Abstract
Background:
Allergic Rhinitis and its Impact on Asthma guidelines recently recommended a treatment strategy for allergic rhinitis (AR) based on disease control rather than symptom severity by using a visual analog scale (VAS) to categorize control.
Objectives:
To evaluate the effectiveness of MP-AzeFlu (Dymista®) by using this VAS in routine clinical practice in Norway. MP-AzeFlu comprises a novel formulation that contains azelastine hydrochloride, fluticasone propionate and excipients delivered in a single spray.
Methods:
This multicenter, prospective, noninterventional study enrolled patients (n = 160) with moderate-to-severe AR and acute symptoms who were eligible to receive treatment with MP-AzeFlu according to its summary of product characteristics. Patients assessed symptom severity by using a VAS from 0 (not at all bothersome) to 100 mm (very bothersome) in the morning before MP-AzeFlu use on days 0, 1, 3, 7, and after ∼14 days. On day 3, the patients assessed their level of disease control as well controlled, partly controlled, or uncontrolled. The proportion of Norwegian patients who achieved defined VAS score cutoffs for “well-controlled” and “partly controlled” AR were also calculated.
Results:
MP-AzeFlu reduced the mean ± standard deviation VAS score from 68.1 ± 16.4 mm at baseline to 37.4 ± 25.9 mm on the last day, a reduction of 30.8 ± 27.2 mm. The results were consistent, irrespective of disease severity, phenotype (i.e., seasonal AR [SAR], perennial AR [PAR], SAR plus PAR, unknown) or age (i.e., 12–17, 18–65, and >65 years). Of the patients (with recorded data), 88.1% considered their symptoms to be partly or well controlled at day 3; and 19.5, 32.0, 50.0, and 61.0% of the patients achieved a ≤38 mm well-controlled VAS score cutoff on days 1, 3, 7, and the last day, respectively.
Conclusions:
MP-AzeFlu provided rapid sustained symptom control in a routine clinical practice in Norway, which provided support for its effectiveness for the treatment of AR in real life.
Keywords: Azelastine, control, effectiveness, fluticasone propionate, MP-AzeFlu, Norway, perennial allergic rhinitis, real-life, seasonal allergic rhinitis, visual analog scale
There are estimates that one in five people in Norway have allergic rhinitis (AR), a disease that is associated with a high symptomatic burden for patients as well as a high socioeconomic cost.1,2 Uncontrolled AR has a profound impact on patients' quality of life and leads to sleep problems, emotional issues, and limitations in daily living or social functioning.3 AR is also associated with a substantial loss in productivity due to absenteeism and presenteeism.4 Current therapies5 do not adequately address the symptom burden for many patients with AR, and, therefore, the disease remains a significant health problem.6,7
In recognition of this fact, Allergic Rhinitis and its Impact on Asthma (ARIA) recommendations are a switch from symptom severity to disease control to guide AR treatment decisions.8 This switch of focus has been facilitated by the introduction of the visual analog scale (VAS) as the common language of AR control and the endorsement of a common AR control concept by using VAS score cutoffs and by incorporating this VAS into a simple computer program application (app) for patients (Allergy Diary [MACVIA-LR, Languedoc-Roussillon, France]), which empowers patients to take control of their own AR. This same VAS has been incorporated into an updated ARIA guideline called the AR clinical decision support system (CDSS), with a score cutoff of 5 of 10 mm used to assess AR control and to guide treatment decisions.8 The VAS is well suited to this purpose because it provides a simple quantitative assessment of the impairment caused by AR9 and can be used to assess both AR severity10 and treatment effect.11,12
However, to achieve registration, all AR treatments must show a significantly greater reduction in symptom severity than placebo, assessed by using traditional efficacy measures (such as the reflective total nasal symptom score). This burden of proof was higher for MP-AzeFlu (Dymista®; Meda AB, Solna, Sweden), a novel intranasal formulation that contains azelastine hydrochloride (AZE), fluticasone propionate (FP), and excipients delivered in a single spray.13 In this instance, significance was required versus not only placebo but also versus AZE and FP monotherapy. Results from large, randomized, double-blind clinical studies show that MP-AzeFlu provides superior symptom relief than AZE or FP monotherapy, regardless of AR severity, AR season, or disease phenotype.14–17 MP-AzeFlu provided twice the overall nasal and ocular symptom relief compared with an intranasal corticosteroid (INS) or intranasal antihistamine, with superiority noted from the first day of assessment and sustained for the study duration.16
Further analyses of the clinical study data showed that one in six patients with moderate-to-severe seasonal AR (SAR) achieved complete to near complete symptom relief16 and that seven in ten patients with mild-to-moderate perennial AR (PAR) achieved complete relief in the first month and about a week faster than with INS monotherapy.15 The improved clinical efficacy of MP-AzeFlu compared with monotherapy with AZE and FP is reported to result from the improved biopharmaceutical characteristics of MP-AzeFlu that produce both an enhanced nasal-mucosal distribution and a larger nasal-mucosal surface area for FP.13 Given that randomized controlled trials (RCT) exclude many patients with AR who present in primary care,18 it is important to determine whether the results of such studies are generalizable to everyday practice. However, to date, none of the studies provided evidence for AR control in real life in line with ARIA recommendations. The aim of the present noninterventional study (NIS) was to assess the effectiveness of MP-AzeFlu in achieving AR control in real-life clinical practice in Norway by using the VAS, in line with ARIA recommendations.
