Abstract
This study evaluated the capability of Serratia marcescens ABHI001 to effectively degrade p-cresol through different techniques. The molecular identity of the laboratory isolate S. marcescens ABHI001 was confirmed through the 16S ribosomal DNA gene pattern, and its morphological features were investigated through field-emission scanning electron microscopy. In addition, the degradation behavior of the isolate for cresol was verified using several techniques, including UV–visible spectroscopy, followed by high-performance liquid chromatography (HPLC), gas chromatography, and Fourier transform infrared spectroscopy. The maximum degradation percentage of 85% for p-cresol could be achieved after 18 h of incubation with S. marcescens ABHI001. The formation of p-hydroxybenzaldehyde, p-hydroxybenzoate, and protocatechuate metabolites was confirmed through HPLC. The study results indicate that S. marcescens ABHI001 may have applications in the bioremediation of organic pollutants, such as p-cresol.
Keywords: Organic pollutants, p-Cresol, Biodegradation, Serratia marcescens
Introduction
Industrial effluents in the form of wastewater contain several toxic pollutants, among which phenolic compounds, such as cresols, are well-known pollutants (Ansari et al. 2016). A molecule of cresol has a methyl group substituted onto the phenol ring (Michałowicz and Duda 2007). Cresol is produced by the methylation of phenol or by the hydrolysis of chlorotoluenes (Choquette-Labbé et al. 2014). Based on the position of the methyl group on the carbon–carbon bond, cresols are characterized as ortho-, meta-, and para-cresol (o-, m-, and p-cresol, respectively). Among these isoforms, p-cresol is a highly toxic pollutant and a carcinogen; thus, exposure to even a low concentration of p-cresol results in adverse effects on the central nervous system, cardiovascular system, lungs, and the kidneys (Basheer and Farooqi 2012; ATSDR 2008). Moreover, p-cresol is often used in disinfectants, fumigants, and explosives and for manufacturing synthetic resins (Balarak and Mahdavi 2016; Berge-Lefranc et al. 2012). The Environmental Protection Agency (EPA) has classified p-cresol as a group C pollutant (ATSDR 1990). Moreover, the World Health Organization recommends that the acceptable concentration of p-cresol in portable water is 0.001 mg/L (WHO 1963). Because of its potentially toxic nature, there is an urgent need to reduce p-cresol levels in wastewater before it is discharged into the environment (Fang and Zhou 2000).
The shortcomings of physical and chemical treatment methods have long been argued, including the production of toxic intermediates, high cost of disposing final effluents, and partial mineralization of compounds (Saxena et al. 2013; Lim et al. 2014). By contrast, the biodegradation method has several advantages, including relatively low cost, no chemical use, and high public acceptance. Therefore, this method offers more favorable opportunities to completely destroy the pollutants or at least transform them into nontoxic substances (Al-Khalid and El-Naas 2012). Furthermore, biodegradation is emerging as one of the ideal technologies for removing phenolic pollutants from the environment through the action of microorganisms (Bisht et al. 2015). The final product generated by different biodegradation methods varies considerably, but the most frequent final product generated by the degradation of pollutants is carbon dioxide (Joutey et al. 2013).
Extensive research has been conducted on the microbial degradation of phenolic compounds due to its viable characteristics in removing environmental toxins (Krastanov et al. 2013). Numerous microorganisms exhibit high potential to utilize cresol as sole carbon and energy sources for their growth and survival (Ren et al. 2014; Reshma and Mathew 2014; Murthy and Gayathri 2017). In a recent study, Xenofontos et al. reported an alkalophilic Advenella species (LVX-4) for the degradation of p-cresol (Xenofontos et al. 2016). They noted that the LVX-4 strain possesses the ability to degrade p-cresol at a high concentration of 750 mg/L, and that this strain utilizes p-cresol for its growth at an optimum pH of 9 (Xenofontos et al. 2016). Pseudomonas putida is another species that has been identified and characterized as a p-cresol-degrading organism. It is a Gram-negative aerobic bacterium that can grow optimally even at room temperature (Bouallegue et al. 2004). Surkatti and El-Naas (2014) examined the p-cresol degradation ability of P. putida immobilized in polyvinyl alcohol gel. Furthermore, they conducted batch experiments at different concentrations of p-cresol and found that the maximum degradation occurs at a concentration of 200 mg/L (Surkatti and El-Naas 2014).
