Abstract
Introduction:
Allergic rhinitis (AR) is one of the most common allergic diseases, which affects ∼20% of the world's population. T-helper (Th) type 2 cells produce interleukin (IL) 4 and IL-13, and mediate allergic responses, and these cytokines have been extensively studied as key players in the atopic airway diseases. However, the involvement of Th17 cells and IL-17 in AR has not been clearly examined.
Aim:
To reevaluate AR clinical severity with serum IL-17, whether IL-17 affects the disease alone or in contribution with the atopic predisposition.
Patients and Methods:
During an 18-month period, 39 individuals were divided into three groups: A, (13 control), B (13 with mild-to-moderate AR), and C (13 with severe AR). Both group B and group C patients (26) were subjected to clinical examination and allergy skin testing, and to measurement of both total serum immunoglobulin E (IgE) and IL-17 levels. Eleven patients with AR then were exposed to 6 months of cluster immunotherapy, whereas the rest of the patients were not exposed.
Results:
Revealed a significant elevation of serum IL-17 levels with an associated increase in serum IgE in the patients with AR compared with controls and revealed that the serum levels of both total serum IgE and IL-17 decreased significantly after cluster immunotherapy.
Conclusion:
These preliminary results added new data about the use of injective immunotherapy as well as reported on the use of sublingual immunotherapy.
Keywords: Allergic rhinitis, correlation between IL-17 and severity, IgE, IL-17 relation to immunotherapy, immunotherapy, interleukin 17, severity of allergy
Allergic rhinitis (AR) is one of the most common allergic diseases and affects ∼20% of the world's population.1 In Western countries, between 10 and 30% of people are affected in a given year. It is most common between the ages of 20 and 40 years.2 The prevalence of AR among Egyptian school children is high, and estimates among schoolchildren in the Al Maasara and Al Maadi regions were 12.33%.3 AR has become increasingly prevalent in the Middle East Gulf region; estimates indicate that up to 36% of the region's population may be affected.4 Approximately 10–40% of patients have asthma comorbidity, whereas most patients with asthma have AR. AR is the most common cause of rhinitis.5
Regulation between T-helper (Th) type 1 and Th2 cells (Th1/Th2 balance) has been considered to be important for the homeostasis of the body's immune regulation, and dysregulation of the Th1/Th2 balance leads to excessive Th1 or Th2 cell activation, which results in autoimmune diseases or allergic diseases, respectively. This Th1/Th2 paradigm has been widely accepted for the past 2 decades. For many years, it has been known that, in AR, after deposition of allergens into the mucus layer, the allergens are taken up by antigen-presenting cells and are processed and presented to helper T lymphocytes. Activated helper T lymphocytes release cytokines, such as interleukin (IL) 4 and IL-13, and interact with B lymphocytes to induce the synthesis of allergen specific IgE. Thereafter, the allergen-specific IgE binds to the high affinity receptor for IgE on the surface of mast cells.6
Allergen induces Th2 lymphocyte proliferation in persons with allergies with the release of their characteristic combination of cytokines, including IL-3, IL-4, IL-5, IL-9, IL-10, and IL-13. These substances promote IgE and mast cell production.7 So, a diagnosis of AR is performed by clinical parameters as well as measurement of specific IgE level and even the total serum IgE level. There currently is no accepted screening test for differentiating AR from diseases with similar symptoms. The measurement of the total serum IgE level is a low-cost test that is used in the diagnosis of AR.8 The new T-helper cell subset, Th17, was identified and characterized. Th17 cells are known to be involved with autoimmune disease and neutrophil infiltration. The discovery of Th17 added additional complexity to the existing Th1/Th2 balance paradigm. The role of Th17 cells in AR is not clear and is still controversial.9 IL-17, as a major family cytokine, is usually associated with autoimmune reaction or neutrophil inflammation. Nevertheless, it has been demonstrated that allergic sensitization through the airway promotes a strong Th17 response and acute bronchial hyperactivity in mouse model of asthma.10
The role of IL-17 and/or Th17 cells in asthma has been extensively studied in mouse models.11 However, human data are scarce. Studies to unravel the role of IL-17A in asthma started in the late 1990s by expression studies in airway cells. IL-17A was shown to be expressed in bronchial biopsy specimens, bronchoalveolar lavage fluid, and sputum of patients with asthma.12 Furthermore, a recent study showed that, on bronchial house-dust mite challenge, systemic IL-17A levels increase in individuals with house-dust mite allergy.13 Th2 cells produce IL-4 and IL-13, and mediate allergic responses, and these cytokines have been extensively studied as key players in atopic airway diseases. The roles of IL-4 in IgE production and IL-13 in bronchial hyperresponsiveness and tissue remodeling are evident.14 However, the involvement of Th17 cells and IL-17 in AR has not been clearly examined.15 In this study, we investigated the possible role of IL-17 in the severity of AR.