METHODS
Study Design
This multicenter, prospective NIS was conducted in Norway between March and December 2014. The study consisted of two visits: an inclusion visit and an optional follow-up visit ∼14 days later. The timing of the latter visit was flexible, to allow for normal clinical practice. As an alternative to the follow-up visit, the patients were allowed to return their completed diary card by mail to the physician after finishing the study. During the study, the patients were treated with MP-AzeFlu (one spray in each nostril twice daily). There were no restrictions regarding concomitant treatments, apart from ritonavir, which was to be avoided. The study was performed in line with current Norwegian laws and guidelines, and the study documents were approved by a central ethics committee.
Patients
Patients could enter into this study if they were eligible to receive treatment for AR with MP-AzeFlu according to the medication's approved indication in Norway (i.e., if they were ages ≥12 years, had moderate-to-severe SAR or PAR, and if monotherapy with either an intranasal antihistamine or glucocorticoid was not considered sufficient.)19 Patients were required to have acute AR symptoms on the study inclusion day, defined as a recommended VAS score of ≥50 mm. However, if physicians rated the patients' symptoms as moderate to severe, the patients could still be enrolled in the study, irrespective of their VAS score. Patients were excluded if they had hypersensitivity to MP-AzeFlu or any of its excipients. Female patients were excluded if they were pregnant or breast-feeding. All patients (if <18 years old, then their caregivers) provided written informed consent.
Physicians
Physicians (general practitioners; allergists; ear, nose, and throat specialists; pulmonologists; dermatologists; and pediatricians) who were usually involved in AR management participated in this study. Each physician could enroll up to 10 patients. The decision of whether to include a patient in the study was made by the physician independently from and after the decision to prescribe MP-AzeFlu had been made.
Data Collection and Assessments
MP-AzeFlu Use in Routine Clinical Practice.
Information on patient demographics, clinical symptoms, and previous AR treatments was documented by the physician at the inclusion visit. The physicians also recorded information on AR history, the number of visits in the current calendar year due to AR, predominant symptoms, and ARIA-defined AR severity. SAR was defined as allergy to at least one pollen allergen (i.e., spring, summer, and/or autumn pollen) but no nonpollen allergens. PAR was defined as allergy to at least one nonpollen allergen (i.e., dust mites, pet dander, and/or mold) but no pollen allergens. SAR plus PAR was defined as allergy to at least one pollen and at least one nonpollen allergen. AR of unknown origin was defined as allergy to other or unknown allergens (i.e., not one of the allergens listed above) or unknown allergens (i.e., rhinitis indicated from the patient's history but not specific immunoglobulin E data). The reason for the patient's visit (“acute AR symptoms,” “expected allergen exposure in near future,” or “other”) and the reason for prescribing MP-AzeFlu (“other therapies were not sufficient in the past,” “other therapies are not considered to be sufficient to treat acute symptoms,” or “other”) was documented by the physician. All data were recorded by the physicians in an English language electronic case report form (eCRF) (Trium Analysis Online GmbH, München, Germany).
MP-AzeFlu Effectiveness Assessment.
The patients recorded AR symptom severity (on days 0 [i.e., the inclusion visit], 1, 3, 7, and the last day) and disease control (on day 3) on a patient card (in Norwegian language), which was returned to the physician at the follow-up visit or sent by mail. The patients used a VAS that ranged from 0 mm (not at all bothersome) to 100 mm (very bothersome) to evaluate how bothersome their current symptoms had been in response to the statement “please reflect on how bothersome your symptoms were within the previous 24 hours.” The patients recorded their VAS score in the morning before administration of MP-AzeFlu. The patients recorded the level of disease control within the previous 24 hours on day 3 as “well controlled,” “partly controlled,” or “uncontrolled.” On receipt of the patient cards, the physicians transcribed this information into the eCRF. Data were electronically signed by the physicians and saved in the study data base located at Trium Analysis Online.
Safety
All suspected adverse drug reactions (ADR) and special situations (i.e., pregnancy; breast-feeding; any overdose, abuse, off-label use, misuse, or medication error; an adverse reaction related to occupational exposure; lack of efficacy) were documented by the physicians and recorded in the eCRF. An ADR was defined as an adverse event with a reasonable possibility that the event may have been caused by MP-AzeFlu. Adverse events were coded by using the Medical Dictionary for Regulatory Activities coding system (version 17.0).20
Statistics
It was planned to enroll 150 patients, which was deemed sufficient to provide insight into the real-life use of MP-AzeFlu in Norway. The baseline and efficacy analyses were conducted on the safety population, defined as all patients who were treated at least once with MP-AzeFlu and whose physician provided an electronic signature to confirm data accuracy. All the data were reported with descriptive statistics. Analyses were performed by the contract research organization Syneed Medidata GmbH (Berlin, Germany) with SAS version 9.1.3 (Cary, NC).
