Abstract
Background:
Hereditary angioedema (HAE) is a life-long disease that often manifests by puberty. Treatment of attacks is essential to improve quality of life and to decrease morbidity and mortality. During pregnancy, treatment is limited because multiple treatment options, including icatibant, are not approved for use during pregnancy.
Objective:
We report the outcomes of three pregnancies during which icatibant was used by a patient with HAE with normal C1-inhibitor for treatment of attacks. We also reviewed the literature for reports of icatibant use during pregnancy for outcomes and adverse events.
Methods:
We report on a patient who treated herself with icatibant during three separate pregnancies. Postpartum follow-up verified the health of the mother and children. We also performed a complete literature search of medical literature data bases on icatibant use during pregnancy.
Results:
The patient in our report administered multiple doses of icatibant during three pregnancies. The child born from the first pregnancy and the child from the third pregnancy were born at term and without congenital anomalies. The child from the second pregnancy was 1-month preterm. All three children were developmentally normal. The literature search identified two case reports and one abstract of limited icatibant use without adverse events during pregnancy in patients with HAE. These pregnancies resulted in the births of healthy infants.
Conclusion:
From a search of the literature, three cases of icatibant use during pregnancy resulted in healthy infants. In addition, we report that from icatibant use in three separate pregnancies, one infant was born prematurely, but there were no birth defects. From follow-up, the children continued meeting developmental milestones. This report adds to the acquisition of knowledge for drug adverse events during postmarketing surveillance for icatibant use during pregnancy.
Keywords: Hereditary angioedema, pregnancy, icatibant, adverse events, gestation, C1-inhibitor, bradykinin, decidua
Hereditary angioedema (HAE) is a rare disease, characterized by recurrent localized swelling, inflammation, and pain without urticaria or pruritus.1,2 HAE pathogenesis involves increased bradykinin levels that activate the bradykinin B2 receptor, which results in vasodilation and edema. Edema can occur at multiple locations, including the skin, gastrointestinal tract, and upper airway. Laryngeal swelling is concerning due to the risk of mortality from airway obstruction. There are three types of HAE all of which present similarly.1 All three, HAE with deficient C1-inhibitor (C1INH) (type 1), HAE with dysfunctional C1INH (type 2), and HAE with normal C1INH have been treated similarly; however, data to support the effectiveness of therapy in HAE with normal C1INH are of lower evidence standards because no double-blind, controlled research trial is possible in a disease that currently has no diagnostic test.
Therapy of HAE is divided into treatment for swelling attacks and prophylaxis to prevent future attacks. Treatment for attacks includes plasma-derived human C1 inhibitor concentrate (pdhC1INH), a bradykinin B2 receptor antagonist (icatibant), an inhibitor of kallikrein (ecallantide), and recombinant human C1INH.3 Short-term prophylaxis for attacks includes pdhC1INH, attenuated androgens (danazol, stanozolol, oxandrolone), and fresh frozen plasma.3 Therapies for long-term prophylaxis include attenuated androgens, antifibrinolytics (tranexamic acid), epsilon amino caproic acid, and pdhC1INH.3
During pregnancy, HAE attacks may worsen, improve, or remain unchanged.4 Anatomic locations of attacks remain similar during pregnancy, but the frequency of abdominal attacks may be increased.3–5 For treatment of HAE during pregnancy, pdhC1INH is indicated as preferred therapy for attacks and for short- and long-term prophylaxis.3 The attenuated androgens are contraindicated for use during pregnancy, and there is currently a lack of data available on the use and safety of icatibant (category C), ecallantide (category C), and recombinant human C1INH (category B) during pregnancy.