METHODS
The study was conducted on patients with persistent allergic rhinitis (AR) who Attend Ear Nose and Throat outpatient clinics, allergy, and immunology clinics at Suez Canal's university hospital. The study was carried out from August 2014 to January 2016. The patients were selected by simple random sampling.
Study Population
The study included 39 individuals who were divided into three groups: group A included 13 healthy individuals used as a control group for both groups B and C; group B included 13 patients with mild-to-moderate AR; and group C included 13 patients with severe AR. The patients were classified according to Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines16 for classification of severity of AR. The patients in groups B and C were subjected to clinical examination to verify the severity of their AR. The patients in both group B and group C (26) were subjected to allergy skin testing, and, based on these results, the 26 patients were divided into two subgroups as follows: subgroup 1, 11 patients whose skin tests results were positive for house-dust mite; subgroup 2, 15 patients composed of 9 patients with negative skin tests results and 6 patients with positive skin test results to allergens other than house-dust mite The patients of both subgroups received medical treatment according to ARIA guidelines. The patients in subgroup 1 received cluster immunotherapy for 6 months, and the patients in subgroup 2 were exposed to immunotherapy for allergic rhinitis. The patients in subgroups 1 and 2 were then reevaluated with regard to total serum IgE and serum IL-17 levels after 6 months. Because subgroup 1 received 6 months of cluster immunotherapy and subgroup 2 did not, subgroup 2 was used as the control to subgroup 1 with regard to immunotherapy.
Tools
The used tools was the following:
A structured questionnaire according to the ARIA scoring system
True Visual Analogue Scale (VAS) scores for global assessment of severity of nasal and non-nasal symptoms
Skin scratch testing according to the European Academy of Allergy and Clinical Immunology
Total serum IgE level by using VIDAS (Omega Laboratories Ltd., Montreal, Canada)
Enzyme-linked immunosorbent assay (ELISA) for measuring of serum IL-17 levels
Allergenic extracts used for cluster immunotherapy
Scoring of clinical symptoms by VAS
The patients evaluated their clinical status subjectively on a seven-point VAS; they were asked to globally rate the combination of the nasal and non-nasal symptoms on this seven-point scale: 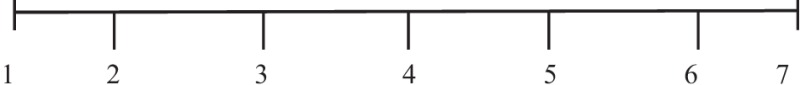 . We recorded the score in the results of this study as the following: none, 1–2; mild, 3–4; moderate, 5–6; severe, 7.
. We recorded the score in the results of this study as the following: none, 1–2; mild, 3–4; moderate, 5–6; severe, 7.
Skin Tests for Allergy
Skin testing was performed according to the practice parameters by Bernstein et al.17 for the following allergens using the vaccine of Omega Laboratory Ltd:

Measurement of Skin Reaction
The longest and orthogonal diameters of any resultant wheal and erythema were measured by using the practice parameters by Bernstein et al.,17 and the results were interpreted as shown in (Table 1).