The mean VAS scores on days 0, 1, 3, 7, and the last day were assessed for the total population (n = 160) according to baseline AR severity (less severe, baseline VAS of 50–74 mm [n = 73]; more severe, baseline VAS of 75–100 mm [n = 49]), AR phenotype (SAR [n = 49], PAR [n = 16], SAR plus PAR [n = 42], unknown [n = 53]) and age group (12–17 years [n = 21], 18–65 years [n = 135], and >65 years [n = 4]). Symptom control on day 3 was analyzed for the total population and according to AR phenotype, excluding patients with missing control data from the analysis (n = 59).
Post Hoc Analyses
A weighted mean of country-specific VAS cutoffs (Youden index) to define well-controlled and partly controlled AR on day 3 were calculated from a pooled data set by incorporating data from Germany, Sweden, Denmark, Norway, and Romania21 and were 38 and 55 mm, respectively. Response was defined as achievement of these cutoffs on days 0, 1, 3, 7, and last day. Responder rates were derived from time-to-response analysis as Kaplan-Meier estimates. The time at which patients achieved the AR CDSS–defined well-controlled VAS score threshold (i.e., 50 mm) was also assessed.
RESULTS
Patient Disposition
This study was conducted by 37 physicians in Norway who enrolled 172 patients. Of these, 12 patients were excluded from the analyses due to unconfirmed data documentation, which left 160 patients in the safety population.
Patient Demographics and Baseline Characteristics
A summary of baseline patient demographics and clinical characteristics is presented in Table 1. There were slightly more female patients (n = 87 [54.4%]) than male patients in the safety population. The mean ± standard deviation (SD) age of the study population was 35.2 ± 15.7 years, with most of the patients ages 18–65 years (n = 135 [84.4%]). Most patients had SAR, either alone (n = 49 [30.6%]) or in combination with PAR (n = 42 [26.3%]). There were very few patients with PAR only (n = 16 [10.0%]) and a relatively high proportion of patients with etiology of unknown origin (n = 53 [33.1%]). The vast majority of patients had confirmed ARIA-defined moderate-to-severe disease (n = 154 [96.3%]). The mean ± SD duration of AR was 14.3 ± 13.4 years. Overall, 122 patients (76.3%) had a baseline VAS score of ≥50 mm. Nasal congestion was by far the most common predominant symptom (n = 124 [77.5%]). Ocular symptoms were present in 37.5% of the patients (n = 60).
Table 1.
Patient demographics and baseline clinical characteristics (N = 160)
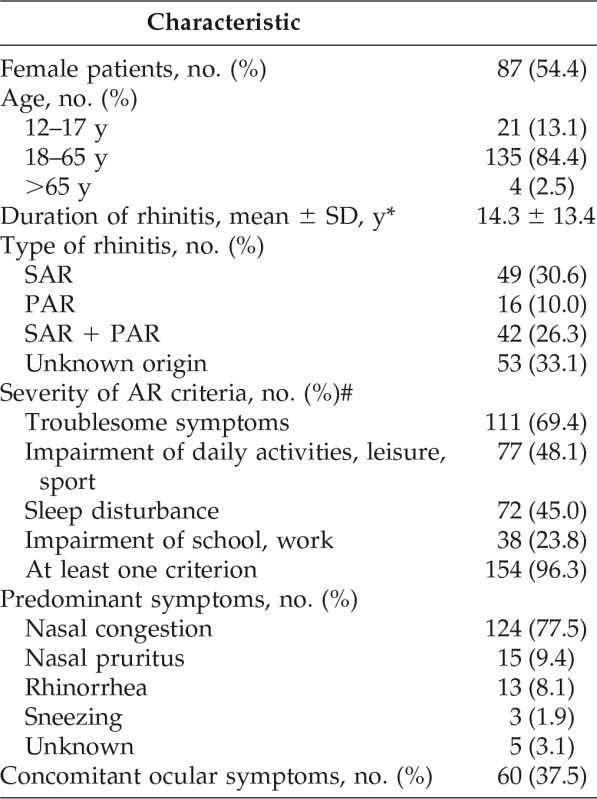
SD = Standard deviation; SAR = seasonal allergic rhinitis; PAR = perennial allergic rhinitis; AR = allergic rhinitis.
*n = 81.
#Moderate-to-severe AR if at least one criterion was met.
