Abstract
Background:
There is an ongoing discussion concerning the potential origins of chronic rhinosinusitis with nasal polyposis (CRSwNP).
Objective:
The aim of this study was to quantify subpopulations of T cells in peripheral blood and nasal polyps in CRSwNP to examine their influence on the etiology of this disease.
Methods:
Tissue and blood samples were collected from 11 patients who underwent nasal sinus surgery, and these samples were analyzed by multicolor flow cytometry.
Results:
There was a significantly lower frequency of CD4+ T-helper (Th) cells and a significantly higher frequency of CD8+ T cells among lymphocytes isolated from nasal polyps compared with peripheral blood mononuclear cells (PBMC). In both T-cell subpopulations, a shift mainly from naive T cells among peripheral blood lymphocytes toward an effector memory and terminally differentiated subtype predominance in nasal polyps was observed. Among CD4+ T cells, the frequencies of cluster of differentiation (CD) 45RA- Forkhead-Box-Protein P3high (FoxP3high) cytotoxic T-lymphocyte-associated Protein 4high (CTLA-4high) activated regulatory T (Treg) cells, and CD45RA- Forkhead-Box-Protein P3low (FoxP3low) memory T cells were significantly increased in nasal polyps compared with PBMC.
Conclusion:
In this study, we presented a detailed characterization of CD4+ and CD8+ T-cell subpopulations in patients with CRSwNP. CD8+ T cells were more prominent in nasal polyps than in CD4+ T cells. Both nasal CD8+ T cells and CD4+ T cells predominantly had an effector memory phenotype. Among CD4+ T cells, activated Treg cells were increased in nasal polyps compared with PBMC. The data point toward a local regulation of T-cell composition within the microenvironment of nasal polyps, which might be further exploited in the future to develop novel immunotherapeutic strategies.
Keywords: Chronic rhinosinusitis with nasal polyps, chronic rhinosinusitis without nasal polyps, T cell subpopulations, CD4+ T cells, CD8+ T cells, regulatory T cells, memory T cells, conventional T cells, Forkhead box protein 3, human leukocyte antigen–antigen D related
Chronic rhinosinusitis (CRS) is an inflammatory condition of the mucosal nasal tissue that persists over at least 12 weeks or that demonstrates more than four episodes of infections per annum. The development of nasal polyps subdivides CRS into two different groups: CRS with nasal polyposis (CRSwNP) and CRS without nasal polyposis (CRSsNP).1 CRSwNP can be further subclassified into two endotypes, depending on the histologic findings with either eosinophilic or fibrotic (noneosinophilic) cell infiltration. Among white patients in Europe and the United States, the most common subtype is eosinophilic, whereas Asian patients most often develop fibrotic nasal polyps.2 The statistics on CRS are heterogeneously discussed in the literature: the prevalence of CRS is described to be ∼5% (range, 1–19%) whereas the subgroup CRSwNP has a prevalence of ∼2%.3 There is still an ongoing discussion about the potential origins of nasal polyps, including a chronic inflammatory reaction of the nasal mucosa, anatomic variations that lead to constrictions of draining passages, or an allergic diathesis.
Recently, noninvasive colonization with fungi, including Aspergillus fumigatus and Candida albicans4–6 or with the bacterium Staphylococcus aureus, has been indicated to act as triggers for the development of nasal polyps.7,8 Moreover, it is believed that fungal proteins4 and/or bacterial superantigens9–11 induce recruitment of T cells into the nasal mucosa. Apart from the sheer number of T cells recruited, variations in T-lymphocyte subpopulations, especially of regulatory T (Treg) cells,12 are also considered to crucially contribute to the development of nasal polyps. Immunomodulatory properties that induce variations in T-lymphocyte subpopulations, indeed, have been noted both for fungal13,14 and for staphylococcal proteins.15 It is possible to classify Treg cells into two subpopulations, depending on their origin: natural Treg cells from the thymus and peripherally induced Treg cells, which develop from peripheral naive T-helper (Th) cells.16,17 A deficiency of peripheral Treg cells can generate a chronic T-cell–mediated immunopathology. Reduced numbers and/or the function of Treg cells were demonstrated to be responsible for different kinds of autoimmune disorders and chronic infections.16,18 Even in oncologic studies, the importance of Treg cells has been shown, and many researchers have focused on their influence.19,20
For many years, Treg cells were identified by the surface markers CD4, CD25, and the intracellular transcription factor Forkhead-Box-Protein P3 (FoxP3).21 FoxP3 is a key regulator of Treg cell function and development.16 Miyara et al.,22 however, managed to further differentiate Treg cells into subpopulations of CD4+ FoxP3+ T cells based on the surface marker CD45RA and the checkpoint inhibitor cytotoxic T-lymphocyte-associated Protein 4 (CTLA-4). In this context, resting Treg (rTreg) cells, activated Treg (aTreg) cells, and conventional nonsuppressive memory T cells with a low expression of Forkhead-Box-Protein P3 (FoxP3low) were defined.22,23 For identifying these subpopulations and analyzing the differences between peripheral and edaphic T cells, flow cytometry analysis is the method of choice.18,22,24,25 The aim of this study was to compare subpopulations of T cells in peripheral blood and nasal polyps of patients with CRSwNP by using an up-to-date panel of markers for effector and Treg cell subsets to elucidate their influence on the etiology of this disease.