Yao et al. (2011) identified a novel m-cresol-degrading strain named Lysinibacillus cresolivorans. They investigated the degradation rate of m-cresol at concentrations ranging from 54.1 to 529.1 mg/L. The experimental results suggested that the maximum reaction rate of 46.80 mg/h could be achieved at the initial concentration of 224.2 mg/L for m-cresol (Yao et al. 2011). However, to the best of our knowledge, the potential of Serratia marcescens to degrade p-cresol in wastewater has not yet been studied.
Therefore, the present study evaluated the p-cresol degradation ability of the bacterial strain S. marcescens ABHI001 that was isolated from the adjoining area of Mathura Refinery, Mathura, India. In addition, to confirm the capability of S. marcescens ABHI001 to effectively degrade p-cresol, various analytical techniques, such as UV–visible spectroscopy, scanning electron microscopy (SEM), high-performance liquid chromatography (HPLC), gas chromatography (GC), and Fourier transform infrared spectroscopy (FT-IR), were performed.
Materials and methods
Materials
p-Cresol, phosphate buffer saline (PBS), glutaraldehyde (2.5%), ethanol (99.5%), sulphuric acid (H2SO4; 98.0%), and dichloromethane (99.5%) were purchased from Sigma-Aldrich Chemie Riedster (Steinheim). All solvents used for HPLC were of HPLC grade and were sourced from Fisher Scientific (Mumbai, India). Nutrient media purchased from Hi-Media (India) was used as the growth medium for the bacterial strain.
Isolation, screening, and identification of the bacterial strain
A soil sample was collected from the adjoining area of Mathura Refinery, Mathura, India. The soil sample was aseptically transferred to a 250-mL Erlenmeyer flask containing 50 mL of sterile distilled water. The flask was vigorously vortexed for 30 min on a vortex shaker. Microbial colonies were isolated on sterilized nutrient agar plates by using the serial dilution method. These plates were incubated at 30 °C until morphologically distinct bacterial colonies appeared. Seven colonies exhibiting different morphological features, comprising two fungi and five bacteria, were screened, and bacterial colonies were further isolated for purification and degradation screening (p-cresol concentration of 0.003 mg/L). Time-bound subculturing of pure colonies was performed for preservation and experimental studies. Pure microbial colonies were stored at 4 °C in a nutrient agar slant, and the glycerol stock of these colonies was preserved at −20 °C. Subsequently, to screen for the isolate with the optimal degradation ability among the selected isolates, biodegradation screening studies were performed with each isolate (ABHI001, ABHI002, ABHI003, ABHI004, and ABHI005) at a p-cresol concentration of 0.003 mg/L. Furthermore, the molecular identification of the selected isolate was performed.
Molecular identification of the screened bacterial isolate
To confirm the molecular identity of the selected bacterial strain designated ABHI001, genomic DNA was extracted and used as the template for the polymerase chain reaction (PCR) amplification of the 16S ribosomal DNA (rDNA) gene. The PCR-amplified products were then purified and subjected to sequence analysis. A similarity search for the identified sequence was performed using the BLAST program of the National Center of Biotechnology Information. Furthermore, phylogenetic investigation was performed using the neighbor-joining method with the MEGA software program (Tamura et al. 2007), as shown in Fig. 1.
Fig. 1.
Phylogenetic tree neighbor-joining based on 16S rDNA gene sequence showing the relationship between bacterial strain Serratia marcescens ABHI001 and other relatives within genus
Biodegradation study of p-cresol
For the biodegradation study, in a 250-mL conical flask, 10% inoculum of the isolated bacterial strain was inoculated into 100 mL of nutrient broth containing p-cresol concentrations ranging from 50 to 500 mg/L; the inoculated broth was incubated at 30 °C with continuous shaking at 110 rpm. Furthermore, 3.0-mL samples were taken at a regular time interval of 2 from 0 to 26 h and were centrifuged at 8000 rpm to estimate the degree of degradation using a UV–visible spectrophotometer at the wavelength of 710 nm. The degradation percentage was calculated as follows:
Analytical investigation
Morphological analysis of the laboratory isolate through field-emission SEM
For the morphological analysis through field-emission SEM (FE-SEM) (SIRIDN-2000), the selected bacterial sample was subcultured for 24 h on a nutrient agar plate and then transferred to a centrifuge tube containing PBS at pH 7.0 after achieving full growth. The sample was centrifuged, and the resultant pellet was washed three times with PBS. Furthermore, 2.5% glutaraldehyde was added to this sample and incubated overnight at room temperature. Thereafter, the sample was again washed three times with sodium phosphate and cells were harvested. For dehydration, the cells were treated with serial ethanol dilutions (30, 50, 70, 80, 90, and 100%) for 30 min each. A small amount of dehydrated cells was transferred over a clean silicon substrate and dried at room temperature for 30 min. For the visual characterization of microorganisms, FE-SEM was performed, and images were collected at an accelerating voltage of 30 kV.
