Abstract
First-line antibiotic treatment for eradicating Helicobacter pylori (HP) infection is effective in HP-positive low-grade gastric mucosa-associated lymphoid tissue lymphoma (MALToma), but its role in HP-negative cases is uncertain. In this exploratory retrospective study, we assessed the outcome and potential predictive biomarkers for 25 patients with HP-negative localized gastric MALToma who received first-line HP eradication (HPE) therapy. An HP-negative status was defined as negative results on histology, rapid urease test, 13C urea breath test, and serology. We observed an antibiotic response (complete remission [CR], number = 8; partial remission, number = 1) in 9 (36.0%) out of 25 patients. A t(11;18)(q21;q21) translocation was detected in 7 (43.8%) of 16 antibiotic-unresponsive cases, but in none of the 9 antibiotic-responsive cases (P = 0.027). Nuclear BCL10 expression was significantly higher in antibiotic-unresponsive tumors than in antibiotic-responsive tumors (14/16 [87.5%] vs. 1/9 [11.1%]; P = 0.001). Nuclear NF-κB expression was also significantly higher in antibiotic-unresponsive tumors than in antibiotic-responsive tumors (12/16 [75.0%] vs. 1/9 [11.1%]; P = 0.004). A substantial portion of patients with HP-negative gastric MALToma responded to first-line HPE. In addition to t(11;18)(q21;q21), BCL10 and NF-κB are useful immunohistochemical biomarkers to predict antibiotic-unresponsive status in this group of tumors.
Introduction
Most low-grade gastric mucosa-associated lymphoid tissue lymphomas (MALT lymphomas) are characterized by close association with Helicobacter pylori (HP) infection, and eradication of HP can cure approximately 70% of these tumors1–4. In contrast to HP-positive gastric MALT lymphomas, the role of first-line antibiotics in the treatment of HP-negative gastric MALT lymphomas remains uncertain4–8. Previous sporadic reports have revealed that certain types of HP-negative gastric MALT lymphomas can respond to common regimens that are used for HP eradication (HPE) therapy, i.e., a proton-pump inhibitor (PPI) plus clarithromycin, amoxicillin, metronidazole, or other antibiotics9–11.
As mentioned in these reports, some HP-negative patients might still have HP-associated tumors because previous use of bismuth, PPIs, and antibiotics could lead to pseudo-negative results on conventional HP tests such as the rapid urease test, the urea breath test, and histology12–14. In addition, one cannot completely exclude the possibility of a false HP-negative status if histomorphological findings disclose atrophic gastritis or intestinal metaplasia15,16. Furthermore, infection with coccoid forms of HP, which are difficult to be cultured and detected by immunohistochemical staining, and produce less urease, may cause pseudo-negative results on HP tests17–19.
The t(1;14)(p22;q32) chromosomal translocation, juxtaposing the BCL10 gene of chromosome 1p to the immunoglobulin gene locus of chromosome 14q, results in strong expression of a truncated BCL10 protein in the nuclei and cytoplasm in MALT lymphoma1,20,21. However, t(1;14)(p22;q32) is rarely found in gastric MALT lymphoma. Several studies have demonstrated that the t(11;18)(q21;q21) translocation can predict HP independence (tumor unresponsive to HPE) in patients with HP-positive gastric MALT lymphoma21–24. Furthermore, the presence of t(11;18)(q21;q21) translocation was more frequently found in HP-negative gastric MALT lymphoma than in HP-positive gastric MALT lymphoma7,25,26. Previously, we showed that regardless of the status of the t(11;18)(q21;q21) translocation, nuclear expression of BCL10 and NF-κB is closely associated with HP independence in gastric MALT lymphoma3,27.
Epidemiologic studies have shown that the presence of cytotoxin-associated gene A (CagA) protein, the most important HP virulence factor, is associated with the formation of lymphoid follicles and MALT lymphoma of the stomach28,29. Previous studies reported that the CagA-seropositive rate in patients with gastric MALT lymphoma ranged from 89% to 96%30,31.
Lehours et al. reported detection of the CagA gene in 47.4% of the HP strains obtained from 90 cases of gastric MALT lymphoma32. Among t(11;18)(q21;q21)-negative gastric MALT lymphoma cases, Sumida et al. found that titers of anti-CagA were significantly higher in HP-dependent cases than in HP-independent cases33. We recently found that 11 HP strains isolated from patients with HP-dependent gastric lymphomas (5 gastric diffuse large B-cell lymphomas with histologic evidence of MALT and 6 gastric MALT lymphomas) were CagA positive34. We and other investigators demonstrated that CagA can promote cellular proliferation and attenuate apoptosis of B-cells through activation of CagA-signaling such as SRC homology-2 domain-containing phosphatase (SHP2) and extracellular signal-regulated kinase (ERK)-related signaling, or BAD phosphorylation and p53 accumulation35–38. Furthermore, we reported that HP CagA protein and its signaling pathway proteins, such as phospho (p)-SHP2, p-ERK, p-38 mitogen-activated protein kinase (MAPK), BCL-2, and BCL-XL, can be detected in tumors of gastric MALT lymphoma39,40. The expression of CagA and CagA-signaling molecules is closely associated with HP-dependence of these tumors40, indicating CagA may serve as a marker for the presence of HP for gastric MALT lymphoma.
In this study, we assessed the response rate and the long-term disease-free status of patients with localized HP-negative gastric MALT lymphoma (all negative for histology [including HP, atrophic gastritis, and intestinal metaplasia], rapid urease test, 13C urea breath test, and serology as well as for CagA expression in tumor cells and gastric microenvironments) who received first-line HPE regimens consisting of PPIs plus clarithromycin and amoxicillin. We also investigated the association between potential biomarkers, including t(11;18)(q21;q21), nuclear BCL10 expression, and nuclear NF-κB expression, and antibiotic-unresponsive status of the same type of tumors.