METHODS
Preparation of Human Lymphocytes
Heparinized blood samples (10 mL) were obtained during surgery by venous puncture from 11 patients who were undergoing nasal sinus surgery, and the samples were transferred to the laboratory. Lymphocytes were separated by density-gradient centrifugation (10 minutes, 1000 × g) at room temperature on equal amounts of Ficoll (Biochrom GmbH, Berlin, Germany) by using a membrane-containing 10-mL cell tube (Greiner Bio-One GmbH, Frickenhausen, Germany). After washing twice in phosphate-buffered saline (PBS) solution (Gibco; BRL Life Technologies, Eggenstein, Germany), the cell number and viability were determined by using a cell counter plus analyzer system (CASY TT; Roche Innovatis AG, Reutlingen, Germany) according to the manufacturer's protocol. After centrifugation at 1600 rpm, the cells were frozen to −80°C with 1 mL of freezing medium, which contained 10 parts of fetal calf serum (Linaris Biologische Produkte GmbH, Dossenheim, Germany) and one part of Dimethyl sulfoxide (DMSO) (Carl Roth GmbH + Co. KG, Karlsruhe, Germany).
Preparation of Tissue Samples
All tissue samples were collected during surgery from the 11 patients who were undergoing regular nasal sinus surgery. Polyp tissue specimens were processed to obtain tissue-associated lymphocytes; healthy nasal mucosa served as the control tissue. The polyps were cut into small fragments and mashed through a cell stainer (Greiner Bio-One, Frickenhausen, Germany) from 100 to 40 μm in PBS solution (Gibco; BRL Life Technologies). After washing twice in PBS solution, the cell number and viability were determined by using a CASY TT system according to the manufacturer's protocol. After centrifugation (5 minutes, 1600 rpm), the cells were frozen to −80°C with 1 mL of freezing medium.
Flow Cytometry Analysis
The following antibodies were used: anti-CD45 Pacific Orange, anti-CD3 R-Phycoerythrin.cyanine dye 7 (PE.Cy7), anti-CD4 Pacific Blue, anti-CD8a Alexa 700, anti-CD28 R-Phycoerythrin (PE), anti-CD45RA Peridinin.Chlorophyll protein-Cyanin5.5 (Per.CP-Cy5.5), anti-C-C chemokine receptor type 7 (CCR7) Alexa 488, anti-CD25 Allophycocyanin (anti-CD25 APC), anti-Human Leukocyte Antigen - antigen D Related (HLA-DR) Alexa 700, anti-CD4 Fluorescein isothiocyanate (FITC), anti-Forkhead-Box-Protein P3 (FoxP3) Pacific Blue and anti-cytotoxic T-lymphocyte-associated Protein 4 (CTLA-4) R-Phycoerythrin (PE) (all from BioLegend, San Diego, California). Gating started on forward and side scatter properties, then CD45 was identified. Within this population, CD3+ cells were detected, in addition, with Viability Dye 780 (eBioscence, Inc., San Diego, CA) in this step to detect apoptotic cells. Afterward, gating on viable CD3+ CD4+ or CD3+ CD8+ T cells followed, and CD4+ and CD8+ T-cell subsets were identified. Isotype control was performed by using mouse immunoglobulin G (mouse-IgG) Allophycocyanin and mouse-IgG R-Phycoerythrin (BioLegend) for a better discrimination of CTLA-4 and CD25.