HPLC analysis
HPLC (Dionex Ultimate 3000, Sunnyvale, California) was conducted to evaluate the p-cresol degradation ability of the screened bacterial isolate. In brief, 10% inoculum of S. marcescens ABHI001 was inoculated into the degradation broth flask containing medium and 85 mg/L p-cresol. Simultaneously, a control broth flask was also set up with aforesaid concentration of p-cresol and medium, but without any bacterial inoculum. Both these broths were incubated for 18 h. A 10-mL sample was collected from the degradation broth and control broth flasks, transferred to an Eppendorf tube, and centrifuged for 10 min at 5000 rpm (Eltec Research Centrifuge TC 4100 F). Subsequently, the obtained supernatant was filtered through a 0.2-μm microporous membrane. Furthermore, HPLC was conducted on a C-18 column at 30 °C, and detection was performed on a UV detector at 270 nm. The mobile phase was composed of 60% methanol and 40% H2O, with a flow rate of 0.7 mL/min. The degradation percentage was calculated by comparing the peak area and retention time (RT) of the degraded and control samples.
GC analysis
GC (Nucon 5765) was performed to further corroborate the p-cresol degradation ability and to identify the number of products formed. As described in Sect. 3.2, the sample was used for the estimation of the p-cresol degradation ability of S. marcescens ABHI001 after 18-h incubation; the control sample was also prepared as described in “HPLC analysis” section. For GC analysis, 10 mL of the p-cresol degraded sample was centrifuged for 5 min at 5000 rpm at a constant temperature of 25 °C. The resultant supernatant was acidified to pH 2.0 using 0.5-mol/L H2SO4 (98.0%). Subsequently, it was extracted three times using dichloromethane (99.5%) in an equal ratio in a separating funnel, with shaking at irregular gaps. Moreover, 1 μL of the control and degraded samples were injected into GC equipped with a splitless injector. The temperature of the oven was maintained at 90 °C using a thermal conductivity detector, and GC analysis was performed on a Perkin Elmer column (Porapak Q, 60–80 mesh, 2 M × 1.8 × 2 mm). The detector temperature and auxiliary temperature were set to 150 °C, and nitrogen was used as the carrier gas, with a flow rate of 40 mL/min. The degradation percentage of p-cresol was calculated by comparing the number of peaks and RT of the degraded and control samples.
FT-IR analysis
As described in “HPLC analysis” section, the FT-IR analysis of the p-cresol degraded sample and control samples was conducted on a Perkin Elmer Spectrophotometer (Perkin Elmer, USA) with a scanning range of 4000–500 cm−1. The samples were dried for 30 min in an oven; thereafter, they were directly placed in the sample cell of the spectrophotometer after pelletization with KBr. For degradation analysis, data on peaks were collected from the spectra of the samples.
Results and discussion
Identification of bacterial strains
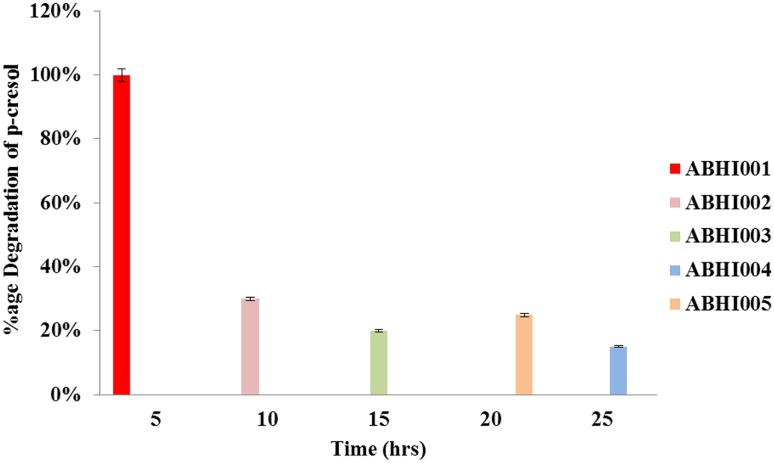
The degradation percentage of p-cresol for all five selected bacterial isolates named ABHI001, ABHI002, ABHI003, ABHI004, and ABHI005 was estimated. As shown in Fig. 2, in contrast to other four isolates, the maximum degradation percentage of p-cresol was observed for ABHI001, showing 100% degradation. Therefore, this strain was selected for further study. The identity of the isolate ABHI001 was confirmed through molecular characterization. The obtained 16S ribosomal DNA sequence of isolated ABHI001 was submitted to GenBank under accession no. KR866113. Phylogenetic analysis of the 16S rDNA sequence was performed using BLAST; the results indicated that the strain exhibited 100% homology with S. marcescens strain ITBB B5-1 (GenBank accession no.: JN896750.1). Based on the results of cladistic analysis and homology assessment, the selected bacterial isolate was designated as S. marcescens ABHI001, belonging to the class Gammaproteobacteria. Similar to all proteobacteria, Gammaproteobacteria are Gram-negative and known for their biodegradation ability of organic compounds (Zhang et al. 2015).