Results
Clinicopathological features and tumor response to HP eradication therapy
Between January 1, 2005, and June 30, 2014, 25 patients with newly diagnosed stage IE/IIE1 primary HP-negative (results of the histology [including HP, atrophic gastritis, and intestinal metaplasia], rapid urease test, 13C urea breath test, and serology were all negative) gastric MALT lymphoma who received HPE as first-line treatment were included. Among them, 18 cases were also negative for HP cultures. Furthermore, CagA expression was not detected in tumor cells of all patients, indicating that HP is not present in these 25 cases (Fig. 1). We also showed that there was no CagA gene detected in gastric tumor biopsies obtained from patients with antibiotic-responsive tumors (Supplementary method, data not shown). Among these, 22 (88.0%) were at stage IE and three (12.0%) were at stage IIE1 (Table 1). Regarding the underlying diseases, four patients had hepatitis B virus infection, one patient had a hepatitis C virus infection, and one patient had an autoimmune disease (Sicca syndrome). Twenty-one (84.0%) of the 25 patients were treated with amoxicillin, clarithromycin, and omeprazole, whereas 4 (16.0%) patients received amoxicillin, clarithromycin, and lansoprazole.
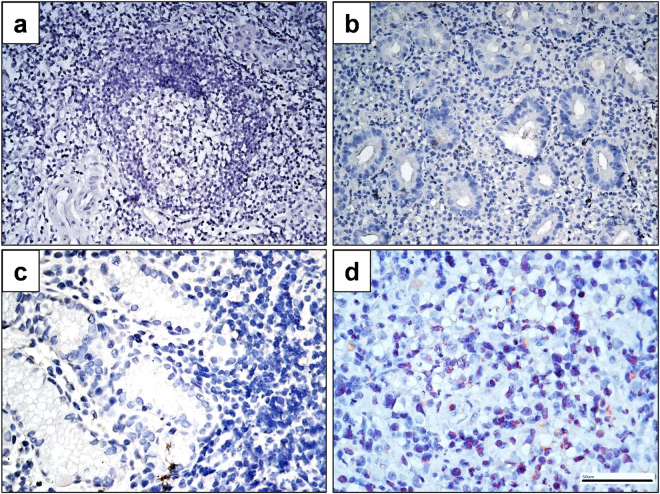
Figure 1.
Immunohistochemical analysis of CagA expression in tumor cells of HP-negative gastric MALT lymphoma. (a) An antibiotic-responsive case (Case 9#, time to complete remission [CR] after completing HPE, 5 months) displaying no CagA expression in the tumor cells of the gastric mucosa (b) An antibiotic-responsive case (Case 19#, time to CR after completing HPE, 10 months) displaying no CagA expression in the tumor cells of the gastric mucosa (c) An antibiotic-responsive case (Case 23#, time to CR after completing HPE, 4 months) displaying no CagA expression in the tumor cells of the gastric mucosa (d) An antibiotic-responsive case of HP-positive gastric MALT lymphoma (time to CR after completing HPE, 1 month) displaying nuclear CagA expression in the tumor cells of the gastric mucosa (served as positive control).
Table 1.
Characteristics, first-line antibiotics responses, and second-line treatment responses of HP-negative gastric MALT lymphoma patients.
| Case | Sex/Age | Stage | Response | Time to CR | 2nd-line Tx/time to 2nd-line Tx after diagnosis | Response to 2nd-line Tx | Relapse | Survival* | Serum IFE** |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/54 | I | PD | R-COP (6)/12.5 mos | CR | Parotid gland | +106 mos | NA | |
| 2 | M/53 | I | SD | Chlorambucil*/7 mos | SD | +102 mos | NO | ||
| 3 | F/49 | I | SD | Chlorambucil/14 mos | CR | +98 mos | NO | ||
| 4 | M/65 | I | SD | Chlorambucil/12 mos | CR | +46 mos | IgM/lambda | ||
| 5 | M/48 | I | SD | Chlorambucil + Prednisolone/15 mos | CR | Stomach | +94 mos | NO | |
| 6 | F/33 | I | PD | Riuximab/43 mos | SD | +89 mos | NO | ||
| 7 | M/39 | I | SD | Radiotherapy/12 mos | CR | +88 mos | NA | ||
| 8 | M/57 | I | SD | Radiotherapy/12 mos | CR | +82 mos | NA | ||
| 9 | F/69 | I | CR | 5 mos | +75 mos | NO | |||
| 10 | F/20 | I | SD | Radiotherapy/5 mos | CR | +65 mos | NA | ||
| 11 | F/76 | I | CR | 12 mos | +64 mos | NO | |||
| 12 | M/76 | IIE | SD | Chlorambucil/12 mos | CR | +59 mos | NO | ||
| 13 | M/88 | IE | CR | 24 mos | +56 mos | NA | |||
| 14 | M/58 | I | SD | Observation | +51 mos | NO | |||
| 15 | F/59 | I | PR | Observation | +48 mos | NO | |||
| 16 | F/49 | I | SD | Chlorambucil/11 mos | CR | +42 mos | NO | ||
| 17 | M/61 | I | CR | 15 mos | +36 mos | NO | |||
| 18 | M/62 | I | PD | R-COP/18 mos | CR | +31 mos | IgM/lambda | ||
| 19 | M/61 | I | CR | 10 mos | +24 mos | NO | |||
| 20 | M/74 | IIE1 | SD | Chlorambucil/6 mos | CR | +23 mos | IgM/kappa | ||
| 21 | M/71 | I | SD | Chlorambucil/8 mos | CR | +22 mos | NO | ||
| 22 | F/51 | I | CR | 1 mos | +21 mos | NO | |||
| 23 | F/77 | I | CR | 4 mos | +20 mos | NO | |||
| 24 | M/48 | IIE1 | SD | Observation | +18 mos | NO | |||
| 25 | F/32 | I | CR | 7 mos | +18 mos | NO |
Abbreviation: HP, H. pylori; CR, complete remission; Tx, treatment; IFE, immunofixation electrophoresis; M, men; F, women; PD, progressive disease; SD, stable disease; PR, partial remission; mos, months; R, rituximab; COP, cyclophosphamide, vincristine, and prednisolone; NO, no monoclonal gammopathy; NA, no analyses. Case information: Hepatitis B virus carrier, cases #3, #6, #16, and #19; Sicca syndrome, case # 14; Hepatitis C virus infection, case #22. *Survival, alive with follow-up time after treatment (months). **Immunofixation electrophoresis(IFE) showed IgM or IgG monoclonal gammopathy.