The other populations could be easily discriminated. After blocking with 25 μg/mL of normal mouse IgG (Sigma-Aldrich, Co., St. Louis, MO) for 15 minutes on ice, all the cells underwent cell surface staining, followed by intracellular staining. For intracellular staining of FoxP3 and CTLA-4, all the cells were treated with fixation buffer for 30 minutes at room temperature (eBioscence). Afterward, permeabilization buffer was applied (eBioscence), followed by staining with anti-FoxP3 and anti–CTLA-4 for 45 minutes at room temperature. All the antibodies were used according to the manufacturers' instructions. Flow cytometry analysis was performed by using an LSR II flow cytometer (Becton, Dickinson and Co., San Diego, CA), and data were analyzed by using FlowJo software (TreeStar, Ashland, OR).
Ethics Issues
The study was approved by the ethics board of the Medical Faculty, Julius-Maximilian-University Wuerzburg (12/06), and all the participants gave written informed consent. All the authors significantly contributed to this work.
Statistics
Data are presented as mean ± standard deviation (SD). Statistical significance was analyzed by a two-tailed paired t-test by using GraphPad Prism Software 6.0c (GraphPad Software Inc., La Jolla, California). For nonparametric distribution, the Wilcoxon test was applied. Values of p < 0.05 were considered to be statistically significant.
RESULTS
Patient Characteristics
Eleven patients were included in the study group (seven male and four female patients). All the patients received intranasal topical steroids before surgery. The treatment started weeks before surgery, and, if the patients did not benefit from this medical therapy, then a surgical intervention was planned, according to the European guideline suggestions.26 Patients with Churg-Strauss syndrome, primary ciliary dyskinesia, or cystic fibrosis were excluded. The mean ± SD age was 53.27 ± 15.84 years. Eosinophilic polyposis was described in the histologic evaluation of most of the patients (10/11). The characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of the study group

SD = Standard deviation.
Predominance of Effector and/or Memory Cells among CD4+ and CD8+ T-cell Subsets in Nasal Polyps
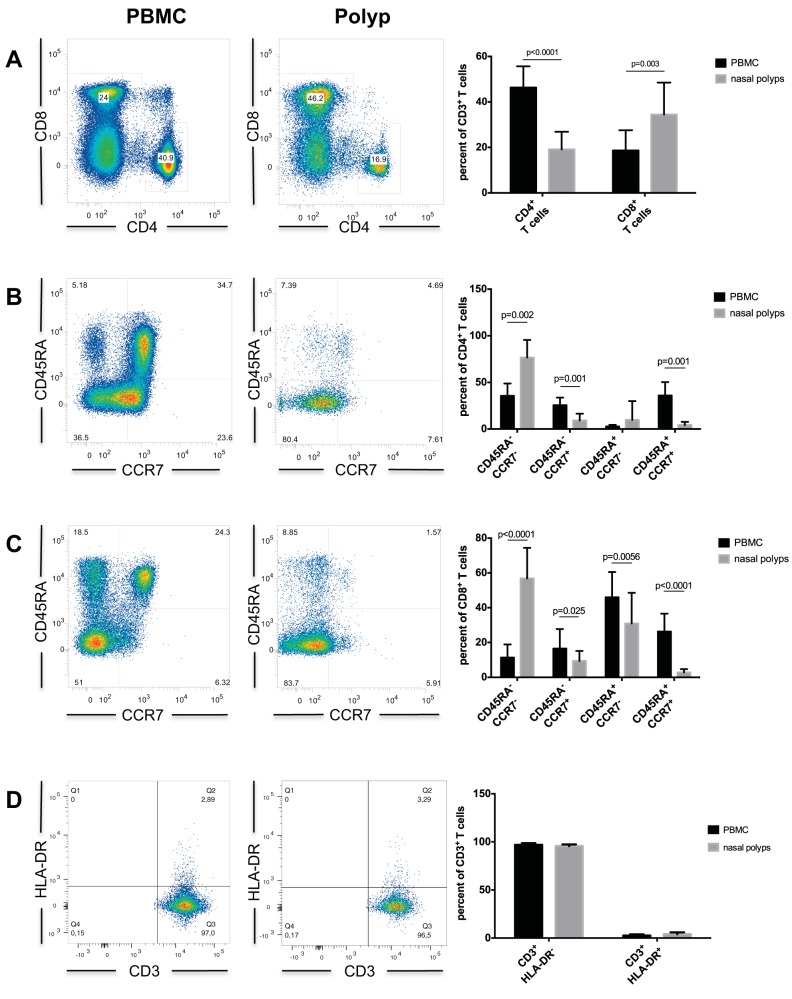
The number of isolated lymphocytes from healthy nasal mucosa was too low for the multicolor flow cytometry panel. Lymphocytes of nasal polyps and peripheral blood from the 11 patients were analyzed by flow cytometry. After separating all apoptotic cells, there was a significantly lower amount of CD3+ CD4+ T cells and a significantly higher amount of CD3+ CD8+ T cells in nasal polyps compared with peripheral blood mononuclear cells (PBMCs) (Fig. 1 A; Table 2).