In the current calendar year, the mean ± SD number of physician visits due to AR was 1.5 ± 3.1. Of the patients, 47.5% (n = 76) had visited their physician due to their AR at least once in the current calendar year before inclusion into the study; 13.8% (n = 22) of the patients had attended once before, 8.8% (n = 14) had attended twice before, and 15.6% (n = 25) had made three or more visits before the current visit. The most frequent reasons for the physician visit were “acute AR symptoms” (n = 69 [43.1%]), “expected allergen exposure in the near future” (n = 18 [11.3%]), and “other” (n = 76 [47.5%]). The most frequent reason for prescribing MP-AzeFlu was that “other therapies were not sufficient in the past” (n = 114 [71.3%]). For the remaining patients, other reasons were cited, including “other therapies were not considered sufficient to treat acute symptoms.”
AR Treatments in the Past Year
As shown in Table 2, the AR medications most frequently used in the past year were INS (n = 119 [74.4%]), oral antihistamines (n = 96 [60.0%]), and oral and/or intranasal decongestants (n = 44 [27.5%]). Eye drops, either antihistamine or a mast cell stabilizer, were used by 26 patients (16.3%). Overall, 104 patients (65.0%) had used multiple AR treatments in the past year. Eight patients (5.0%) had undergone previous immunotherapy, and a further eight (5.0%) were undergoing immunotherapy at the time of the inclusion visit.
Table 2.
AR treatments in the past year (N = 160)
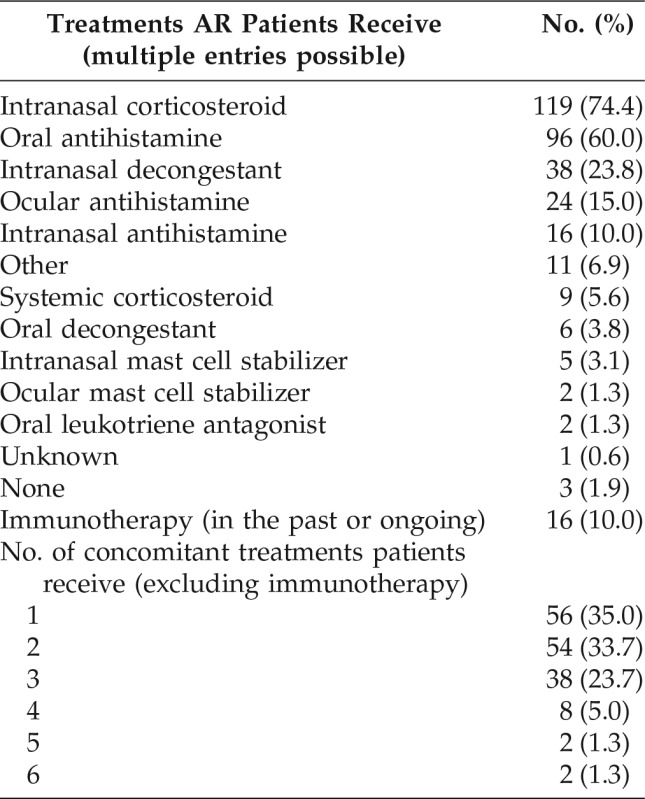
AR = Allergic rhinitis.
Effectiveness
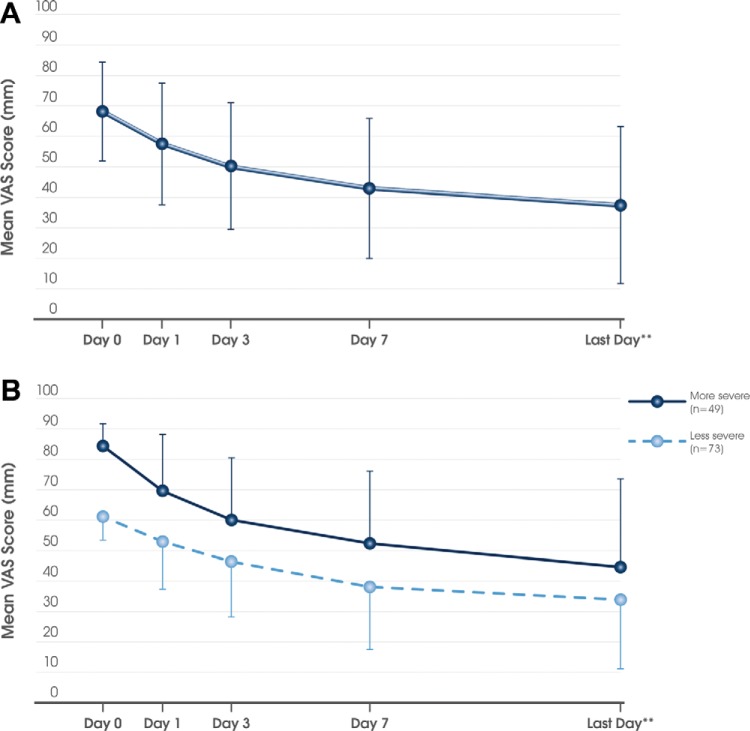
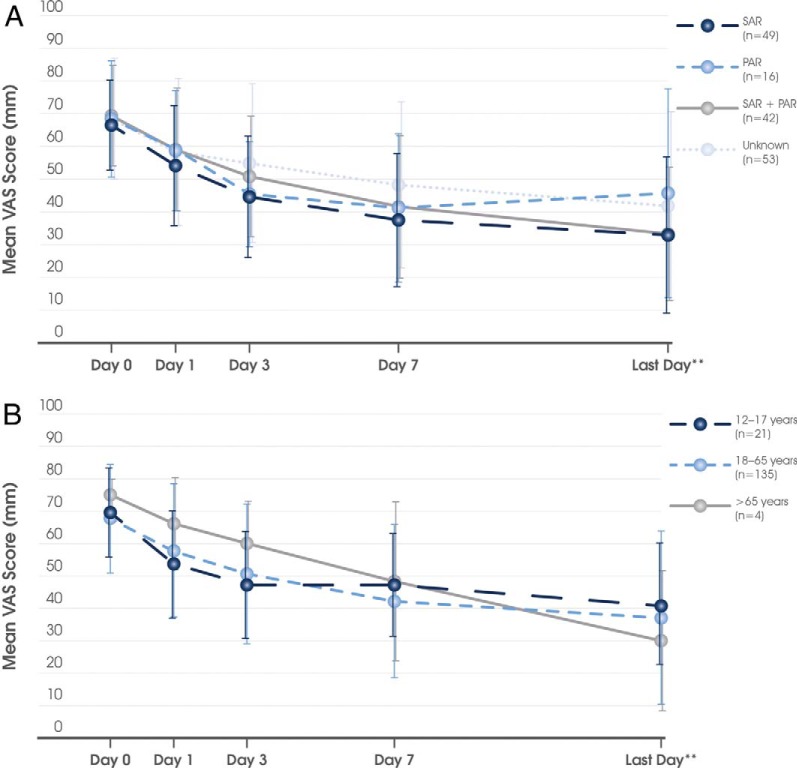