Table 1.
Interpretation of results of the skin test
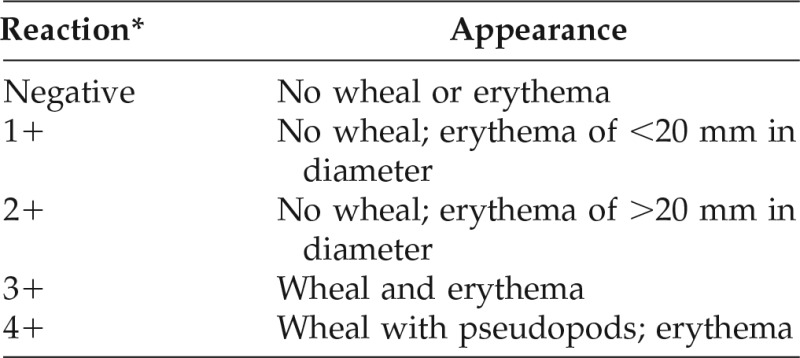
*A result of ≥2+ is considered positive.
In Vitro Parameters
Measurement of Total Serum IgE.
VIDAS total IgE is an automated immunoenzymatic quantitative test for determination of total human IgE levels in serum and plasma. All assay steps are performed automatically by the VIDAS instrument. They consist of a succession of suction cycles and discharge of the reaction medium. At the end of the test, the results are calculated automatically by the instrument relative to a calibration curve stored and then are printed.
Measurement of Serum IL-17.
Blood samples were taken from the patients in each group for measurement of IL-17 levels by ELISA before and after immunotherapy. The ELISA kit for IL-17 by using the multiple antibody sandwich principle was purchased from E. Bioscience (Omega Laboratories Ltd.), affymatrix. This kit includes a monoclonal antibody as a first antibody, rabbit polyclonal a, and biotin-labeled goat-antirabbit antibody as a second antibody, followed by horseradish-peroxidase-conjugated streptavidin.
Materials of Immunotherapy.
Bulk extract to mite mix for subcutaneous cluster immunotherapy (10,000 AU/mL) was purchased from Omega Laboratory.
Administration of Subcutaneous Cluster Immunotherapy.
Administration of subcutaneous cluster immunotherapy was assessed according to parameters of Cox et al.18 Bulk allergen extracts of Mite Mix (Dermatophagoides farinae, 50%; Dermatophagoides pteronyssinus, 50% [10,000 AU/mL]) were aseptically diluted in sterile 10% glycerol–saline solution plus 0.5% phenol under aseptic condition. The preparation begins with the preparation of the vial that contains the maintenance concentrate by adding equal volumes of both the bulk allergen extract brought from the company plus the same volume of the diluent so that the concentration was 1:1 (5000 AU/mL) (vial 1). Diluted extract was administrated subcutaneously in the deltoid region of the arm by using a 1-mL tuberculin syringe, and the course of immunotherapy was composed of 2 stages: stage 1 (buildup phase), there was a buildup of doses from the lowest dose until we reached the maintenance dose. This was done over 9 visits at weekly intervals as illustrated in Table 2; and stage 2 (maintenance phase), the patients received regular dose weekly, starting with the last dose in stage 1; this should be continued for at least 2–3 years.18
Table 2.