Icatibant, a competitive antagonist of the bradykinin B2 receptor, was evaluated in clinical trials for treatment of patients >18 years of age with type 1 or type 2 HAE.6–8 Icatibant reduces the time to symptom relief during attacks.7–9 The most common adverse reaction to icatibant is injection site reaction, and less frequent adverse reactions include pyrexia, diarrhea, dizziness, nausea, and headache.6–8 For most attacks, a single icatibant injection is needed for treatment.10 There have not been any studies in humans of icatibant use during pregnancy. Because of the lack of data on the effects of icatibant use in humans during pregnancy, icatibant is pregnancy category C. Results of animal studies showed adverse events, including fetal death and preimplantation loss, related to icatibant use.6 In these animal studies, icatibant was not teratogenic.6 A concern for the use of icatibant during pregnancy is that cells of the human decidua express bradykinin B2 receptors, and the effects of inhibition on these receptors, even for transient periods, are unknown.11,12 Cases of short-term use of icatibant during pregnancy have been documented13–15 but with limited use compared with our patient in this report.
METHODS
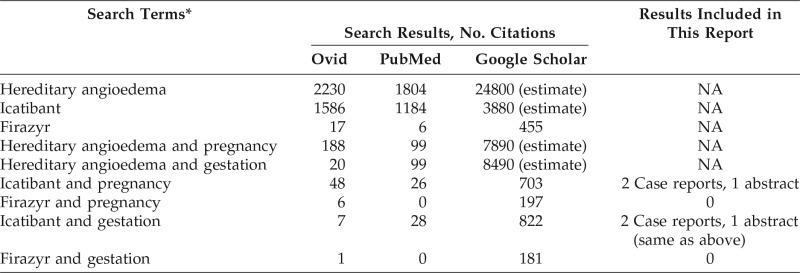
We performed a complete history and physical examination of the patient reported in this review and a complete history of the children for congenital anomalies and physical or developmental abnormalities. We reviewed the pregnancies and queried about adverse events during and after the pregnancies. In addition, a search of the English-language literature was conducted through Google Scholar (Google, Mountain View, CA), PubMed, and Ovid (Wolters Kluwer Health, New York, NY) by using the following keywords: “pregnancy” or “gestation” and “icatibant” or “Firazyr (Shire, Lexington, MA).” The searches were last updated July 29, 2016. The search findings are listed in Table 1 for comparison. The publications were reviewed for data related to icatibant use in pregnancy to include in this article.
Table 1.
Search results for Ovid, PubMed, and Google Scholar
NA = Not assessed.
*The terms in the first five rows were for reference only; the terms in the last four rows were used to find reports of icatibant use during human pregnancy and were included in this report.
RESULTS
We report on the treatment of a 29-year-old woman with HAE with normal C1INH. Severe abdominal attacks began 7 years before initiating care at our clinic. Initially, she was having attacks an average of twice per month, with each attack lasting several days. The attack frequency and severity worsened over time to twice weekly, with numerous emergency department visits and hospitalizations. At that point, a trial of danazol was attempted for 2 months, without improvement in symptoms. Icatibant provided significant relief. Following presentation to our clinic, laboratory evaluation showed normal C4 value (19 mg/dL), C1INH level (11 mg/dL), and C1-functional level (>100%). A diagnosis of HAE with normal C1INH was given due to her characteristic history of recurrent abdominal attacks, skin swelling, and airway attacks, along with the absence of abnormal laboratory results. She also noted a family history of swelling episodes in her paternal grandfather.
Initially, she was treated for attacks with pdhC1INH and/or icatibant; however, on-demand pdhC1INH did not provide any benefit. Subsequent use of prophylactic pdhC1INH resulted in less severe attacks but was discontinued due to the inability to improve her quality of life or to reduce the burden of her disease. After becoming pregnant with her first child, she experienced a reduced severity and frequency of attacks. Because her symptoms improved with pregnancy, she purposefully became pregnant two more times to prevent attacks. During pregnancy, she continued to have attacks with significant abdominal swelling, which occurred once or sometimes twice per week. She reportedly treated these attacks with icatibant during the first trimester of her first pregnancy and throughout all three trimesters of her second and third pregnancies. Despite previous counsel against the use of icatibant during pregnancy, the patient revealed that she used icatibant numerous times during all three of her pregnancies. She had received icatibant from multiple sources and had episodes of noncompliance to follow-up. Two of her three pregnancies were uneventful. Her middle child was born 1-month preterm. There were no observed birth defects in her three children, who are all now meeting developmental milestones at 19 months, 3 years, and 4 years of age.