The histological scoring system proposed by the Groupe d’Etude des Lymphomes de l’Adult (GELA) is currently recommended to improve the consistency between the findings of different studies regarding first-line HPE for gastric MALT lymphoma41. The European Gastro-Intestinal Lymphoma Study (EGILS) consensus and the International Extranodal Lymphoma Study Group (IELSG) study therefore recommend the routine use of the GELA criteria in evaluating the response to treatment, including HPE, of gastric MALT lymphoma42,43. A complete remission (CR) is defined by the GELA grading system as the total disappearance of gross lymphoma and a negative histologic finding (CR or probable minimal residual disease [pMRD]), whereas partial remission (PR) is defined as normalization or reduction of macroscopic findings, histologic signs of lymphoma regression, and no signs of progression42,43. Previously, Fishbach et al. reported that 32 (32%) of 101 patients with pMRD or PR of tumors (according to the GELA criteria) after successful HPE achieved a histologic CR and 62% of patients had stable disease during the second-year of follow-up44. Another international randomized LY03 trial showed that the addition of an alkylating agent, chlorambucil, did not result in a better recurrence/progression-free survival or overall survival for patients with gastric MALT lymphoma who responded to HPE (including a CR or PR) compared with those who received “watch and wait”45.
Therefore, based on the GELA criteria for evaluating responses of gastric MALT lymphomas to HPE, we categorized our patients into two subgroups, those having antibiotic-responsive tumors (including CR and PR) and those having antibiotic-unresponsive tumors (including stable disease [SD] and progressive disease [PD]). The clinicopathological features of 9 patients (CR, number = 8; PR, number = 1) with antibiotic-responsive tumors (Fig. 2a–d) and 16 patients with antibiotic-unresponsive tumors, and the responses of their tumors to HPE are summarized in Table 1. We observed antibiotic responses in 9 (36.0%; 95% confidence interval [CI], 17.2–54.8%) out of 25 patients, and the median time to a CR (number = 8) was 7 months (95% CI, 0.1–13.9 months) (Fig. 3a). The antibiotic-responsive rate for 22 patients with stage IE was 40.9% (9/22), whereas the antibiotic-responsive rate for three patients with stage IIE1 was 0%. Of 20 patients receiving serum immunofixation electrophoresis (IFE) assessments, immunoglobulin M lambda monoclonal gammopathy was detected in 3 (25.0%) of the 12 antibiotic-unresponsive tumors, but not in the 8 antibiotic-responsive tumors (P = 0.242, Table 1).
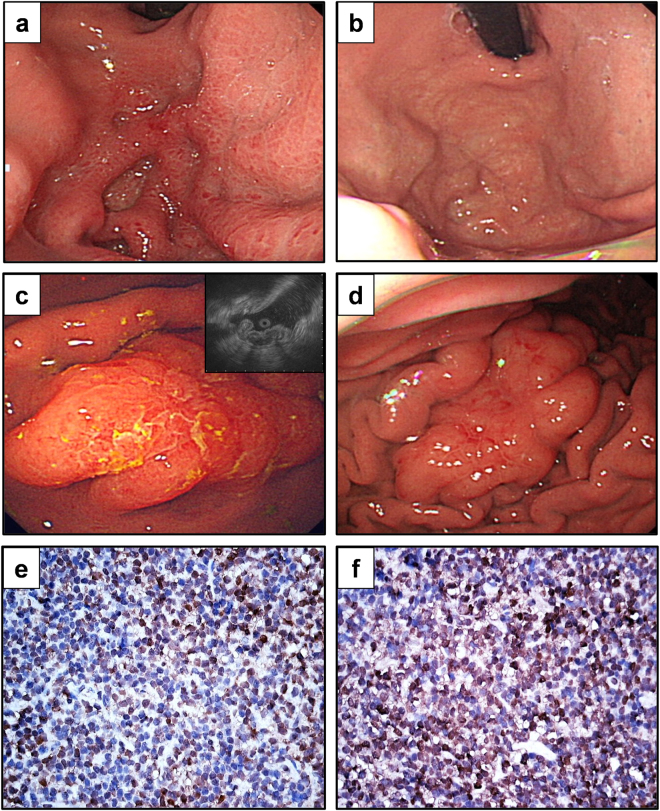
Figure 2.
Endoscopic features and immunohistochemical analysis of BCL10 and NF-κB expression in HP-negative gastric MALT lymphoma. (a) Endoscopy showing a 2 cm slightly raised lesion with hyperemic patches in the antrum of the stomach of a 51-year-old woman (Case 22#). (b) One month after the completion of an HPE regimen, CR was achieved. (c) Endoscopy showing elevated and enlarged folds lesions measuring about 3~4 cm in the upper body of a 59-year-old woman. Right upper bottom, endoscopic ultrasound showing tumor with mucosa involvement (thickness up to 7.2 mm at second layer) (Case #15). (d) Four months after the completion of an HPE regimen, partial remission was achieved. (e) Nuclear expression of BCL10 in tumor cells of an antibiotic-unresponsive case (Case #6). (f) Nuclear expression of NF-κB in tumor cells of an antibiotic-unresponsive case (Case #6). HPE, H. pylori eradication therapy.
Figure 3.
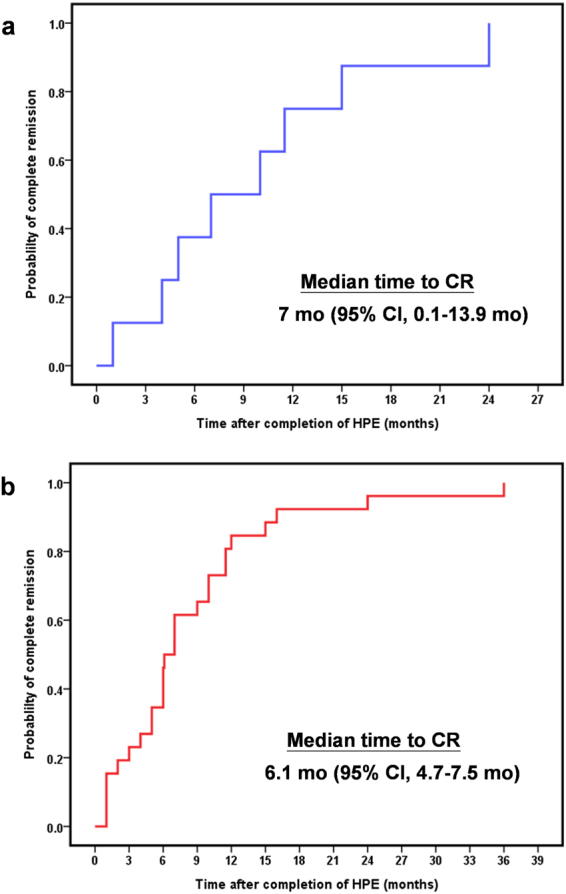
The time to complete remission of patients with antibiotic-responsive tumors. (a) Time to CR in our 8 cases was calculated from the completion of antibiotic treatment to the first evidence of CR through Kaplan–Meier analysis. (b) Time to CR in 26 cases of our series and that of five other investigators (refs9,16,55,57,58) was calculated from the completion of antibiotic treatment to the first evidence of CR through Kaplan–Meier analysis. Mo, month; CI, confidence interval; HPE, H. pylori eradication therapy.