Figure 1.
(A) CD8+ and CD4+ T cells in PBMC and nasal polyps. (B) CD4+ T cell subsets in PBMC and nasal polyps. (C) CD8+ T cell subsets in PBMC and nasal polyps. (D) Upregulation of HLA-DR on CD3+ T cells in PBMC and nasal polyps. Data are shown as means ± standard deviations (SD) of the 11 patients. PBMC = peripheral blood mononuclear cells; HLA-DR = human leukocyte antigen - antigen D related.
Table 2.
CD3+ T cell subpopulations
PBMC = peripheral blood mononuclear cells; SD = standard deviation; CD45RA = cluster of differentiation 45RA; CCR7 = C-C chemokine receptor type 7.
*Of the 11 patients.
#Paired t-test.
Further analyses of CD3+ CD4+ T cells showed significantly lower frequencies of CCR7+ CD45RA+ naive and CCR7+ CD45− central memory T cells in nasal polyps compared with PBMCs (Table 2). The proportion of CCR7− CD45RA− effector memory Th cells was significantly higher in nasal polyps (Fig. 1 B; Table 2). There was no statistically significant difference in the frequency of CCR7− CD45RA+ terminally differentiated T cells (Fig. 1 B; Table 2). This result was consistent with the amounts of CD45RA+ CD28− terminally differentiated T cells without statistical significance (0.43 ± 0.75% PBMC, 1.6 ± 2.79% nasal polyps) between both study groups. CD4+ CD25− CD45RA− memory Th cells were significantly higher in nasal polyps (44.11 ± 13.20% PBMC, 77.33 ± 8.00% nasal polyps), and a significantly lower fraction of CD4+ CD25− CD45RA+ naive Th cells was observed in the polyps (36.60 ± 15.57% PBMC, 7.71 ± 2.65% nasal polyps).
Quantitative analysis of CD3+ CD8+ T cells showed significantly lower frequencies of CCR7− CD45RA+ terminally differentiated and CCR7+ CD45RA+ naive subpopulations among these cells in nasal polyps, whereas CCR7− CD45RA− effector memory (cytotoxic) T cells were significantly higher in nasal polyps (Fig. 1 C; Table 2). CCR7+ CD45RA− central memory cytotoxic T cells were significantly lower in nasal polyps (Fig. 1 C; Table 2). CD45RA+ CD28− terminally differentiated T cells were also significantly higher in PBMC (12.73 ± 6.94% PBMC, 4.97 ± 4.95% nasal polyps). However, there was no statistically significant difference in the upregulation of the marker for activation HLA-DR24 on CD3+ T cells (Fig. 1 D) between both groups (2.69 ± 1.16% PBMC, 4.06 ± 1.9% nasal polyps).