With regard to the literature search, the same two case reports of icatibant use during pregnancy by each of the PubMed, Ovid, and Google Scholar searches were found. In addition, one abstract on icatibant use in pregnancy was also identified by Google Scholar search. In one case, a 26-year-old woman with type 2 HAE used icatibant five times for treatment of four acute attacks during the first 6 weeks of pregnancy.13 Pregnancy was confirmed in the sixth week, and icatibant use was discontinued. There were no reported adverse effects during pregnancy, and the baby was born healthy. In the second case, a 31-year-old woman with type 1 HAE treated herself with icatibant during an acute laryngeal attack during the 16th week of pregnancy.14 The patient delivered a full-term healthy baby. In the abstract, a 40-year-old woman with type 1 HAE had an upper airway attack treated with icatibant at both 32 and 35 weeks of pregnancy.15 At week 36 of pregnancy, she treated abdominal pain with icatibant before the pain was recognized as labor, and she underwent an uncomplicated Caesarian section 6 hours later. She gave birth to a healthy infant.
DISCUSSION
HAE with normal C1INH is rare and can be difficult to treat. In this case, our patient reported the use of numerous doses of icatibant during three separate pregnancies. Our review of the literature found three reports of icatibant use without adverse events during pregnancy in two patients with HAE type 1 and in one patient with HAE type 2. Here, we provided evidence of reported icatibant use in three pregnancies in a patient with HAE with normal C1INH associated with one preterm birth and without any observed birth defects or developmental delay. The patient did not have any spontaneous abortions.
A concern for the use of icatibant during pregnancy is the large number of bradykinin B2 receptors on decidua cells of humans.11,12,16 In addition, receptors have also been isolated in the uterine tissue of animals.17 The purpose of the receptors in the placenta and uterus and the effects of blocking these receptors are unknown at this time; however, bradykinin and lipopolysaccharide can affect the release of arachidonic acid from decidua cells.12 The short, competitive inhibition by icatibant, which is hours, may prevent any physiologic consequences of the inhibition. In turn, there may be no significant benefit of the bradykinin B2 receptors during pregnancy unless there is an active infection of the placenta.
Other research has focused on the offspring of rats treated with a bradykinin B2 receptor inhibitor, Hoe 140, during both pre- and postnatal phases of life.18,19 Litters of rats treated with Hoe 140 were carried to full gestation without noted complications.18 However, there was increased perinatal mortality in the offspring of rats treated with Hoe 140.19 Rats given Hoe 140 in utero and postnatally had higher systolic blood pressure, heart rate, and body weight compared with control animals at 9 weeks of age but had reduced urinary creatinine excretion.19 Results of these studies indicate the necessity for further research into the benefits and significances of the bradykinin B2 receptor for the normal development of the uterus, placenta, and fetus.
CONCLUSION
An extensive literature search in conjunction with our report revealed one preterm birth after icatibant use in pregnancy. Although we could not rule out a role for icatibant, preterm birth is common in the United States, with an estimated incidence of 1 in 10 infants born prematurely according to the Centers for Disease Control and Prevention.20 All three of the children from our report were meeting their developmental milestones. Because these cases and the literature only provided a few examples, more research is needed to investigate the risks of icatibant use during pregnancy. Several reported cases are not enough to comment on the safety of icatibant use in pregnancy. However, only through these reports of icatibant use during pregnancy can the U.S. Food and Drug Administration and the pharmaceutical companies gather data on adverse events and safety in pregnancy. Icatibant use during pregnancy can be documented through publication and in the Icatibant Outcome Survey, an observational study for patients who received icatibant.21 We recommend adhering to the guidelines,1 which suggest the use of pdhC1INH during pregnancy for attacks and for short-term and long-term prophylaxis. Until further data are available for use of icatibant during pregnancy, the utilization of icatibant should be reserved for when the benefit outweighs the risk.