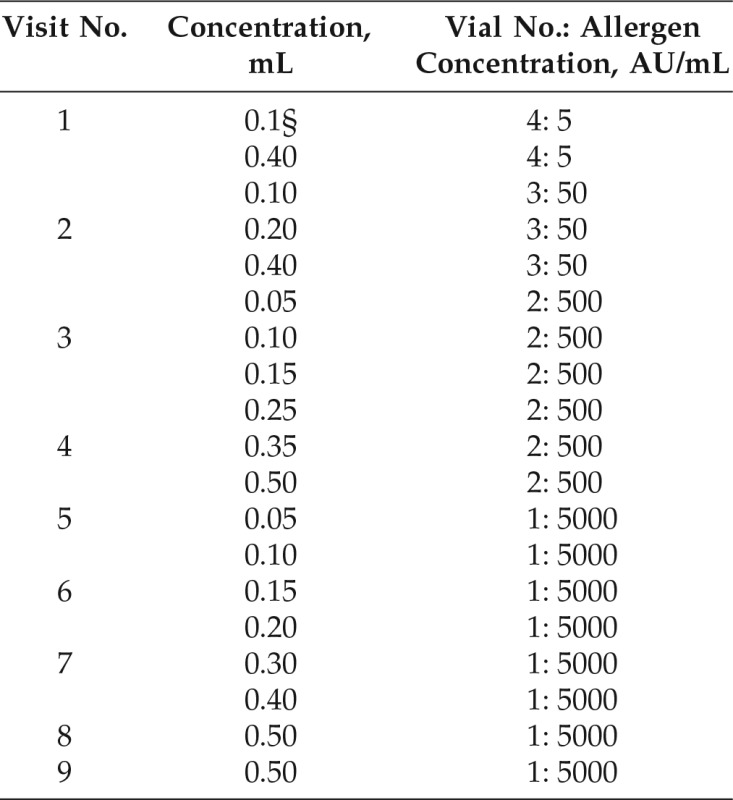
From Ref. 17.
The interval between injections was 30 min; the interval between visits was 1 wk.
Safety Precautions Taken before the Visit.
Premedication, histamine (H1) blocker and montelukast, was given 1 day before and the morning of the procedure during the buildup phase. The patients took both pills at the same time.
Ethics Committee Approval
The Faculty of Medicine, Suez Canal University Research Ethics Committee approved the proposal for the study, entitled “The study of possible correlation between serum levels of IL-17 and clinical severity in patients with allergic rhinitis” (research no. 2253; approval January 14, 2014).
RESULTS
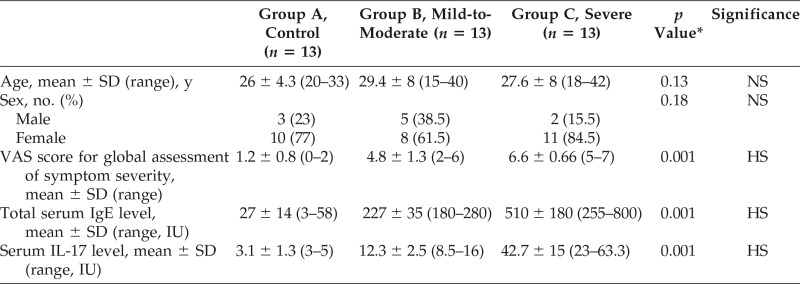
The results of this study showed that the total serum IgE levels were significantly high in patients with mild-to-moderate or severe AR versus controls (Table 3). This study also showed that the serum IL-17 levels were significantly high in the AR mild-to-moderate and AR severe groups versus the control group (Table 3). It was also noted that the total serum IgE levels decreased after 6 months of cluster immunotherapy compared with levels measured before immunotherapy and compared with levels taken without immunotherapy (Table 4). Analysis of the results revealed that the serum IL-17 levels decreased after 6 months of cluster immunotherapy compared with serum IL-17 levels measured before immunotherapy and compared with serum IL-17 levels taken in those who did not have immunotherapy.
Table 3.
Comparison of the control, mild-to-moderate, and severe allergic rhinitis groups with regard to age, sex, VAS score, total serum IgE levels, and serum IL-17 levels
VAS = Visual analog scale; IgE = immunoglobulin E; IL-17 = interleukin 17; NS = non-significant; HS = highly significant.
*The p values for group comparison by using one-way analysis of variance.