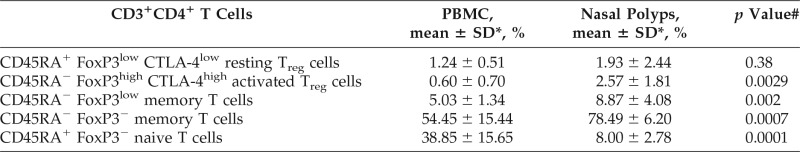
Significant Increase in aTreg Cells and Nonsuppressive Conventional FoxP3low Memory T Cells in Nasal Polyps compared with PBMC
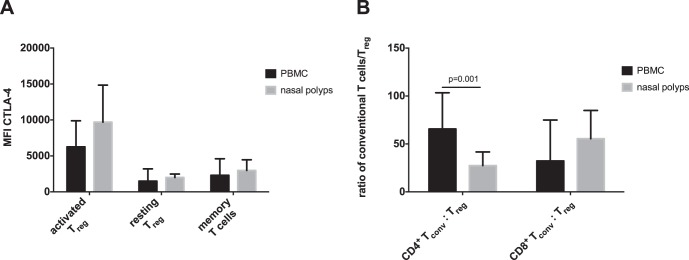
Most of the CD3+ CD4+ T cells were FoxP3− in both groups (Fig. 2 A; Table 3). In detail, there were significantly lower frequencies of CD3+ CD4+ CD45RA+ FoxP3− naive T cells and significantly higher frequencies of CD3+ CD4+ CD45RA− FoxP3− memory T cells in nasal polyps compared with peripheral blood (Fig. 2 A; Table 3). CD3+ CD4+ CD45RA− FoxP3high aTreg and CD3+ CD4+ CD45RA− FoxP3low memory T cells were significantly higher in nasal polyps, but CD3+ CD4+ CD45RA+ FoxP3low rTreg cells were not elevated in nasal polyps (Fig. 2 A; Table 3). The mean fluorescence intensity of CTLA-4 was high on aTreg cells and low on rTreg cells in both groups (Fig. 3 A). To determine whether there is a mismatch of conventional CD45RA− FoxP3low memory, CD45RA− FoxP3− memory, and CD45RA+ FoxP3− naive CD4+ T cells or CD8+ T cells to Treg cells (aTreg and rTreg), the ratio of these cells was calculated (Fig. 3 B). The ratio of conventional CD4+ T cells to Treg cells was significantly higher in peripheral blood than in nasal polyps.
Figure 2.
Regulatory T cell (Treg) subpopulations in PBMC and nasal polyps. (A) Frequency of CD4+ resting Treg (rTreg), activated Treg (aTreg), and FoxP3low memory T cells in PBMC and nasal polyps. (B) Expression of CTLA-4 by FoxP3+ cells in PBMC and nasal polyps. (C) CD4+ CD25+ FoxP3+ activated Treg in PBMC and nasal polyps. Data are shown as means ± standard deviations (SD) of the 11 patients. PBMC = peripheral blood mononuclear cells; FoxP3low = low expressive Forkhead-Box-Protein P3; CTLA-4 = cytotoxic T-lymphocyte-associated Protein 4; FoxP3+ = Forkhead-Box-Protein P3.
Table 3.
Treg subpopulations analyzed by staining of CD4, CD45RA, FoxP3, and CTLA-4
Treg = regulatory T; CD45RA = cluster of differentiation 45RA; FoxP3 = Forkhead-Box-Protein P3; CTLA-4 = cytotoxic T-lymphocyte-associated Protein 4; PBMC = peripheral blood mononuclear cell; SD = standard deviation; FoxP3low = low expression of Forkhead-Box-Protein P3; FoxP3high = high expression of Forkhead-Box-Protein P3; CTLA-4low = low expression of cytotoxic T-lymphocyte-associated Protein 4; CTLA-4high = high expression of cytotoxic T-lymphocyte-associated Protein 4.
*Of the 11 patients.
#Paired t-test.
Figure 3.
(A) Mean fluorescence intensity (MFI) of CTLA-4 on FoxP3+ T cells in PBMC and nasal polyps. (B) Ratio of conventional T cells to regulatory T cells in CD4+ and CD8+ T cells. Data are shown as means ± standard deviations (SD) of the 11 patients. CTLA-4 = cytotoxic T-lymphocyte-associated Protein 4; oxP3high = high expression of Forkhead-Box-Protein P3.
DISCUSSION
In this study, a detailed quantification of the subpopulations of T lymphocytes in peripheral blood and nasal polyps in CRSwNP was presented. As previously shown, there was a switch from mainly CD4+ T cells among peripheral blood αβ T cells to mainly cytotoxic CD8+ T cells in nasal polyps, with a significant domination of effector T cells among both CD4+ and CD8+ T cells in nasal polyps compared with peripheral blood.27–29 Moreover, aTreg cells were significantly increased among CD4+ T cells in nasal polyps compared with peripheral blood in patients with CRSwNP.