Among the 16 cases without a tumor CR or PR, 14 patients who had persistent or increasing epigastric discomfort and were endoscopically or pathologically documented to have progressive tumors during the regular follow-up period were administered salvage treatments including oral alkylating agents (chlorambucil), rituximab-based regimens, and radiotherapy (Table 1). The time to aforementioned rescue therapy following HPE failure in these 14 patients is listed in Table 1. Of the 14 patients receiving second-line treatments, 12 patients achieved a CR while two patients had SD. Notably, two patients with SD undergoing observation alone experienced no progression during the follow-up (18 to 51 months after treatment) (Table 1).
At a median follow-up of 51.0 months (95% CI, 34.7–67.3 months), all patients with responsive tumors after HPE therapy were alive and free of lymphomas and progression, whereas 2 (16.7%) out of 12 patients with responsive tumors after second-line therapy experienced relapse (one in the parotid gland and the other in the stomach) (Table 1).
Correlation of clinicopathological features, t(11;18)(q21;q21), and expression of BCL10 and NF-κB with tumor response to HP eradication therapy
In addition to the patient-related factors (sex, P = 0.087), the endoscopic appearance (ulceration or ulcerated mass, P = 0.671), lesion sites (proximal location or 2-component involvement; P = 0.100), clinical stage (IIE1, P = 0.280), and endoscopic staging (tumors extending into the muscularis propria or beyond; P = 0.345) were not associated with the antibiotic-unresponsive status (Table 2 ).
Table 2.
Correlation between clinicopathologic features, t(11;18)(q21;q21), and nuclear expression of BCL10 and NF-κB with tumor response to HP eradication therapy in HP-negative gastric MALT lymphomas.
| Clinicopathologic characteristics | Tumors response to HPE regimen | P* | |
|---|---|---|---|
| Antibiotic-responsive | Antibiotic-unresponsive | ||
| (no. = 9) | (no. = 16) | ||
| Age (median, range, years) | 61 (32–88) | 53.5 (20–76) | |
| Sex, male/female | 6-Mar | 4-Dec | 0.087 |
| Stage, no. (%) | 0.28 | ||
| IE | 9 (100%) | 13 (81.2%) | |
| IIE1 | 0 (0.0%) | 3 (18.8%) | |
| Endoscopic features, no. (%) | 0.671 | ||
| Ulceration, ulcerated mass, or giant nodular folds | 5 (55.6%) | 11 (68.8%) | |
| Gastritis-like or erosions on infiltrative mucosa | 4 (44.4%) | 5 (31.2%) | |
| Location of tumor(s), no. (%) | 0.1 | ||
| Proximala or ≥2 components | 5 (55.6%) | 10 (62.5%) | |
| Distalb | 4 (44.4%) | 6 (37.5%) | |
| Depth of gastric wall involvement, no. (%)¶ | 0.345 | ||
| Submucosa or above | 6/7 (85.7%) | 9/16 (56.2%) | |
| Muscularis propria or beyond | 1/7 (14.3%) | 7/16 (43.8%) | |
| API2-MALT1, no. (%) | 0.027 | ||
| Negative | 9 (100%) | 9 (56.2%) | |
| Positive | 0 (0.0%) | 7 (41.2%) | |
| BCL10 expression, no. (%) | 0.001 | ||
| Cytoplasmic or negative | 8 (88.9%) | 2 (12.5%) | |
| Nuclear | 1 (11.1%) | 14 (87.5%) | |
| NF-κB expression, no. (%) | 0.004 | ||
| Cytoplasmic or negative | 8 (88.9%) | 4 (25.0%) | |
| Nuclear | 1 (11.1%) | 12 (75.0%) | |
| 2nd-line treatment regimen | |||
| Chlorambucil | 8 (50.0%) | ||
| Rituximan-based | 3 (18.7%) | ||
| Radiotherapy | 3 (18.7%) | ||
| Observation | 2 (12.6%) | ||
Abbreviation; HPE, H. pylori eradication therapy; HP, H. pylori; no., number. Proximala: Middle body, upper body, fundus, or cardia. Distalb: Antrum, angle, or lower body. P*: comparison of discrete variables between HP-dependent and HP-independent. P values (two sided) were calculated using Fisher’s exact test. ¶Gastric wall involvement was evaluated by endoscopic ultrasonography or computed tomography in 23 patients.
The API2-MALT1 fusion transcript of t(11;18)(q21;q21) was detected in 7 (43.8%) of the 16 antibiotic-unresponsive tumors, but not in the 9 antibiotic-responsive tumors (P = 0.027, Table 2). Nuclear BCL10 expression was significantly higher in the antibiotic-unresponsive group than in the antibiotic-responsive group (14 out of 16 [87.5%] vs. 1 out of 9 [11.1%]; P = 0.001) (Fig. 2e, Table 2 ). Similarly, nuclear NF-κB expression was detected in 12 (75.0%) out of the 16 antibiotic-unresponsive tumors and in 1 (11.1%) out of the 9 antibiotic-responsive tumors (P = 0.004) (Fig. 2f, Table 2 ). Nuclear BCL10 expression was also more frequently observed in t(11;18)(q21;q21)-positive tumors than in t(11;18)(q21;q21)-negative tumors (6 out of 7 [85.7%] vs. 9 out of 18 [50.0%]; P = 0.179). Furthermore, nuclear NF-κB expression was more frequently observed in t(11;18)(q21;q21)-positive tumors than in t(11;18)(q21;q21)-negative tumors (6 out of 7 [85.7%] vs. 7 out of 18 [38.9%]; P = 0.073).