The mean ± SD time period between commencing MP-AzeFlu treatment and the VAS assessment on the last visit (or the day that the patient returned his or her card) was 16.2 ± 5.9 days (median, 14 days). As shown in Fig. 1 A, with MP-AzeFlu treatment, the mean ± SD VAS score decreased from 68.1 ± 16.4 mm at baseline to 37.4 ± 25.9 mm at the last visit, which corresponded to a mean ± SD reduction of 30.8 ± 27.2 mm. The treatment effect was rapid and sustained and independent of disease severity (Fig. 1 B), phenotype (Fig. 2 A) or patient age class (Fig. 2 B).
Figure 1.
Effect of MP-AzeFlu (Dymista®) on the visual analog scale (VAS) score over time in (A) the total population (n = 160) and (B) according to baseline severity. Less severe, baseline VAS score of 50–74 mm; more severe, baseline VAS score of 75–100 mm. Data are presented as mean ± standard deviation. **The mean of the last day corresponds to day 16.2.
Figure 2.
Effect of MP-AzeFlu (Dymista®) on visual analog scale (VAS) score over time according to (A) allergic rhinitis phenotype and (B) patient age. SAR = seasonal allergic rhinitis; PAR = perennial allergic rhinitis. Data are presented as mean ± standard deviation. **The mean of the last day corresponds to day 16.2.
As shown in Fig. 3, there was a rapid reduction in symptom burden with MP-AzeFlu treatment. After only 3 days, 29.7% (n = 30) of patients (who provided control status data) considered their symptoms were well controlled and 58.4% (n = 59) felt that their symptoms were partly controlled. Only 11.9% of patients (n = 12) indicated that their symptoms were uncontrolled at day 3. More than 78% of patients with SAR, PAR, SAR plus PAR, or AR of unknown origin reported that their symptoms were well or partly controlled after 3 days of treatment (Fig. 3).
Figure 3.
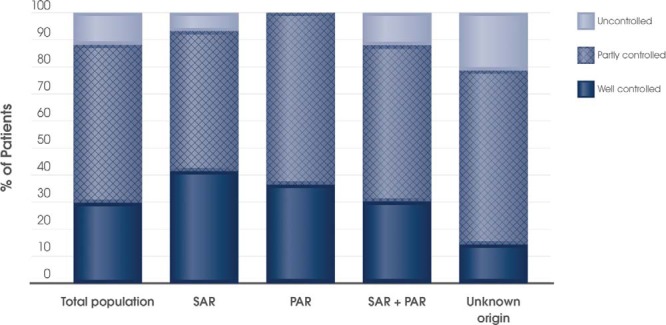
Patient perception of allergic rhinitis (AR) control on day 3 for the total population with present control status (n = 101) and according to AR phenotype (i.e., seasonal AR [SAR], n = 29; perennial AR [PAR], n = 11; SAR + PAR [n = 33]; unknown [n = 28]).