CD4+ Th cells can exhibit different properties, depending on their differentiation into one of the following Th1, Th2 or Th17 subsets. However, newer studies showed a variation in different T-cell subtypes present in the infected nasal mucosa but with a predominant Th1 subset in CRSsNP and Th2 subset in CRSwNP.29–31 Th17 T cells act synergistically with the other subsets and produce, especially interleukin (IL) 17, IL-21, IL-22, and IL-26. Among patients with CRSwNP, demographic variation seemed to play an additional role with respect to T-cell subdivision: Th2-biased cytokine profiles were mostly found in patients from Europe and the United States, whereas Th1:Th17 profiles were predominantly found in Asian patients.25
In the present study, the majority of T lymphocytes in nasal polyps were CD8+ T cells with an effector memory phenotype. These findings underscored the importance of CD8+ cytotoxic T-cell responses in the pathogenesis of nasal polyps. The role of CD8+ T cells in the defense against viruses and intracellular bacteria is clearly understood, but their role in allergic diseases is not yet completely known. Tang et al.32 described effector CD8+ T cells as dampening allergic responses in the effector phase. In contrast, there are other studies that identify effector memory CD8+ T cells as pathogenic in allergic responses.33,34
Because fungal and bacterial colonization of the local nasal mucosa has been identified to have at least contributed to T-cell recruitment into polyps,4–11 we were curious to study HLA-DR expression as a marker of recent T-cell activation. In contrast to their overall activated phenotype, the edaphic CD3+ T cells did not show any increased HLA-DR expression compared with blood T cells. This lack in HLA-DR upregulation might be caused by differentiation of the CD4+ and CD8+ T-cell subsets outside the polyp tissue in lymphoid organs without a restimulation of the T cells within the nasal polyp itself. Tissue-resident CD8+ T cells have been shown in mice to be maintained in situ, without local antigenic restimulation,35 and antifungal memory CD8+ T cells have also been shown to survive in the absence of antigen or CD4+ T-cell help.36 Alternatively, the preoperative treatment with local or systemic corticoids inhibits the upregulation of HLA-DR and reactivation of the T cells. Moreover, corticoid treatment might also have had a differential impact on Treg cells and conventional T cells, although information about the effects of glucocorticoids on functional properties of Treg cells is inconsistent in the literature. Some investigators see an increase in Treg cells after glucocorticoid therapy.37 However, in contrast, de Paz et al.38 described interindividually different responses of Treg cells to glucocorticoids among different patients, and Tabares et al.39 showed Treg cells to be relatively resistant to the immunosuppressive actions of glucocorticoids, which seemed to be in line with the present study findings.
Treg cells play an important role in maintaining peripheral tolerance to self-antigens and in counteracting the inflammatory activity of effector Th cell subsets.37 Classically, Treg cells were analyzed by staining for CD4, CD25, and FoxP3 expression. As previously mentioned, Miyara et al.22 described a revised classification of CD4+ FoxP3+ T cells into FoxP3high aTreg, FoxP3low rTreg, and conventionally nonsuppressive Foxp3low memory T cells. The FoxP3low memory T cells contain Th17 T cells and are not suppressive. Moreover, within the three subpopulations described, FoxP3low memory T cells had the highest frequencies in our study, which indicated that the Th17 pathway might also be important in the CRSwNP pathogenesis, even in patients of European descent. Apart from FoxP3low memory T cells, we found significantly more aTreg cells among CD4+ T cells in polyp tissue compared with T cells from peripheral blood (Fig. 2). Furthermore, we found a significantly lower ratio of conventional memory and naive CD4+ T cells to Treg cells in nasal polyps than in PBMC. This indicated that Treg cells should be able to efficiently suppress local CD4+ T-cell responses in the polyp and might thereby prevent further exacerbation of the disease. In contrast, the CD8+:Treg ratio was not significantly different in nasal polyps compared with peripheral blood, which indicated that Treg cells might be less able to control CD8+ T cells than conventional CD4+ T cells in polyps.
To our knowledge, this study provided the first comparison of different Treg cell subpopulations in peripheral blood and nasal polyps when using multicolor flow cytometry. A previous study on Treg cells in CRSwNP used flow cytometry to characterize Treg cells in peripheral blood and two-color immunohistochemistry to detect Treg in nasal polyps.17 Other studies focused on the nasal mucosa and/or polyps by using immunohistochemistry to detect Treg cells2,12 Although Treg cell frequencies in peripheral blood in healthy subjects and in patients with CRSwNP do not seem to differ,17 the two studies that analyzed Treg cells in the mucosa reported a decrease in absolute as well as relative numbers compared with patients with CRSsNP and mucosal tissue from controls.2,12 In these studies, however, FoxP3 expression alone was used to identify Treg cells. Because our own analyses revealed that the majority of CD4+ FoxP3+ cells in nasal polyps were nonregulatory memory T cells, further studies are needed to determine the abundance of Treg cells in polyps versus mucosal tissues of patients with CRSsNP and “healthy” controls.