Discussion
In this study, we demonstrated that nine (36.0%) out of 25 patients with HP-negative gastric MALT lymphoma were responsive to HPE, and remained lymphoma-free and progression-free at the longest follow-up. Our findings are consistent with a systematic review of published articles that demonstrated that a first-line HPE regimen resulted in a CR rate of 15.5% in 110 patients with HP-negative gastric MALT lymphoma10. As an addition to Zullo et al.10, who analyzed the CR rate after first-line antibiotic treatment, the diagnostic methods for HP, and the administration of HP regimens, we assessed the time to CR, the potential markers, including clinical stage, t(11;18)(q21;q21), and BCL10 expression, in 22 published results from 1999 through 2016 (summarized in Table 3)5–7,9,15,16,25,26,46–58. Overall, including our report, the CR rate after completing HP eradication treatments was observed in 68 (27.9%) out of 244 patients. The most commonly used HPE regimens consisted of PPIs plus at least two antibiotics such as amoxicillin, clarithromycin, or metronidazole for 7 to 14 days. Examinations for the presence of HP were mostly based on positive results from histology, rapid urease tests, 13C urea breath tests, and serology, and culture and stool antigen tests had been evaluated in five studies7,16,25,47,53, including our study.
Table 3.
Published reports on the efficacies of first-line antibiotics treatment in HP-negative gastric MALT lymphomas.
| Author | Year | No. of patients | Stage | FU after Tx (mo) | CR rate No. (%) | Time to CR (mo) | Makers | HP test | HPE-like regimen |
|---|---|---|---|---|---|---|---|---|---|
| Steinbach et al.5 | 1999 | 6 | IE | 5 or more | 0 (0) | ND | NA | H, RUT, S | A + C + P, C + T, T + M 21D |
| Nakamura T et al.25 | 2000 | 4 | ND | ND | 1 (25) | ND | t(11;18): 2/2 (+), non-CR | H, UBT, S, C | ND |
| Ruskone-Fourmestraux et al.6 | 2001 | 10 | IE (6) IIE1 (4) | 2–21 (8) | 0 (0) | ND | NA | H, S, C, PCR | A + C + P for 14 days |
| Ye et al.7 | 2003 | 5 | IE | 4–12 (4.5) | 0 (0) | ND | t(11;18): 2/5 (+), non-CR; BCL10 (N): 3/5 (+), non-CR | H, RUT, S, C | ND |
| Raderer et al.46 | 2006 | 6 | IE | 12–19 (17) | 5 (83) | ND | Included in ref.38 | H, UBT, S, SAT | C + M + P (7D) |
| Nakamura S et al.15 ,# | 2006 | 7 | IE(6) IIE1 (1) | 1–15 (4) | 2 (29) | ND | t(11;18): 0/1 CR; 3/3(+),non-CR | H, RUT, UBT, S | A + C + P with and without M |
| Akamatsu et al.16 ,# | 2006 | 9 | IE-IIE1 | 6 or more | 1 (11) | 6 | NA | H, S, C | A + C + P (7D) |
| Nozaki et al.47 | 2006 | 1 | IE | 5 years | 1 (0) | t(11;18): negative | H, UBT, C | A + C + P (7D) | |
| Terai et al.48 | 2008 | 4 | IE-IIE1 | ND | 1 (25) | ND | t(11;18): 3/3(+), non-CR | H, RUT, UBT, S | A + C + M + P (7D) |
| Nakamura T et al.49 | 2008 | 17 | IE (16) IIE1 (1) | 1 case: 43 | 2 | ND | t(11;18): 1/2(+), CR; 7/15(+), non-CR | H, RUT, S | A + C + P with and without M |
| Dong et al.50 | 2008 | 1 | IE | ND | 0 (0) | NA | H, UBT, S | A + C + P for 28D | |
| Stathis et al.51 | 2009 | 14 | IE (9) IIE1 (5) | ND | 5 (35) | ND | I: 5/9 (+), CR; II: 0/5 (+), CR | H, UBT, S | A + C + P, C + M + P, or A + M + P |
| Sumida et al.33 | 2009 | 9 | IE | ND | 0 (0) | ND | t(11;18): 4/9(+), non-CR; BCL10(N): 5/9 (+), non-CR | H, UBT, S | A + C + P |
| Park et al.9 | 2010 | 6 | IE | 27-Jun | 3 (50) | 2-Jan | NA | H, RUT, UBT, S | A + C + P (7 or 14D) |
| Asano et al.52 | 2012 | 17 | IE (15) IIE1 (2) | 0.3–12.7 years | 5 (29) | ND | Single lesion or antrum location t(11;18):1/1(+), CR | H, RUT, UBT, S | A + C + P (16) (7D) A + M + P (1) (7D) |
| 6/9 (+), non-CR | |||||||||
| Nakamura S et al.53 | 2012 | 44 | ND | ND | 6 (14) | ND | NA | H, RUT, UBT, S, C | A + C + P, C + M + P, or A + M + P |
| Choi et al.26 | 2013 | 5 | IE (4) IIE1 (1) | ND | 2 (40) | ND | t(11;18): 0/2(+), CR; 2/3 (+), non-CR | H, RUT, UBT, S | A + C + P (7D) |
| (7D) | |||||||||
| Ryu et al.54 | 2014 | 9 | IE-IIE1 | ND | 5 (56) | ND | NA | H, RUT, UBT, S | A + C + P (7 or 14D) |
| Raderer et al. 46,55 | 2015 | 13 | IE (8)IIE1 (5) | 42–181* | 5 (46) | Mar-36 | IE: 5/8 (+), CR; IIE1: 0/5 (+), CR; t(11;18): 0/5(+), CR 1/1(+), PR; 2/7(+), non-CR | H, UBT, S | C + M + P or C + A + P |
| (7 or 14D) | |||||||||
| Li et al.56 | 2016 | 4 | ND | 2 (50) | NA | H, UBT | A + C + M + P (7 or 14D) | ||
| Kim et al.57 | 2016 | 6 | ND | 3 (50) | 6.1 (median) | NA | H, RUT, UBT | A + C + P (7 or 14D) | |
| Gong et al.58 | 2016 | 28 | IE (24) | 16 (57) | 11.5 (median) | NA | H, RUT, UBT, S | A + C + P (7 or 14D) | |
| IIE (1)/IV (3) | |||||||||
| Present study | 2016 | 25 | IE (22) | 18–106 | 8 (32) | 24-Jan | IE: 8/22 (+), CR; IIE1: 0/3 (+), CR; t(11;18): 0/7(+), CR 6/13 (+), non-CR; BCL10 (N): 13/16(+), non-CR; NF-κB (N): 11/16(+), non-CR | H, RUT, UBT, S, C | A + C + P (14D) |
| [kuo et al.] | IIE1 (3) | ||||||||
| Overall | 244 & | 68 (27.9) | Median 6 mo | t(11;18): 39/74(+), non-CR; 2/19(+), CR; P = 0.001 |
Abbreviation: No, number; FU, follow-up; Tx, treatment; Mo, months; CR, complete remission; HP, Helicobacter pylori; HPE, HP eradication;; ND, non-described; NA, non-analysis; t(11;18), t(11;18)(q21;q21); (+), positive; BCL(N), nuclear BCL10 expression; NF-κB (N), nuclear NF-κB expression. D, days. HP examination test: H, histology; RUT, rapid urease test; UBT, urea breath test; S, serological test; C, culture; SAT, stool antigen test; PCR, protein chain reaction. HPE-like regimen: A, amoxicillin; C, clarithromycin; M, metronidazole; T, tetracycline; P, proton-pump inhibitor, including lansoprazole, pantoprazole or esomeprazole for 7 to 21 days (D). #Refs15,16, included 1 case with transformed high-grade MALT lymphoma, renamed as diffuse large B-cell lymphoma with MALT (DLBCL[MALT]). *Follow-up after diagnosis. &Raderer et al.55: 13 patients (6 patients had been previously reported in ref.46.