Fig. 2.
Percentage of p-cresol degradation by isolated bacterial strains named as ABHI001, ABHI002, ABHI003, ABHI004, and ABHI005. Initial conditions: concentration of p-cresol = 0.003 mg/L, inoculum concentration = 10%, incubation time = 24 h
Morphological and biochemical analyses of S. marcescens ABHI001 through FE-SEM
An FE-SEM micrograph of S. marcescens ABHI001 is shown in Fig. 3. The micrograph suggests that it is a motile, rod-shaped bacterium with rounded ends, which are the most common characteristic features of S. marcescens. Zarei et al. (2010) and Khanam and Chandra (2015) also reported the same morphological features for S. marcescens B4A and S. marcescens KC1. To characterize and identify the bacterial isolate S. marcescens ABHI001 that exhibited the maximum degradation percentage of p-cresol, both its biochemical and physiological characteristics were examined. After Gram staining, the isolate was examined under a microscope with 1000× magnification. The isolate was found to be a Gram-negative, spore-forming, and rod-shaped bacterium of varying lengths (Fig. 3). Furthermore, biochemical tests, such as urease, catalase, oxidase, and indole production; methyl-red; Voges–Proskauer; and citrate (IMViC) utilization tests were performed, and the results are summarized in Table 1. Zarei et al. (2010) performed biochemical and microbiological analyses to characterize the newly isolated S. marcescens B4A and found that this isolate was indole negative, methyl-red negative, and citrate positive. They also performed the Voges–Proskauer test and found red color formation, indicating its positive reaction.
Fig. 3.

SEM image of isolated bacterial strain Serratia marcescens ABHI001
Table 1.
Characteristics of isolated bacteria strain Serratia marcescens ABHI001
| Characteristics | Serratia marcescens ABHI001 |
|---|---|
| Gram staining | −ve |
| Shape | Short, rod-shaped |
| SIM motility | Motile |
| Voges-Proskaeur | +ve |
| H2S production | −ve |
| Citrate | +ve |
| Catalase production | +ve |
| Oxidase | −ve |
| Indole | −ve |
Biodegradation of p-cresol through UV–visible spectroscopy
UV–visible spectrophotometer analysis was performed to screen the p-cresol degradation ability of the bacterial isolate S. marcescens ABHI001. It was observed that the time required by S. marcescens ABHI001 to degrade p-cresol depended on the initial concentration of p-cresol. The maximum degradation percentage was observed at a p-cresol concentration of 85 mg/L (85%) in 18 h. Degradation decreased as the initial concentration of p-cresol increased. For the initial concentration of 500 mg/L, p-cresol degradation declined to 30% in 18 h, as shown in Fig. 4. Such a reduction in the degradation rate at a higher concentration has been attributed to the toxic effect of p-cresol. Liu et al. (2016) utilized Acinetobacter calcoaceticus PA for the biodegradation of phenol at the initial concentrations ranging between 200 and 2500 mg/L. The strain effectively removed 91.6% of phenol at the initial concentration of 800 mg/L within 48 h and showed an acceptance of phenol concentrations up to 1700 mg/L at a degradation rate of 46.2%. In a previous study, phenol-degrading bacteria incubated with an initial phenol concentration higher than 2000 mg/L showed no growth; this finding was attributed to the inhibitory effect of higher phenol concentrations (Liu et al. 2016). A similar observation was documented by Singh et al. (2008), who demonstrated the p-cresol degradation behavior of Gliomastix indicus over a wide range of the initial p-cresol concentrations (10–700 mg/L). They found that the rate of substrate consumption reduced as the initial concentration increased due to the augmentation of inhibition by the substrate p-cresol (Singh et al. 2008).