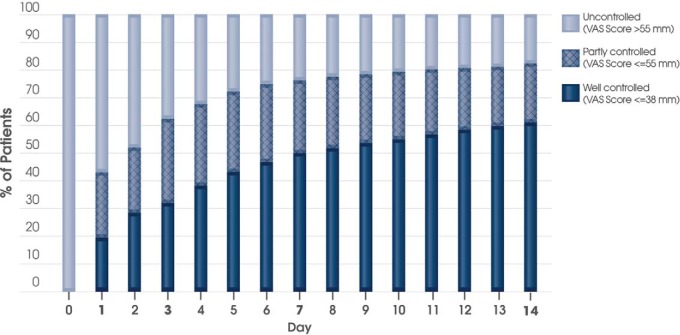
Response
The perception of “well-controlled” symptoms corresponded to a VAS score cutoff of 38 mm21; 19.5% of the patients achieved this cutoff on day 1, 32.0% on day 3, 50.0% on day 7, and 61.0% on the last day (Fig. 4). Similarly, the feeling of “partly controlled” symptoms corresponded to a VAS score cutoff of 55 mm.21 Of the Norwegian patients, 43.1, 62.3, 76.2, and 82.3% achieved at least this cutoff on days 1, 3, 7, and the last day, respectively (Fig. 4). This response was relatively independent of phenotype. More or less, a similar proportion of SAR, PAR, SAR plus PAR, and those with unknown phenotype achieved these well-controlled and partly controlled VAS score cutoffs on days 1, 3, 7, and the last day. On average, the patients treated with MP-AzeFlu achieved the AR CDSS control cutoff (i.e., 50 mm) between day 3 and day 7.
Figure 4.
The proportion of patients treated with MP-AzeFlu (Dymista®) who had well-controlled (i.e., a visual analog scale [VAS] score of ≤38 mm), partly controlled (i.e., VAS score of ≤55 mm), and uncontrolled allergic rhinitis over time. Data are presented as Kaplan Meier estimates for days 1, 3, 7, and 14, and are interpolated for the other days.
Safety
No serious ADRs were observed, and only one patient in this study (0.6%) had a nonserious ADR (worsening of asthma), which led to treatment discontinuation. There were no special situations reported.
DISCUSSION
This study was important for three reasons. First, it provided the first evidence of the effectiveness and safety of MP-AzeFlu in Norwegian patients seen in everyday clinical practice. Second, it used a simple VAS to track symptoms, the same VAS incorporated into the updated AR guideline to guide treatment decisions,8 and third, it showed the burden of AR in Norway (before widespread use of MP-AzeFlu). Norwegian patients with AR treated with MP-AzeFlu experienced rapid and sustained symptom control, with consistent response noted, irrespective of disease severity, phenotype, or patient age class. In addition, 6 of 10 patients (61.0%) achieved the patient defined well-controlled VAS score cutoff (i.e., ≤38 mm by the last day of MP-AzeFlu treatment. On average, patients treated with MP-AzeFlu achieved the AR CDSS control cutoff (i.e., 50 mm) between day 3 and day 7.8
Although data generated from the many RCTs on MP-AzeFlu are considered to be the criterion standard evidence to inform treatment recommendations,14–16,22 this NIS provides important information on the effectiveness of MP-AzeFlu in patients' real life and in a way that more closely represents everyday clinical care.23 The patients enrolled in real-life studies are more heterogeneous and representative of those seeking medical care in daily clinical practice than the homogeneous populations evaluated in RCTs, which have to meet rigorous inclusion and exclusion criteria.23 Furthermore, the level of clinical care in real-life studies is closer to that of everyday practice than in RCTs that follow up patients more intensively. The results of well-designed real-life studies, therefore, extend the evidence base on which guideline recommendations are based and show the benefit that patients may expect to achieve with MP-AzeFlu.
The population of patients enrolled in this study corresponded well with the specifications given in the Summary of Product Characteristics for MP-AzeFlu regarding indication and target population.19 None of the patients were <12 years of age, and >95% had moderate-to-severe AR according to the ARIA classification.3 MP-AzeFlu was prescribed both as first-line therapy in those patients for whom it was considered that monotherapy with either an intranasal antihistamine or an INS would provide insufficient symptom relief and also as second-line therapy in those patients who had a history of previous treatment failure.
Patients included in this real-life study had an average baseline VAS score of 68.1 mm, which confirmed both the suboptimal AR symptom control achievable with other AR therapies (even multiple therapies) and the need for new treatment options, e.g., MP-AzeFlu. Insufficiency of previously used AR treatments was also indicated by the fact that approximately two-thirds of the patients in this study were using multiple treatments in an attempt to control their AR symptoms, and approximately seven of ten patients were prescribed MP-AzeFlu because previously used therapies were considered insufficient (the most common of which was INS used by 74.4% of patients in the past year). Furthermore, almost half of the patients had made multiple visits to their physician due to AR in the current calendar year. By providing rapid and effective AR symptom relief, MP-AzeFlu has the potential to reduce the clinical impact of the disease. Future pharmacoeconomic studies are needed to determine the impact of MP-AzeFlu on both patient and economic burden in Norway.