Although the aforementioned published series (some series comprising less cases and some series comprising more cases than our present cases) demonstrating a CR rate of 27.4% (60/219) (Table 3), our current data underline the reality that a proportion of patients without evidence of HP infection can be cured by first-line HPE. First, we showed that none of our patients had histologic evidence of atrophic gastritis or intestinal metaplasia (the aforementioned histomorphological findings are clues of a previous HP infection) in their specimens before HPE15,16, even if they had undergone previous eradication therapy or antibiotics treatment. Second, we showed that none of our patients exhibited CagA expression in tumor cells or in the gastric microenvironment. We also showed that there was no CagA gene detected in gastric tumor biopsies obtained from patients with antibiotic-responsive tumors. These findings indicated that the CagA-negativity of the tumors of our cases is actually just another suggestion that it is a real HP-negative gastric MALT lymphoma. Third, we demonstrated that the time to response for patients with antibiotic-responsive tumors was 7 months (range: 1 to 24 months), and importantly, after the median long-term follow up of 51 months, all patients with responsive tumors were free of lymphoma or progression. Combining our results with that of five other investigators (Table 3)9,16,55,57,58, the median time to a CR for patients with HP-negative gastric MALT lymphoma who received first-line HPE was 6.1 months (95% CI, 4.7–7.5 months) (Fig. 3b).
In the current study, we reported that 29 (31.5%) of 93 patients with gastric MALT lymphoma were HP-negative. However, in a systematic review of gastric MALT lymphoma (including diffuse large B-cell lymphoma), Zullo et al. demonstrated that 117 (11.2%) of 1146 cases were negative for HP infection59. The decreased level of HP infection in the general population and the widespread use of antibiotics in treating gastric-related diseases may alter the epidemiology of gastric MALT lymphoma and contribute to the higher HP-negative rate in our population. Our results are consistent with previous studies that have reported an increase in the prevalence of HP-negative infection in gastric MALT lymphoma cases over the past decade. For example, Choi et al.26 reported a 19.7% HP-negative rate in 66 cases of gastric MALT lymphoma, in which patients received first-line antibiotics during a study of the assessment of remission rate and the incidence of API2-MALT1. Luminari et al. reported that the prevalence of HP infection in gastric MALT lymphoma decreased from 61% (cases diagnosed between 1997 and 2001) to 17% (cases diagnosed between 2002 and 2007)60. In a retrospective analysis of 97 cases of gastric MALT lymphoma, Raderer et al. showed an increased prevalence of HP-negative infection in cases diagnosed after 2004 compared with those diagnosed before 2004 (31.2% versus 18%)55. Mendes et al. also found that the prevalence of HP-negative infection in gastric MALT lymphoma diagnosed between 2005 and 2013 was 67.6% (25/37)61.
In addition to PPIs, the most commonly used antibiotics in the first-line treatment of HP in gastric MALT lymphoma include amoxicillin, clarithromycin, and metronidazole. In the present study, all patients received PPI plus clarithromycin and amoxicillin. It should be noted that increasing antimicrobial resistance of HP, especially for clarithromycin, has been observed in patients with HP infections worldwide62–64. In Europe, the prevalence of clarithromycin resistance by HP ranges from 5.6% to 36.6%65. In a nationwide study of primary resistance to HP in Taiwan after implementation of a national policy to restrict antibiotic consumption since 2001, Liu et al. showed that the prevalence of primary resistance to clarithromycin was 11.2% (95% CI 9.6–13%)66. In a similar time period, in the Asia-Pacific region, the prevalence of primary resistance to clarithromycin in China (2000–2009)67, Japan (2000–2013)68, and Korea (2009–2012)69 was 23.8% (69/290), 31.1% (334/1073), and 23.7% (27/114), respectively. Nevertheless, the prevalence of antimicrobial resistance of HP to clarithromycin in gastric MALT lymphoma is rarely studied because the sensitivity of HP culture is obviously lower than the sensitivity to histologic detection of HP in gastric MALT lymphoma32. Recently, Bilgilier et al. found that the rate of clarithromycin resistance in 13 cases of gastric MALT lymphoma was 15% through analyses of the HP 23S rRNA gene containing genotypic clarithromycin resistance70.