Fig. 4.
Effect of the initial p-cresol concentration on degradation of p-cresol by bacteria Serratia marcescens ABHI001. Initial conditions: concentration of p-cresol = 50-500 mg/L, inoculum concentration = 10%, incubation time = 26 h
Biodegradation analysis of p-cresol through HPLC
To understand the degradation product and degradation pathway, HPLC analysis of p-cresol degraded samples was performed against the control. Before degradation, the sample exhibited a single peak with an RT of 1.57 min, confirming the characteristics of pure p-cresol. By contrast, after 18 h of incubation, the sample exhibited various peaks with the RTs of 2.29, 2.34, and 2.56 min, suggesting that the degradation of p-cresol resulted in the formation of different metabolites (Fig. 5). Based on the aforementioned observation, it can be hypothesized that the bacterial strain S. marcescens ABHI001 utilizes p-cresol as the sole carbon and energy sources by converting it into p-hydroxybenzaldehyde through the p-cresol methylhydroxylase enzyme. Thereafter, p-hydroxybenzaldehyde is oxidized into p-hydroxybenzoate by dehydrogenase; subsequently, p-hydroxybenzoate hydroxylated to form protocatechuate through the ortho-cleavage pathway. These results are in agreement with those of Saxena et al. (2013) who performed the HPLC analysis of phenol degradation. They noticed that the peak completely disappeared after 70 h of incubation, indicating almost complete degradation of phenol and the simultaneous accumulation of a new compound with an RT of 7.62. This may be due to the production of catechol as a result of the ortho-ring cleavage of phenol (Saxena et al. 2013). In addition, Bossert et al. reported the ability of a denitrifying bacterial isolate (PC-07) to oxidize p-cresol to p-hydroxybenzoate (pOHB). They established that PC-07 performed the anaerobic oxidation of p-cresol to pOHB through p-hydroxybenzyl alcohol and p-hydroxybenzaldehyde (pHBZ) intermediates (Bossert et al. 1989).
Fig. 5.
Graph shows formation of various metabolites from p-cresol degradation by bacteria Serratia marcescens ABHI001. Initial conditions: concentration of p-cresol = 85 mg/L, inoculum concentration = 10%, incubation time = 18 h
Biodegradation analysis of p-cresol through GC analysis
Before degradation, the p-cresol degraded sample exhibited a single peak with an RT of 1.9 min (Fig. 6a). However, two peaks were obtained after 18 h of incubation, with RTs of 1.72 and 5.28 min, respectively, as shown in Fig. 6b. The transformation in the shape of absorption peaks at varied RT not only showed the alteration of p-cresol into other products, but also suggested the high biodegradability of p-cresol. Lin et al. (2008) performed GC analysis of chlorinated phenols produced from the degradation of an industrial effluent by P. fluorescens isolate. The analysis showed the existence of two phenolic compounds in the effluent. The major compound was observed at 6.10 min, and the minor compound was observed at 7.73 min, indicating the degradation percentage of phenol (Lin et al. 2008). Furthermore, Basheer and Farooqi (2012) conducted chromatographic analysis to ascertain the number of metabolites formed from the biodegradation of p-cresol on the basis of the RT and peak area.
Fig. 6.