The effectiveness of MP-AzeFlu observed in this real-life setting was better than its efficacy assessed in RCTs.15,16 In RCTs, patients treated with MP-AzeFlu experienced twice the nasal and ocular symptom relief compared with those treated with an INS.16 Superiority over INS was noted from the first day of assessment and treatment difference sustained for 1 year.15,16 A responder analysis of the data from RCTs demonstrated that one in six patients with moderate-to-severe SAR achieved complete or near-complete symptom relief (defined as ≤1 point remaining in each nasal symptom score), with this response achieved about a week faster than with AZE or FP monotherapy.16 In this real-life study, 61.0% of patients achieved a VAS score of ≤38 mm (i.e., well controlled) by treatment end, and >88% of the patients treated with MP-AzeFlu considered that their symptoms at least partly controlled after just 3 days of treatment. The rapid cross-over below the AR CDSS control threshold (i.e., 5 cm) after MP-AzeFlu treatment corroborated this finding.8
The real-life effectiveness and safety of MP-AzeFlu has also been investigated in two other Scandinavian countries (i.e., Sweden and Denmark).24,25 In all of these studies, MP-AzeFlu was well tolerated and provided rapid and effective AR symptom relief from the first day of treatment, which was consistent, regardless of patient age class, disease severity, or phenotype. The mean change from baseline in the VAS score to the last visit was 30.8 mm in this study compared with 36.1 mm in the Swedish study24 and 38.8 mm in the Danish study.25 The percentage of Norwegian patients who reported well-controlled or partly controlled disease after 3 days of MP-AzeFlu treatment (88.1%) was comparable with that reported by patients from Sweden or Denmark, 84.0 and 85.6%, respectively.24,25
The main limitations of this study were those typically associated with noninterventional, observational studies (i.e., the lack of placebo or active comparator and random assignment). In addition, the conclusions that can be drawn from the subgroup data, in particular, the PAR and >65 years of age subgroups, were limited by the small sample size. A limitation of the day 3 symptom control data was the high proportion of patients whose symptom control was unknown (36.9%). The accuracy and completeness of data in this NIS relied on the quality of the patients' medical records and the ability of the patients to recall information. Missing data were a source of potential bias when analyzing clinical study data. However, in this NIS, data were complete for most variables and rates of missing data were within acceptable limits (9–26%) for others. A strength of the study was the use of the ARIA recommended VAS to assess disease control.8 This VAS has also been incorporated into a free app for patients (Allergy Diary) to enable patients to monitor their own disease control, remind them to take their medication, and encourage them to visit a health care provider, when appropriate.26 A similar physician- and pharmacist-focused app (Allergy Diary Companion [MACVIA-LR]) is currently under development. This app will use the same VAS as the patient app to assess control and will also include the AR CDSS, which thus links all key stakeholders with a common language and a common aim (i.e., disease control).
CONCLUSION
MP-AzeFlu provided effective and rapid symptom control in a real-world setting in Norwegian patients with AR and with uncontrolled disease, despite monotherapy and multiple therapy usage and multiple physician visits. The results were consistent, irrespective of disease severity, phenotype, or age class, and supported the effectiveness of MP-AzeFlu for the treatment of AR in real life.
ACKNOWLEDGMENTS
The authors thank the following investigators for their participation in the study: Thorarin Saevarsson, M.D., Fredrik Eng, M.D., Mikal Gjellan, M.D., Vidar Holth, M.D., Jan Philip Junker Eikeland, M.D., Morten Pettersen, M.D., Espen Kolsrud, M.D., Stein Helge Glad Nordahl, M.D., Gregor Bachmann Harildstad, M.D., Ketil Olsholt, M.D., Michael Strand, M.D., Atle Kristian Søreide, M.D., Hogen Vaagland, M.D., Ingebjørg Skrindo, M.D., Ingrid Skarheim, M.D. We thank DunTung Nguyen, Ph.D., and Hans-Christian Kuhl, Ph.D., for critical assessment of the data and manuscript review.