The results of this study were limited by (1) the small number of subjects, and (2) there not being a control group of healthy nasal mucosa. The study was declared to be a pilot project with a limited amount of samples. Because there was no control group with healthy mucosa, interpretation of the data was affected. For example, a significantly lower ratio of conventional memory and naive CD4+ T cells to Treg cells in nasal polyps compared with PBMC in patients with CRSwNP was observed. But, if the ratio of conventional T cells to Treg cells were much lower in healthy control tissue than in polyps, this would indicate that there are not enough Treg cells in the polyps. Without a control group of healthy mucosa, it was difficult to provide further mechanistic insight. Unfortunately, analysis of lymphocytes from healthy nasal mucosa failed due to low amounts of these cells in this noninflammatory tissue, and high cell amounts are necessary for this multicolor flow cytometry analysis. When assuming that most of the lymphocytes in healthy nasal mucosa were circulating intravascular cells, a control group of PBMC seemed to be equivalent, and, furthermore, a comparison of isolated T cells from tissue with PBMC is well established in the literature.23,40
CONCLUSION
In this study, a detailed contemporary characterization of T-cell subpopulations was presented. CD8+ (cytotoxic) T cells are the main subpopulation in nasal polyps. Both CD8+ T cells and CD4+ T cells showed differentiation into a more effector memory phenotype. Treg cells are important for immune homeostasis and aTreg cells were increased relative to conventional CD4+ T cells but not to CD8+ T cells in nasal polyps compared with peripheral blood, which indicated that a failure of Treg cells to control the CD8+ T cell response in the polyp might contribute to the development of nasal polyposis.
Footnotes
This work was, in part, supported by a grant from the Deutsche Forschungsgemeinschaft (SFB/TR 124 FungiNet, project C6)
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Van Crombruggen K, Zhang N, Gevaert P, et al. Pathogenesis of chronic rhinosinusitis: Inflammation. J Allergy Clin Immunol 128:728–732, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Shi J, Fan Y, Xu R, et al. Characterizing T-cell phenotypes in nasal polyposis in Chinese patients. J Invest Allergol Clin Immunol 19:276–282, 2009. [PubMed] [Google Scholar]
- 3. Stuck BA, Bachert C, Federspil P, et al. Rhinosinusitis guidelines of the German Society for Otorhinolaryngology, Head and Neck Surgery [in German]. HNO 55:758–760, 762–764, 766–777, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Pant H, Beroukas D, Kette FE, et al. Nasal polyp cell populations and fungal-specific peripheral blood lymphocyte proliferation in allergic fungal sinusitis. Am J Rhinol Allergy 23:453–460, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Pant H, Macardle P. CD8(+) T cells implicated in the pathogenesis of allergic fungal rhinosinusitis. Allergy Rhinol (Providence) 5:146–156, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clinic Proc 74:877–884, 1999. [DOI] [PubMed] [Google Scholar]
- 7. Bachert C, Zhang N, Patou J, et al. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol 8:34–38, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Van Zele T, Gevaert P, Watelet JB, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol 114:981–983, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Wang M, Shi P, Yue Z, et al. Superantigens and the expression of T-cell receptor repertoire in chronic rhinosinusitis with nasal polyps. Acta Otolaryngol 128:901–908, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Conley DB, Tripathi A, Seiberling KA, et al. Superantigens and chronic rhinosinusitis: Skewing of T-cell receptor V beta-distributions in polyp-derived CD4+ and CD8+ T cells. Am J Rhinol 20:534–539, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conley DB, Tripathi A, Seiberling KA, et al. Superantigens and chronic rhinosinusitis II: Analysis of T-cell receptor V beta domains in nasal polyps. Am J Rhinol 20:451–455, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Kim YM, Munoz A, Hwang PH, et al. Migration of regulatory T cells toward airway epithelial cells is impaired in chronic rhinosinusitis with nasal polyposis. Clin Immunol 137:111–121, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bacher P, Kniemeyer O, Schonbrunn A, et al. Antigen-specific expansion of human regulatory T cells as a major tolerance mechanism against mucosal fungi. Mucosal Immunol 7:916–928, 2014. [DOI] [PubMed] [Google Scholar]