a GC analysis of p-cresol (control) before degradation; b GC analysis of p-cresol degradation after 18 h incubation with Serratia marcescens ABHI001
Biodegradation analysis of p-cresol through FT-IR
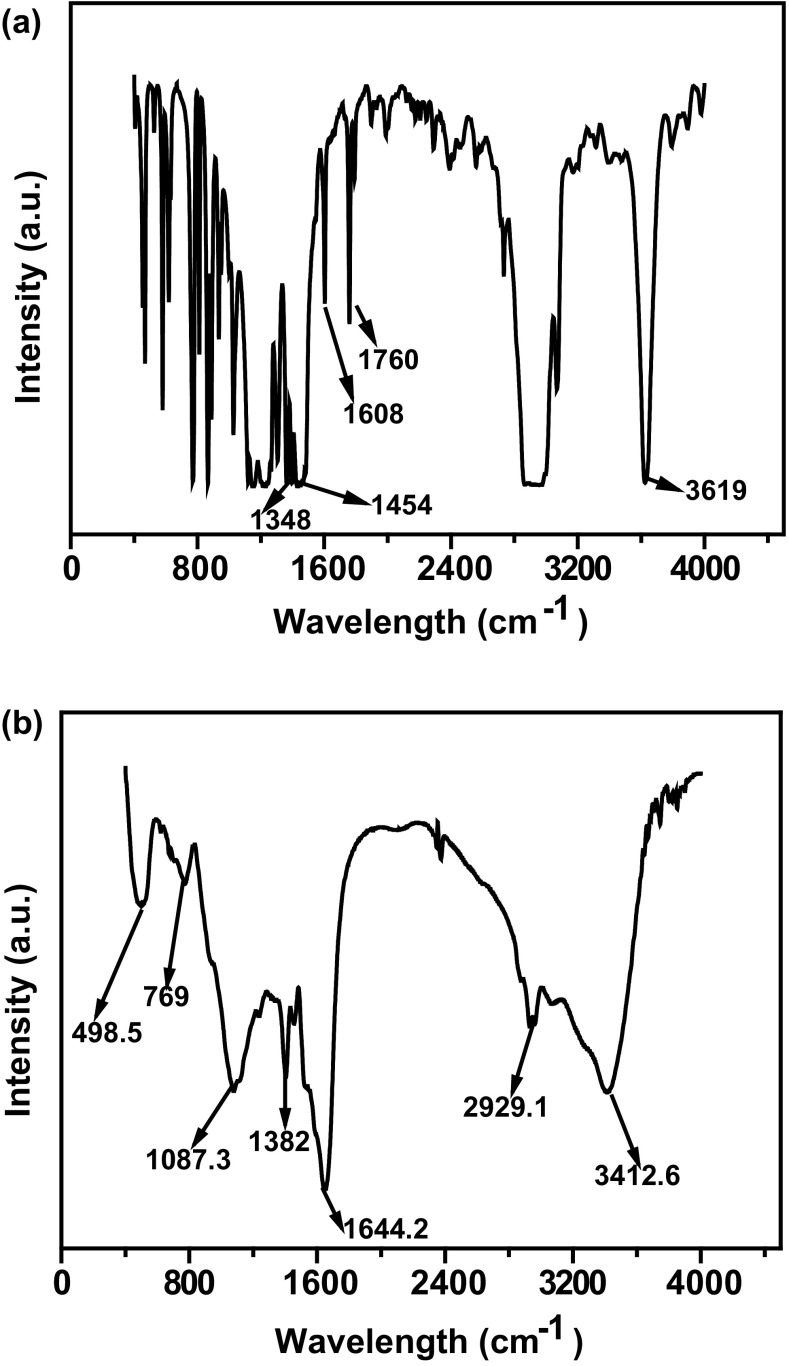
IR is a versatile analytical tool for the characterization of the molecular structure of compounds through characteristic vibrational absorption. Before degradation, the FT-IR spectra of p-cresol (Fig. 7(a)) exhibited a characteristic peak at 3625 cm−1, which was assigned to the presence of an OH group, and a peak at 2956 cm−1, which was assigned to C–H stretching. Peaks at 1750 and 1603 cm−1 were assigned to C=C and C=O stretching, respectively. The peak at 1264 cm−1 was assigned to the presence of a C–O group, and the peaks between 1305 and 1395 cm−1 were assigned to the presence of CH3 bending. Figure 7b depicts significant changes in the FT-IR spectra of p-cresol after 18 h of incubation, showing a shift in the peak at 1644, 1403, 1077, and 2961 cm−1. A broad peak at 3416 cm−1 was evident in the spectra, which was assigned to the presence of an OH group. Thus, the FT-IR analysis confirmed that p-cresol was degraded into other compounds. This phenomenon has also been reported in the study by Edalli et al., in which the IR spectrum of metabolites produced by the degradation of p-cresol showed shifting of characteristic absorption bands vis-à-vis at its initiate state (Edalli et al. 2016). Furthermore, Huang et al. investigated the microbial biodegradation of 3,5-xylenol or m-cresol by P. putida NCIMB 9869 through FT-IR spectroscopy (Huang et al. 2006).