Footnotes
Presented at the European Academy of Allergy and Immunology Congress, Vienna, Austria, June 11–15, 2016
Medical writing assistance in the preparation of this manuscript was provided by Ruth Murray, M.D., and David Harrison, D.Phil., Medscript Ltd, and Roger Hill, Ph.D., Ashfield Health Care Communications, funded by Meda Pharma GmbH and Co. KG
This study was funded by Meda
R. Dollner is an advisory board member for Meda AS, Norway. The remaining authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Selnes A, Odland JO, Bolle R, et al. Asthma and allergy in Russian and Norwegian schoolchildren: Results from two questionnaire-based studies in the Kola Peninsula, Russia, and northern Norway. Allergy 56:344–348, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Dotterud LK, Kvammen B, Bolle R, Falk ES. A survey of atopic diseases among school children in Sor-Varanger community. Possible effects of subarctic climate and industrial pollution from Russia. Acta Derm Venereol 74:124–128, 1994. [DOI] [PubMed] [Google Scholar]
- 3. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA[2]LEN and AllerGen). Allergy 63(suppl. 86):8–160, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Cardell LO, Olsson P, Andersson M, et al. TOTALL: High cost of allergic rhinitis—A national Swedish population-based questionnaire study. NPJ Prim Care Respir Med 26:15082, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berger WE, Meltzer EO. Intranasal spray medications for maintenance therapy of allergic rhinitis. Am J Rhinol Allergy 29:273–282, 2015. [DOI] [PubMed] [Google Scholar]
- 6. Canonica GW, Bousquet J, Mullol J, et al. A survey of the burden of allergic rhinitis in Europe. Allergy 62(suppl. 85):17–25, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Mullol J. A survey of the burden of allergic rhinitis in Spain. J Investig Allergol Clin Immunol 19:27–34, 2009. [PubMed] [Google Scholar]
- 8. Bousquet J, Schunemann HJ, Hellings PW, et al. MACVIA clinical decision algorithm in adolescents and adults with allergic rhinitis. J Allergy Clin Immunol 138:367–374.e2, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Demoly P, Bousquet PJ, Mesbah K, et al. Visual analogue scale in patients treated for allergic rhinitis: An observational prospective study in primary care: Asthma and rhinitis. Clin Exp Allergy 43:881–888, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Bousquet PJ, Combescure C, Neukirch F, et al. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy 62:367–372, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Bousquet J, Bachert C, Canonica GW, et al. Efficacy of desloratadine in intermittent allergic rhinitis: A GA(2)LEN study. Allergy 64:1516–1523, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Bousquet J, Bachert C, Canonica GW, et al. Efficacy of desloratadine in persistent allergic rhinitis—A GA2LEN study. Int Arch Allergy Immunol 153:395–402, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Derendorf H, Munzel U, Petzold U, et al. Bioavailability and disposition of azelastine and fluticasone propionate when delivered by MP29–02, a novel aqueous nasal spray. Br J Clin Pharmacol 74:125–133, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carr W, Bernstein J, Lieberman P, et al. A novel intranasal therapy of azelastine with fluticasone for the treatment of allergic rhinitis. J Allergy Clin Immunol 129:1282–1289.e10, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Price D, Shah S, Bhatia S, et al. A new therapy (MP29–02) is effective for the long-term treatment of chronic rhinitis. J Investig Allergol Clin Immunol 23:495–503, 2013. [PubMed] [Google Scholar]
- 16. Meltzer E, Ratner P, Bachert C, et al. Clinically relevant effect of a new intranasal therapy (MP29–02) in allergic rhinitis assessed by responder analysis. Int Arch Allergy Immunol 161:369–377, 2013. [DOI] [PubMed] [Google Scholar]
- 17. Hampel FC, Ratner PH, Van Bavel J, et al. Double-blind, placebo-controlled study of azelastine and fluticasone in a single nasal spray delivery device. Ann Allergy Asthma Immunol 105:168–173, 2010. [DOI] [PubMed] [Google Scholar]
- 18. Costa DJ, Amouyal M, Lambert P, et al. How representative are clinical study patients with allergic rhinitis in primary care? J Allergy Clin Immunol 127:920–926.e1, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Dymista. Summary of product characteristics. Available online at http://tinyurl.com/ydc6gdzh; Accessed July 11, 2017.
- 20. Klimek L, Bachert C, Stjarne P, et al. MP-AzeFlu provides rapid and effective allergic rhinitis control in real-life: A pan-European study. Allergy Asthma Proc 37:376–386, 2016. [DOI] [PubMed] [Google Scholar]
- 21. The Medical Dictionary for Regulatory Activities (MedDRA). Available online at http://www.meddra.org; accessed July 7, 2017.
- 22. Berger W, Bousquet J, Fox AT, et al. MP-AzeFlu is more effective than fluticasone propionate for the treatment of allergic rhinitis in children. Allergy 71:1219–1222, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Price D, Smith P, Hellings P, et al. Current controversies and challenges in allergic rhinitis management. Expert Rev Clin Immunol 11:1205–1217, 2015. [DOI] [PubMed] [Google Scholar]
- 24. Stjarne P, Strand V, Theman K, et al. Real-life effectiveness of a new allergic rhinitis therapy (MP29–02) in Sweden. Clin Transl Allergy 5:P37, 2015. [Google Scholar]
- 25. Haahr P, Jacobsen C, Blegvad S, et al. Real life effectiveness of a new allergic rhinitis therapy (MP29–02) in Denmark. Allergy 70:484–485, 2015. [Google Scholar]
- 26. Bousquet J, Bachert C, Price D, et al. Assessing allergic rhinitis symptom control using a simple visual analogue scale: The digital solution. Allergy 69:134, 2014. [Google Scholar]