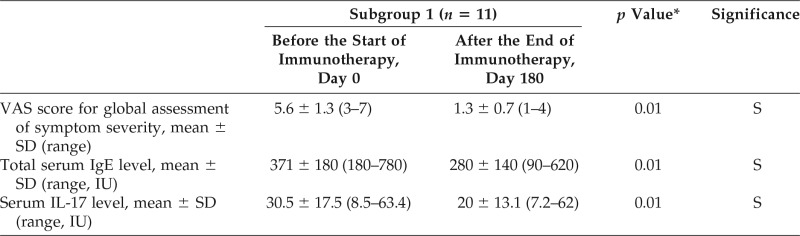
Table 4.
Comparison of the VAS score, total serum IgE, and serum IL-17 levels in group 1 before and after treatment of the group by cluster immunotherapy for 6 months
VAS = Visual analog scale; IgE = immunoglobulin E; IL-17 = interleukin 17; SD = standard deviation; S = significance.
*The p values for between group comparison assed by the paired sample t-test.
DISCUSSION
The results of this study indicated that the total serum IgE levels were significantly high in patients with AR of either mild-to-moderate or severe groups versus controls (Table 3). This result agreed with previous studies that showed a positive relationship of raised IgE levels with atopic dermatitis, conjunctivitis, and AR.19 Kerkhof et al.20 also stated that sensitization to common allergens is usually assessed by measuring specific IgE levels in serum or by performing a skin test to identify the specific allergens. Cox et al.21 reported that a diagnosis of allergy is most often done by reviewing a person's medical history and when finding a positive result for the presence of allergen-specific IgE when conducting a skin or blood test.
The results of this study also showed that the serum IL-17 levels were significantly high in the AR of the mild-to-moderate and severe groups versus the control group (Table 3). This result agreed with Ba et al.,22 who showed that the number of IL-17 cells in tissues of patients with AR was significantly higher than that of the controls. They also showed that the eosinophilic cell count correlated with the number of IL-17 cells. Meanwhile, this result disagreed with Liu et al.,23 who reported that there were no significant differences between patients with AR and patients without AR in the protein expression of IL-17 in inferior turbinate tissues. Our results also agreed with Nieminen et al.,24 who showed that serum IL-17A levels and allergen-induced IL-17A messenger RNA expression correlate with symptom severity, as assessed via a VAS and symptom medication score, respectively.
Furthermore, results of a recent study indicated that, on bronchial house-dust mite challenge, systemic IL-17A levels increase in individuals with house-dust mite allergy.13 Recently, the relationship between IL-17 levels and the severity of atopic eczema/dermatitis syndrome was demonstrated, and, furthermore, the relationship with different phenotypes in children indicates IL 17 level and relation to severitys' role as marker of “atopic march” and disease severity. Serum IL-17, IL-23, and IL-10 values in children with atopic eczema/dermatitis syndrome have an association with clinical severity and phenotype.25
Recent studies demonstrated that both Th2 and Th17 are involved in the pathogenesis of allergic airway inflammation through releasing specific cytokines.26 The specific T cells stimulate the production of allergen-specific IgE, production of IgE is essential step in AR.27 A recent study about IgE production in human B cells found that IL-17 could induce B cells switching to IgE, which implies Th17 involvement in the atopic phenomenon.28 In fact, allergic inflammation is typically continuous and persistent until allergen exposure occurs.
In conclusion, this study provides evidence that serum IL-17 levels are significantly related to allergy severity. Thus, increased IL-17 serum levels might be considered a marker of allergy severity in patients with AR. The atopic state of the individual is the major determining factor that affects both the development and severity of AR as has been known for many years. One of the new function of IL-17 is its role in autoimmune disease.
Results of our study showed that IL-17 levels were not only associated with AR but also that the levels increased with increasing severity of the disease, so the function of IL-17 works together with IgE for the development of an atopic state in a way that may need further research to be fully understood. The results of our study revealed that the total serum IgE levels decreased after 6 months of cluster immunotherapy compared with levels measured before immunotherapy and compared with levels taken of those patients who did not receive immunotherapy (Table 4).