Fig. 7.
a FT-IR analysis of p-cresol (control) before degradation b FT-IR analysis of p-cresol degradation after 18 h incubation with bacteria Serratia marcescens ABHI001
Conclusion
In this study, a laboratory isolate, S. marcescens ABHI001, was reported to degrade p-cresol effectively. The p-cresol degradation efficiency of the strain was confirmed through various analytical studies. The maximum degradation ability of the isolate was found at a p-cresol concentration of 85 mg/L at 18 h, which was confirmed through UV–visible spectroscopy, HPLC, GC, and FT-IR. Hence, the laboratory isolate S. marcescens ABHI001 may be utilized for the industrial-scale degradation of p-cresol discharged from industrial wastewater.
Acknowledgements
The authors T.S., A.K.B., N.S., and P.K.M. acknowledge the Department of Chemical Engineering & Technology IIT (BHU), Varanasi, India, for conducting this study. The authors N.S. and P.K.M. acknowledge IIT (BHU), Varanasi, India, for providing the Institute Post-Doctoral Fellowship.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for cresols (Draft) Altanta: US Public Health Service, US Department of Health & Human Services; 1990. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for cresols. Altanta: U.S. Department of Health and Human Services, Public Health Service; 2008. [PubMed] [Google Scholar]
- Al-Khalid T, El-Naas MH. Aerobic biodegradation of phenols: a comprehensive review. Crit Rev Environ Sci Technol. 2012;42:1631–1690. doi: 10.1080/10643389.2011.569872. [DOI] [Google Scholar]
- Ansari AA, Gill SS, Gill R, Lanza GR, Newman L. Phytoremediation: Management of environmental contaminants. Switzerland: Springer International Publishing AG; 2016. [Google Scholar]
- Balarak D, Mahdavi Y. Survey of efficiency agricultural waste as adsorbent for removal of p-Cresol from aqueous solution. Int Res J Pure Appl Chem. 2016;10:1–11. doi: 10.9734/IRJPAC/2016/20507. [DOI] [Google Scholar]
- Basheer F, Farooqi IH. Biodegradation of p-cresol by aerobic granules in sequencing batch reactor. J Environ Sci. 2012;24:2012–2018. doi: 10.1016/S1001-0742(11)60988-1. [DOI] [PubMed] [Google Scholar]
- Berge-Lefranc D, Vagner C, Calaf R, Pizzala H, Denoyel R, Brunet P, et al. In vitro elimination of protein bound uremic toxin p-cresol by MFI-type zeolites. Microporous Mesoporous Mater. 2012;153:288–293. doi: 10.1016/j.micromeso.2011.11.024. [DOI] [Google Scholar]
- Bisht S, Pandey P, Bhargava B, Sharma S, Kumar V, Sharma KD. Bioremediation of polyaromatic hydrocarbons (PAHs) using rhizosphere technology. Braz J Microbiol. 2015;46:7–21. doi: 10.1590/S1517-838246120131354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert ID, Whited G, Gibson DT, Young LY. Anaerobic oxidation of p-cresol mediated by a partially purified methylhydroxylase from a denitrifying bacterium. J Bacteriol. 1989;171:2956–2962. doi: 10.1128/jb.171.6.2956-2962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouallegue O, Mzoughi R, Weill FX, Mahdhaoui N, Ben Salem Y, Sboui H, Grimont F, Grimont PA. Outbreak of Pseudomonas putida bacteraemia in a neonatal intensive care unit. J Hosp Infect. 2004;57:88–91. doi: 10.1016/j.jhin.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Choquette-Labbé M, Shewa WA, Lalman JA, Shanmugam SR. Photocatalytic degradation of phenol and phenol derivatives using a nano-TiO2 catalyst: integrating quantitative and qualitative factors using response surface methodology. Water. 2014;6:1785–1806. doi: 10.3390/w6061785. [DOI] [Google Scholar]
- Edalli VA, Mulla SI, Eqani SAMAS, Mahadevan GD, Sharma R, Shouche Y, Kamanavall CM. Evaluation of p-cresol degradation with polyphenol oxidase (PPO) immobilized in various matrices. Biotech. 2016;6:1–8. doi: 10.1007/s13205-016-0547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang HHP, Zhou GM. Degradation of phenol and p-cresol in reactors. Water Sci Technol. 2000;42:237–244. [Google Scholar]