- 14. Bacher P, Kniemeyer O, Teutschbein J, et al. Identification of immunogenic antigens from Aspergillus fumigatus by direct multiparameter characterization of specific conventional and regulatory CD4+ T cells. J Immunol 193:3332–3343, 2014. [DOI] [PubMed] [Google Scholar]
- 15. Broker BM, Mrochen D, Peton V. The T cell response to Staphylococcus aureus. Pathogens 5:pii: E31, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell 133:775–787, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Sharma S, Watanabe S, Sivam A, et al. Peripheral blood and tissue T regulatory cells in chronic rhinosinusitis. Am J Rhinol Allergy 26:371–379, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robinson DS. The role of regulatory T lymphocytes in asthma pathogenesis. Curr Allergy Asthma Rep 5:136–141, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Jie HB, Schuler PJ, Lee SC, et al. CTLA-4(+) regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res 75:2200–2210, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roychoudhuri R, Eil RL, Restifo NP. The interplay of effector and regulatory T cells in cancer. Curr Opin Immunol 33:101–111, 2015. [DOI] [PubMed] [Google Scholar]
- 21. Pant H, Hughes A, Schembri M, et al. CD4(+) and CD8(+) regulatory T cells in chronic rhinosinusitis mucosa. Am J Rhinol Allergy 28:e83–e89, 2014. [DOI] [PubMed] [Google Scholar]
- 22. Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30:899–911, 2009. [DOI] [PubMed] [Google Scholar]
- 23. Rau M, Schilling AK, Meertens J, et al. Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis is marked by a higher frequency of Th17 cells in the liver and an increased Th17/resting regulatory T cell ratio in peripheral blood and in the liver. J Immunol 196:97–105, 2016. [DOI] [PubMed] [Google Scholar]
- 24. Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat Rev Immunol 12:191–200, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: Focus on nasal polyposis. J Allergy Clin Immunol 136:1431–1440; quiz 1441, 2015. [DOI] [PubMed] [Google Scholar]
- 26. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50:1–12, 2012. [DOI] [PubMed] [Google Scholar]
- 27. Huang Z, Nayak JV, Sun Y, et al. Peripheral blood T-helper cells and eosinophil populations in patients with atopic and nonatopic chronic rhinosinusitis. Am J Rhinol Allergy 31:8–12, 2017. [DOI] [PubMed] [Google Scholar]
- 28. Pant H, Hughes A, Miljkovic D, et al. Accumulation of effector memory CD8+ T cells in nasal polyps. Am J Rhinol Allergy 27:e117–e126, 2013. [DOI] [PubMed] [Google Scholar]
- 29. Derycke L, Eyerich S, Van Crombruggen K, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One 9:e97581, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Annunziato F, Romagnani S. Heterogeneity of human effector CD4+ T cells. Arthritis Res Ther 11:257, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Otto BA, Wenzel SE. The role of cytokines in chronic rhinosinusitis with nasal polyps. Curr Opin Otolaryngol Head Neck Surg 16:270–274, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Tang Y, Guan SP, Chua BY, et al. Antigen-specific effector CD8 T cells regulate allergic responses via IFN-gamma and dendritic cell function. J Allergy Clin Immunol 129:1611–1620.e4, 2012. [DOI] [PubMed] [Google Scholar]
- 33. Miyahara N, Swanson BJ, Takeda K, et al. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nature Med 10:865–869, 2004. [DOI] [PubMed] [Google Scholar]
- 34. Taube C, Miyahara N, Ott V, et al. The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness. J Immunol 176:3157–3164, 2006. [DOI] [PubMed] [Google Scholar]
- 35. Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity 41:886–897, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nanjappa SG, Heninger E, Wuthrich M, et al. Protective antifungal memory CD8(+) T cells are maintained in the absence of CD4(+) T cell help and cognate antigen in mice. J Clin Invest 122:987–999, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lochner M, Wang Z, Sparwasser T. The special relationship in the development and function of T helper 17 and regulatory T cells. Prog Mol Biol Transl Sci 136:99–129, 2015. [DOI] [PubMed] [Google Scholar]
- 38. de Paz B, Alperi-Lopez M, Ballina-Garcia FJ, et al. Cytokines and regulatory T cells in rheumatoid arthritis and their relationship with response to corticosteroids. J Rheumatol 37:2502–2510, 2010. [DOI] [PubMed] [Google Scholar]
- 39. Tabares P, Berr S, Romer PS, et al. Human regulatory T cells are selectively activated by low-dose application of the CD28 superagonist TGN1412/TAB08. Eur J Immunol 44:1225–1236, 2014. [DOI] [PubMed] [Google Scholar]
- 40. Murray T, Fuertes Marraco SA, Baumgaertner P, et al. Very late antigen-1 marks functional tumor-resident CD8 T cells and correlates with survival of melanoma patients. Front Immunol 7:573, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]