Our results agreed with Akdis and Akdis,29 who showed that the allergen-specific immunotherapy has effects that include the modulation of T-cell and B-cell responses and related antibody isotypes as well as effector cells of allergic inflammation, such as eosinophils, basophils, and mast cells. The induction of a tolerant state in peripheral T cells represents an essential step in allergen-specific immunotherapy. Peripheral T-cell tolerance is characterized mainly by generation of allergen-specific T regulatory cells, which leads to suppressed T-cell proliferation and Th1 and Th2 cytokine responses against the allergen. This is accompanied by a significant increase in allergen-specific IgG4, and also IgG1 and IgA, and a decrease in IgE levels in the late stage of the disease.29
The results of our study revealed that the serum IL-17 levels decreased after 6 months of cluster immunotherapy compared with levels measured before immunotherapy and compared with levels taken from those who did not receive immunotherapy (Table 4). This result agreed with Ding et al.,30 who showed that the mechanism of action of sublingual immunotherapy (SLIT) for the treatment of AR and asthma may be associated with inhibition of IL-17 expression and also agreed with Sakashita et al.,31 who showed that long-term SLIT reduced the serum levels of IL-17. So, the serum level of IL-17A might prove useful as a biologic parameter to ascertain the effectiveness of SLIT.
CONCLUSION
These preliminary results add new data on the use of injective immunotherapy as well as reported on the use of SLIT.
Footnotes
No external funding sources reported
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Burns P, Powe DG, Jones NS. Idiopathic rhinitis. Curr Opin Otolaryngol Head Neck Surg 20:1–8, 2012. [DOI] [PubMed] [Google Scholar]
- 2. Wheatley LM, Togias A. Clinical practice. Allergic Rhinitis. N Engl J Med 372:456–463, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al Dhduh MA, Sabri NA, Fouda EM. Prevalence and severity of allergic diseases among Egyptian pediatric indifferent Egyptian areas. Int J Pharm Sci Res 2:107, 2015. [Google Scholar]
- 4. Alsowaidi S, Abdulle A, Shehab A, et al. Allergic rhinitis: Prevalence and possible risk factors in a Gulf Arab population. Allergy 65:208–212, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Bousquet J, Schünemann HJ, Samolinski B. World Health Organization Collaborating Center for Asthma and Rhinitis. Allergic Rhinitis and its Impact on Asthma (ARIA): Achievements in 10 years and future needs. J Allergy Clin Immunol 130:1049–1062, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Haberal I, Corey JP. The role of leukotrienes in nasal allergy. Otolaryngol Head Neck Surg 129:274–279, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Rihoux JP. Perennial allergic rhinitis and keratoconjunctivitis. Thorax 55(suppl. 2):S22–S23, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallace WJ. The diagnosis and management of rhinitis: An updated practice parameter. J Allergy Clin Immunol 122 (suppl.):S1–S84, 2008. [DOI] [PubMed] [Google Scholar]
- 9. Oboki K, Ohno T, Saito H, Nakae S. Th17 and allergy. Allergol Int 57:121–134, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Wilson RH, Whitehead GS, Nakano H, et al. Allergic sensitization through the airways primes Th17-dependant neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med 180:720–730, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cosmi L, Liotta F, Maggi E, et al. Th17 cells: New players in asthma pathogenesis. Allergy 66:989–998, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Bullens DM, Truyen E, Coteur L, et al. IL-17 mRNA in sputum of asthmatic patients: Linking T cell driven inflammation and granulocytic influx. Respir Res 7:135, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bajoriuniene I, Malakauskas K, Lavinskiene S, et al. Response of peripheral blood Th17 cells to inhaled Dermatophagoides pteronyssinus in patients with allergic rhinitis and asthma. Lung 190:487–495, 2012. [DOI] [PubMed] [Google Scholar]