Footnotes
No funding sources supported research
T. Craig is a researcher for CSL Behring, Grifols, Shire, and Biocryst; a speaker for CSL Behring and Grifols; and a consultant with CSL Behring and Biocryst. The remaining authors have no conflicts of interest pertaining to this article
REFERENCES
- 1. Craig T, Aygoren-Pursun E, Bork K, et al. WAO Guideline for the Management of Hereditary Angioedema. World Allergy Organ J 5:182–199, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Longhurst H, Cicardi M. Hereditary angio-oedema. Lancet 379:474–481, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Caballero T, Farkas H, Bouillet L, et al. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol 129:308–320, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Czaller I, Visy B, Csuka D, et al. The natural history of hereditary angioedema and the impact of treatment with human C1-inhibitor concentrate during pregnancy: A long-term survey. Eur J Obstet Gynecol Reprod Biol 152:44–49, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Geng B, Riedl MA. HAE update: Special considerations in the female patient with hereditary angioedema. Allergy Asthma Proc 34:13–18, 2013. [DOI] [PubMed] [Google Scholar]
- 6. Firazyr (icatibant acetate) injection [prescribing information]. Shire, Lexington, MA, 2015. [Google Scholar]
- 7. Lumry WR, Li HH, Levy RJ, et al. Randomized placebo-controlled trial of the bradykinin B(2) receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: The FAST-3 trial. Ann Allergy Asthma Immunol 107:529–537, 2011. [DOI] [PubMed] [Google Scholar]
- 8. Cicardi M, Banerji A, Bracho F, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med 363:532–541, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bork K, Frank J, Grundt B, et al. Treatment of acute edema attacks in hereditary angioedema with a bradykinin receptor-2 antagonist (icatibant). J Allergy Clin Immunol 119:1497–1503, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Longhurst HJ, Aberer W, Bouillet L, et al. Analysis of characteristics associated with reinjection of icatibant: Results from the icatibant outcome survey. Allergy Asthma Proc 36:399–406, 2015. [DOI] [PubMed] [Google Scholar]
- 11. Rehbock J, Chondromatidou A, Miska K, et al. Evidence for bradykinin B2-receptors on cultured human decidua cells. Immunopharmacology 36:135–141, 1997. [DOI] [PubMed] [Google Scholar]
- 12. Rehbock J, Chondromatidou A, Buchinger P, et al. The B2 receptor on cultured human decidua cells: Release of arachidonic acid by bradykinin. Immunopharmacology 33:164–166, 1996. [DOI] [PubMed] [Google Scholar]
- 13. Farkas H, Kohalmi KV, Veszeli N, et al. First report of icatibant treatment in a pregnant patient with hereditary angioedema. J Obstet Gynaecol Res 42:1026–1028, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Zanichelli A, Mansi M, Periti G. Icatibant exposure during pregnancy in a patient with hereditary angioedema. J Investig Allergol Clin Immunol 25:447–449, 2015. [PubMed] [Google Scholar]
- 15. Boufleur K, Delcaro L, Cordeiro DL, et al. Successful and safe use of icatibant for life-threatening angioedema attack during pregnancy in a patient with hereditary angioedema type I. J Allergy Clin Immun 133:Ab36, 2014. (Abs). [Google Scholar]
- 16. Buchinger P, Rehbock J. The bradykinin B2-receptor in human decidua. Semin Thromb Hemost 25:543–549, 1999. [DOI] [PubMed] [Google Scholar]
- 17. Murone C, Perich RB, Schlawe I, et al. Localization of bradykinin B2 receptors in sheep uterus. Immunopharmacology 33:108–112, 1996. [DOI] [PubMed] [Google Scholar]
- 18. Madeddu P, Parpaglia PP, Demontis MP, et al. Early blockade of bradykinin B2-receptors alters the adult cardiovascular phenotype in rats. Hypertension 25:453–459, 1995. [DOI] [PubMed] [Google Scholar]
- 19. Madeddu P, Parpaglia PP, Demontis MP, et al. Effects of kinin blockade on the blood pressure of salt-loaded pregnant rats. Hypertension 25:823–827, 1995. [DOI] [PubMed] [Google Scholar]
- 20. Preterm Birth. Centers for Disease Control and Prevention, 2016. Available online at https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm; accessed May 26, 2017.
- 21. Zanichelli A, Maurer M, Aberer W, et al. Long-term safety of icatibant treatment of patients with angioedema in real-world clinical practice. Allergy 72:994–998, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]