- Huang WE, Hopper D, Goodacre R, Beckmann M, Singer A, Draper J. Rapid characterization of microbial biodegradation pathways by FT-IR spectroscopy. J Microbiol Methods. 2006;67:273–280. doi: 10.1016/j.mimet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Joutey NT, Bahafid W, Sayel H, El-Ghachtouli N (2013) Biodegradation: Involved microorganisms and genetically engineered microorganisms. Biodegradation-Life of Science, InTech Open, Croatia, pp 289–320
- Khanam B, Chandra R. Isolation and identification of endophytic bacteria producing bright red pigment from the dye yielding plant Beta vulgaris L. Int J Pharm Pharm Sci. 2015;7:220–224. [Google Scholar]
- Krastanov A, Alexieva Z, Yemendzhiev H. Microbial degradation of phenol and phenolic derivatives. Eng Life Sci. 2013;13:76–87. doi: 10.1002/elsc.201100227. [DOI] [Google Scholar]
- Lim KT, Shukor MY, Wasoh H. Physical, chemical, and biological methods for the removal of arsenic compounds. Biomed Res Int. 2014;2014:1–9. doi: 10.1155/2014/503784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Reddy M, Moorthi V, Qoma BE. Bacterial removal of toxic phenols from an industrial effluent. Afr J Biotechnol. 2008;7:2232–2238. [Google Scholar]
- Liu Z, Xie W, Li D, Peng Y, Li Z, Liu S. Biodegradation of phenol by bacteria strain Acinetobacter calcoaceticus PA isolated from phenolic wastewater. Int J Environ Res Public Health. 2016;13:300. doi: 10.3390/ijerph13030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michałowicz J, Duda W. Phenols–sources and toxicity. Polish J Environ Stud. 2007;16:347–362. [Google Scholar]
- Murthy A, Gayathri KV. Halotolerant bacterial consortium able to degrade substituted phenolic compounds isolated from saline environment. Environ Risk Assess Remediat. 2017;1:22–29. [Google Scholar]
- Ren Y, Peng L, Zhao G, Wei C. Degradation of m-cresol via the ortho cleavage pathway by Citrobacter farmeri SC01. Biochem Eng J. 2014;88:108–114. doi: 10.1016/j.bej.2014.03.021. [DOI] [Google Scholar]
- Reshma JK, Mathew A. Biodegradation of phenol-aerobic and anaerobic pathways. Int J Sci Nat. 2014;5:366–387. [Google Scholar]
- Saxena M, Gupta S, Mahmooduzzafar Kumar R, Kumar A. Identification and genetic characterization of phenol-degrading bacterium isolated from oil contaminated soil. Afr J Biotechnol. 2013;12:791–797. [Google Scholar]
- Singh RK, Kumara S, Kumara S, Kumar A. Biodegradation kinetic studies for the removal of p-cresol from wastewater using Gliomastix indicus MTCC 3869. Biochem Eng J. 2008;40:293–303. doi: 10.1016/j.bej.2007.12.015. [DOI] [Google Scholar]
- Surkatti R, El-Naas M. Biological treatment of wastewater contaminated with p-cresol using Pseudomonas putida immobilized in polyvinyl alcohol (PVA) gel. J Water Process Eng. 2014;1:84–90. doi: 10.1016/j.jwpe.2014.03.008. [DOI] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- World Health Organization . International Standards for Drinking Water. Geneva: World Health Organization; 1963. [Google Scholar]
- Xenofontos E, Tanase AM, Stoica I, Vyrides I. Newly isolated alkalophilic Advenella species bioaugmented in activated sludge for high p-cresol removal. N Biotechnol. 2016;33:305–310. doi: 10.1016/j.nbt.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Yao H, Ren Y, Wei C, Yue S. Biodegradation characterisation and kinetics of m-cresol by Lysinibacillus cresolivorans. Water SA. 2011;37:15–20. doi: 10.4314/wsa.v37i1.64101. [DOI] [Google Scholar]
- Zarei M, Aminzadeh S, Zolgharnein H, Safahieh A, Ghoroghi A, Motallebi A, Daliri M, Lotfi AS. Serratia marcescens B4A chitinase product optimization using Taguchi approach. Iran J Biotechnol. 2010;8:252–262. [Google Scholar]
- Zhang Z, Lo IMC, Yan DYS. An integrated bioremediation process for petroleum hydrocarbons removal and odor mitigation from contaminated marine sediment. Water Res. 2015;83:21–30. doi: 10.1016/j.watres.2015.06.022. [DOI] [PubMed] [Google Scholar]