- 14. Williams CM, Rahman S, Hubeau C, Ma HL. Cytokine pathways in allergic disease. Toxicol Pathol 40:205–215, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Mo JH, Chung YJ, Kim JH. T cell transcriptional factors in allergic rhinitis and its association with clinical features. Asia Pac Allergy 3:186–193, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. (Epub ahead of print June 8, 2017.) [DOI] [PubMed] [Google Scholar]
- 17. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: An updated practice parameter. Ann Allergy Asthma Immunol 100(3 Suppl 3):S1–S148, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: A practice parameter third update. J Allergy Clin Immunol 127(suppl.):S1–S55, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Rathore AW, Randhawa SM, Ain QU, Maqbool S. Wheezing conditions in early childhood: Prevalence and risk factors among preschool children. Ann King Edward Med Coll 11:14–16, 2005. [Google Scholar]
- 20. Kerkhof M, Dubois AE, Postma DS, et al. Role and interpretation of total serum IgE measurements in the diagnosis of allergic airway disease in adults. Allergy 58:905–911, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Cox L, Williams B, Sicherer S, et al. Pearls and pitfalls of allergy diagnostic testing: Report from the American College of Allergy, Asthma and Immunology/American Academy of Allergy, Asthma and Immunology Specific IgE Test Task Force. Ann Allergy Asthma Immunol 101:580–592, 2008. [PubMed] [Google Scholar]
- 22. Ba L, Du J, Liu Y, et al. The expression and significance of interleukin-17 and the infiltrating eosinophils in nasal polyps and nasal mucous of allergic rhinitis [in Chinese with English abstract]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zh 24:53–56, 2010. [PubMed] [Google Scholar]
- 23. Liu Z, Lu X, Wang H, et al. The expression of transforming growth factor beta1, interleukin-6, 11 and 17 in nasal mucosa of allergic rhinitis patients [in Chinese with English abstract]. Lin Chuang Er Bi Yan Hou Ke Za Zhi 20:625–627, 2006. [PubMed] [Google Scholar]
- 24. Nieminen K, Valovirta E, Savolainen J. Clinical outcome and IL-17, IL-23, IL-27 and FOXP3 expression in peripheral blood mononuclear cells of pollen-allergic children during sublingual immunotherapy. Pediatr Allergy Immunol 21(pt. 2):e174–e184, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Tsybikov NN, Egorova EV, Kuznik BI, et al. Biomarker assessment in chronic rhinitis and chronic rhinosinusitis: Endothelin-1, TARC/CCL17, neopterin, and α-defensins. Allergy Asthma Proc 37:35–42, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Lim H, Kim YU, Yun K, et al. Distinct regulation of Th2 and Th17 responses to allergens by pulmonary antigen presenting cells in vivo. Immunol Lett 156:140–148, 2013. [DOI] [PubMed] [Google Scholar]
- 27. Rosenwasser LJ. Current understanding of the pathophysiology of allergic rhinitis. Immunol Allergy Clin North Am 31:433–439, 2011. [DOI] [PubMed] [Google Scholar]
- 28. Milovanovic M, Drozdenko G, Weise C, et al. Interleukin-17A promotes IgE production in human B cells. J Invest Dermatol 130:2621–2628, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol 119:780–791, 2007. [DOI] [PubMed] [Google Scholar]
- 30. Ding LF, Chen Q, Li L, et al. Effects of sublingual immunotherapy on serum IL-17 and IL-35 levels in children with allergic rhinitis or asthma [in Chinese with English abstract]. Zhongguo Dang Dai Er Ke Za Zhi 16:1206–1210, 2014. [PubMed] [Google Scholar]
- 31. Sakashita M, Yamada T, Imoto Y, et al. Cytokine. Long-term sublingual immunotherapy for Japanese cedar pollinosis and the levels of IL-17A and complement components 3a and 5a. Cytokine 75:181–185, 2015. [DOI] [PubMed] [Google Scholar]