265–1269. doi: 10.1016/j.ijom.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 15.White J, Chaudhry S, Kudler J, Sekandari N, Schoelch M, Silverman S Jr. Nd: YAG and CO2 laser therapy of oral mucosal lesions. J Clin Laser Med Surg. 1998;16(6):299–304. doi: 10.1089/clm.1998.16.299. [DOI] [PubMed] [Google Scholar]
- 16.Elshenawy HM, Eldin AM, Abdelmonem MA. Clinical assessment of the efficiency of low level laser therapy in the treatment of oral lichen planus. Open Access Maced J Med Sci. 2015;3(4):717–721. doi: 10.3889/oamjms.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moritz A, Gutknecht N, Doertbudak O, Goharkhay K, Schoop U, Schauer P. et al. Bacterial reduction in periodontal pockets through irradiation with a diode laser: a pilot study. J Clin Laser Med Surg. 1997;15(1):33–37. doi: 10.1089/clm.1997.15.33. [DOI] [PubMed] [Google Scholar]
- 18.Lundström I, Göran K, Anneroth B, Bergstedt HF. Salivary gland function and changes in patients with oral lichen planus . Eur J Oral Sci . 1982;90(6):443–458. doi: 10.1111/j.1600-0722.1982.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 19.Scott J, Huskisson E. Graphic representation of pain . Pain . 1976;2(2):175–184. [PubMed] [Google Scholar]
- 20.GunaShekhar M, Sudhakar R, Shahul M, Tenny J, Ravikanth M, Manikyakumar N. Oral lichen planus in childhood: A rare case report . Dermatol Online J . 2010;16(8):9. [PubMed] [Google Scholar]
- 21.Allen CM, Beck FM, Rossie KM, Kaul TJ. Relation of stress and anxiety to oral lichen planus. Oral Surg Oral Med Oral Pathol. 1986;61(1):44–46. doi: 10.1016/0030-4220(86)90201-x. [DOI] [PubMed] [Google Scholar]
- 22.Lozada F, Silverman S Jr. Topically applied fluocinonide in an adhesive base in the treatment of oral vesiculoerosive diseases. Arch Dermatol. 1980;116(8):898. [PubMed] [Google Scholar]
- 23.Silverman S, Gorsky M, Lozada-Nur F, Giannotti K. A prospective study of findings and management in 214 patients with oral lichen planus . Oral Surg Oral Med Oral Pathol . 1991;72(6):665–670. doi: 10.1016/0030-4220(91)90007-y. [DOI] [PubMed] [Google Scholar]
- 24.Loh H. clinical investigation of the management of oral lichen planus with CO2 laser surgery . J Clin Laser Med Surg . 1992;10(6):445–449. doi: 10.1089/clm.1992.10.445. [DOI] [PubMed] [Google Scholar]
- 25.Trehan M, Taylor CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus . Arch Dermatol . 2004;140(4):415–420. doi: 10.1001/archderm.140.4.415. [DOI] [PubMed] [Google Scholar]
- 26.Aras MH, Göregen M, Güngörmüş M, Akgül HM. Comparison of diode laser and Er: YAG lasers in the treatment of ankyloglossia . Photomed Laser Surg . 2010;28(2):173–177. doi: 10.1089/pho.2009.2498. [DOI] [PubMed] [Google Scholar]
- 27.Raval N, Raju DR, Athota A, Reddy T. Diode laser and white lesions: a clinical study on postoperative recovery, depth control and wound healing. J Indian Acad Oral Med Radiol . 2011;23(5):308. [Google Scholar]
- 28.Goharkhay K, Moritz A, Wilder-Smith P. et al. Effects on oral soft tissue produced by a diode laser in vitro. Lasers Surg Med . 1999;25(5):401–406. doi: 10.1002/(sici)1096-9101(1999)25:5<401::aid-lsm6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 29.Pick RM, Colvard MD. Current status of lasers in soft tissue dental surgery. J Periodontol. 1993;64(7):589–602. doi: 10.1902/jop.1993.64.7.589. [DOI] [PubMed] [Google Scholar]
- 30.Soliman M, El Kharbotly A, Saafan A. Management of oral lichen planus using diode laser (980 nm): a clinical study . Egyptian Dermatol Online J . 2005;1(1):3. [Google Scholar]
- 31.Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: An update on pathogenesis and treatment . J Oral Maxillofac Pathol . 2011;15(2):127–132. doi: 10.4103/0973-029X.84474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jajarm HH, Falaki F, Mahdavi O. A comparative pilot study of low intensity laser versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus. Photomed Laser Surg . 2011;29(6):421–425. doi: 10.1089/pho.2010.2876. [DOI] [PubMed] [Google Scholar]
- 33.Angiero F, Parma L, Crippa R, Benedicenti S. Diode laser (808 nm) applied to oral soft tissue lesions: a retrospective study to assess histopathological diagnosis and evaluate physical damage. Lasers Med Sci . 2012;27(2):383–388. doi: 10.1007/s10103-011-0900-7. [DOI] [PubMed] [Google Scholar]