Although the question of why a certain proportion of HP-negative gastric MALT lymphomas may respond to antibiotics remains unanswered, several crucial findings may support the following speculations: (1) HPE regimens may also eradicate other bacteria or Helicobacter-like bacteria, such as H. heilmannii that is associated with the development of gastric MALT lymphoma in humans71–73. For example, Morgner et al. found that five patients with documented H. heilmannii infection achieved a CR after 14 days of omeprazole and amoxicillin therapy72. However, we cannot exclude that HPE regimens may eradicate intestinal microbiota that may be associated with the development of HP-negative gastric MALT lymphoma74. (2) In addition to eradicating HP and HP-like bacteria, clarithromycin has direct anti-neoplastic or immunomodulatory effects46,55,75. In a B-cell lymphoma cell line derived from a BALB/c mice model, O’Hara et al. showed that clarithromycin inhibited cell viability and induced apoptosis though down-regulating BCL-2 expression76. Mizunoe et al. showed that macrolides, either clarithromycin or azithromycin, caused apoptosis of activated lymphocytes through attenuation of BCL-XL expression77. Another macrolide, erythromycin, was found to have an inhibitory effect on proliferation of T-cells, and a possible mechanism is the down-regulation of NF-κB expression78. In CD4+ T-cells, azithromycin effectively inhibited cell proliferation and cytokine secretion through down-regulation of the activity of mammalian target of rapamycin79. The aforementioned immunosuppressive effect on CD4+ T-cells was also observed at a higher concentration of clarithromycin (40 mg/L)79. Clinically, Ishimatsu et al. reported two cases of pulmonary MALT lymphoma successfully treated using clarithromycin 200 mg per day80. Kiesewetter et al. reported another case of ocular adnexal MALT lymphoma achieving a CR after treatment with first-line clarithromycin 500 mg twice per day for 4 weeks81. Raderer et al. speculated that high-dose clarithromycin (500 mg twice a day for 14 days) may yield a better CR rate of 38.5% in their cases of HP-negative gastric MALT lymphoma55. Our study also revealed a CR rate of 32% in patients receiving a clarithromycin (500 mg twice a day for 14 days)-based regimen. Furthermore, one recently published phase II trial reported that high-dose clarithromycin (2 g a day for 14 days for each course) resulted in a CR rate of 26.9% in patients with relapsed or refractory extranodal MALT lymphoma82.
Previous studies have demonstrated that the API2-MALT1 fusion protein resulting from a t(11;18)(q21;q21) translocation can activate NF-κB through an API2 moiety-mediated auto-oligomerization and thus contribute to the HP-independent growth of gastric MALT lymphoma83–85. However, whether t(11;18)(q21;q21) can predict antibiotic unresponsiveness in HP-negative gastric MALT lymphomas remains unclear. A systematic review of published results (included our present study) revealed that the frequency of t(11;18)(q21;q21) was significantly higher in antibiotic-unresponsive tumors than in antibiotic-responsive tumors (39 out of 74 [52.5%] vs. 2 out of 19 [10.5%], P = 0.001) (Table 3). These findings also indicate that for the other 50% of antibiotic-unresponsive tumors, other predictive markers should be pursued.
In this study, we showed that nuclear expression of BCL10 or NF-κB is closely associated with an antibiotic-unresponsive status and that both molecules are associated with the status of t(11;18)(q21;q21). These findings are consistent with those of Ye et al.7 and Sumida et al.33 who reported on the relationship between an antibiotic-unresponsive status and BCL10 nuclear expression in an HP-negative gastric MALT lymphoma.
As shown in Table 3, notably, two cases of HP-negative gastric MALT lymphomas with t(11;18)(q21;q21) remained antibiotic-responsive49,52. In a large series of HP-positive gastric MALT lymphomas, Liu et al. also demonstrated that 2 (4.5%) out of 44 patients with t(11;18)(q21;q21) remained HP dependent24. In our series of HP-positive gastric MALT lymphomas (data not shown), the pivotal role of BCL10 or NF-κB in HP-independent growth27,50,86,87 was demonstrated by the finding that two cases with t(11;18)(q21;q21) but lacking both nuclear expression of BCL10 and NF-κB responded well to HPE (Supplementary Fig. 1)87,88. Of these two cases with t(11;18)(q21;q21)-positive but no nuclear NF-κB expressing tumors, one case harbored a fusion transcript of t(11;18)(q21;q21) that contained 3 intact BIR domains in the amino terminal API2 region, and an intact caspase-like domain, but none of the immunoglobulin-like domains in the carboxyl terminal MALT1 region. Previous studies showed that the fusion product of t(11;18)(q21;q21) comprising an intact immunoglobulin-like domain had a greater ability to stimulate NF-κB signaling than the fusion product without an intact immunoglobulin-like domain89,90. Several studies have demonstrated that t(11;18)(q21;q21)-mediated NF-κB activation requires an interaction between API2-MALT1 and TRAF2 or TRAF683–85. The lack of an immunoglobulin-like domain and the disruption of the interaction with TRAF2 or TRAF6 of the API2-MALT1 fusion protein may be linked to the absence of nuclear NF-κB expression in some t(11;18)(q21;q21)-positive tumors that remain antibiotic-responsive.
In summary, the results of this study indicate that a substantial proportion of patients with early-stage HP-negative gastric MALT lymphoma remain antibiotic-responsive and can be cured using a first-line HPE regimen. In addition to t(11;18)(q21;q21), nuclear expression of BCL10 or NF-κB can help us predict antibiotics’ unresponsiveness. Further investigations into microbiota associated with the lymphomagenesis of HP-negative gastric MALT lymphoma are warranted.
Patients and Methods
Ethics statement
All experimental protocols were approved by the Institutional Review Board (IRB) of the Research Ethical Committee of National Taiwan University Hospital (NTUH IRB number: 9361700774). All experiments were conducted in accordance with the approved guidelines and regulations. The patients’ medical data were anonymized prior to access and analysis. All patients provided written informed consent to participate in and to provide tissue material for biological studies.
Patients, treatment, and tissue samples
We screened study subjects from the Cancer Registry, Medical Information Management Office, and the lymphoma database of the Department of Pathology of the National Taiwan University Hospital in Taipei, Taiwan between January 1, 2005 and June 30, 2014. We identified 93 patients with stage IE/IIE1 gastric MALT lymphoma from patients diagnosed with primary gastric lymphoma. We retrospectively reviewed the medical records and pathologic records of these patients to evaluate whether these gastric MALT lymphomas were HP-positive or HP-negative tumors. Evidence of HP infection was defined as positive results on biopsy, histology, a urease test, a 13C urea breath test, or serology91–93. An HP-negative status was defined as total negative results on histology (included HP, atrophic gastritis, and intestinal metaplasia)15,16, a rapid urease test, a 13C urea breath test, and serology.
There were 63 patients with HP-positive tumors and 29 patients with HP-negative tumors. Since gastric MALT lymphoma is relatively indolent and pseudo-negative HP tests may occur, most HP-negative patients in our institution were treated with first-line HPE regimens, particularly if their symptoms were insignificant.
From complete medical records, among 29 patients with HP-negative gastric MALT lymphoma, two patients received radiotherapy, 2 patients received alkylating agents-based chemotherapy, and 25 patients received antibiotics as a first-line treatment. The antibiotics regimens were the same as the HPE regimen, which consisted of 500 mg of amoxicillin administered four times a day (or 1000 mg of amoxicillin administered twice a day), 500 mg of clarithromycin administered twice a day, and 20 mg of omeprazole or 30 mg lansoprazole administered twice a day for 2 weeks as first-line treatment.
Diagnosis of gastric MALT lymphoma was made according to the histological criteria described by Isaacson et al. and the European Gastro-Intestinal Lymphoma Study consensus report on gastric extranodal marginal zone B-cell MALT lymphoma42,94. The tumors were staged and classified according to the Musshoff modification of the Ann Arbor staging system. The patients also received an examination for the presence of monoclonal gammopathy using serum IFE. The patients underwent their first follow-up after an upper gastrointestinal endoscopic examination or an ultrasonic endoscopic examination 4 to 8 weeks following HPE. This examination was repeated every 12 to 16 weeks until we observed histological evidence of remission.
The regression of the tumor following HPE was histologically evaluated according to the criteria of the (GELA) histological scoring system42,43. A CR is defined by the GELA grading system as the total disappearance of gross lymphoma and a negative histologic finding (CR or pMRD), whereas PR is defined as normalization or reduction of macroscopic findings, histologic signs of lymphoma regression, and no signs of progression. Tumors that resolved to a CR or PR after HPE were considered antibiotic-responsive42,43. Two subgroups of patients were considered antibiotic-unresponsive: (1) those who had SD but failed to show histologic regression 24 months following HPE, and (2) those with tumors exhibiting objective evidence of PD at any time during the follow-up42,43.
Multiplex reverse transcription polymerase chain reaction for the API2-MALT1 fusion transcript of t(11;18)(q21;q21) in lymphoma cells
Total cellular RNA was extracted from formalin-fixed and paraffin-embedded tissues using an Ambion RNA isolation kit (RecoverAll™ Total Nucleic Acid Isolation Kit, Ambion® | Life Technologies) and was analyzed for the API2-MALT1 fusion transcripts of the t(11;18)(q21;q21) translocation using multiplex reverse transcription polymerase chain reaction, followed by sequencing as described previously27,95,96. Gastric MALT lymphoma samples with API2-MALT1 fusion transcripts served as positive controls.
Immunohistochemistry
Immunohistochemistry for BCL10 (sc-9560; Santa Cruz Biotechnology, Santa Cruz, CA, USA), NF-κB (p65; sc-109; Santa Cruz Biotechnology), and CagA (A10; sc-28368, Santa Cruz Biotechnology) was performed on paraffin-embedded sections of pre-HPE endoscopic biopsies, using an indirect immunoperoxidase method according to the manufacturer’s instructions27,39,96. Paraffin sections with the first, second, or both primary antibodies omitted were used as negative controls to verify the specificity of the staining.
The percentages of positive cells were averaged to yield an immunohistological score of 0–100%. The staining was considered positive for BCL10 and NF-κB (p65) if the protein was detected in more than 10% of the tumor cells; nuclear staining was performed according to the criteria described by Ye et al.97 and Oshima et al.98. For the CagA maker, positive expression was defined as ≥10% of cells with moderate or strong immunostaining (tumor cells with readily appreciable brown staining distinctly marking the tumor cell nucleus or cytoplasm), as previously described39,40.
Statistical analysis
In this study, the Fisher exact test was used to compare the clinical characteristics, the presence of t(11;18)(p21;q21) as well as the expression levels of BCL10 and NF-κB (p65) between the antibiotic-responsive and antibiotic-unresponsive cases. The analyses were conducted using follow-up data that became available on December 31, 2015. Differences between the results of the comparative tests were considered statistically significant if the two-sided P-value was <0.05.
Electronic supplementary material
Supplementary Methods and Supplementary Figure 1
Acknowledgements
The authors thank the Cancer Registry, Office of Medical Records, National Taiwan University Hospital, for providing necessary patient information. This study was supported by the following research grants: MOST 104-2314-B-002-189-MY3, and MOST 106-2811-B-002-075 from the Ministry of Science and Technology, Taiwan, MOHW106-TDU-B-211-144005 and MOHW106-TDU-B-XXXX from the Ministry of Health and Welfare, Taiwan, and NTU 106-S3514 and DOH97-TD-B-111-001 from the National Taiwan University Hospital. The study was also sponsored, conducted and analyzed by the National Center of Excellence for General Clinical Trial and Research at the National Taiwan University Hospital.
Author Contributions
Contribution: S.H.K., K.H.Y., and A.L.C. contributed to the study design; S.H.K., K.H.Y., M.S.W., J.M.L., H.P.W., L.T.C., and A.L.C. treated patients; S.H.K., K.H.Y., M.S.W., C.W.L., J.M.L., L.T.C., and A.L.C. provided tissue sample; S.H.K., C.W.L., and M.F.W. performed research; S.H.K., K.H.Y., C.W.L., and A.L.C. were involved in data analysis and interpretation; S.H.K., and A.L.C. wrote the manuscript, which was revised and approved by all co-authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14102-8.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Du MQ, Isaccson PG. Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol. 2002;3:97–104. doi: 10.1016/S1470-2045(02)00651-4. [DOI] [PubMed] [Google Scholar]
- 2.Zullo A, et al. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissuelymphoma. Clin Gastroenterol Hepatol. 2010;8:105–110. doi: 10.1016/j.cgh.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Kuo SH, Cheng AL. Helicobacter pylori and mucosa-associated lymphoid tissue: what’s new. Hematology Am Soc Hematol Educ Program. 2013;2013:109–117. doi: 10.1182/asheducation-2013.1.109. [DOI] [PubMed] [Google Scholar]
- 4.Zucca E, et al. ESMO Guidelines Working Group. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi144–148. doi: 10.1093/annonc/mdt343. [DOI] [PubMed] [Google Scholar]
- 5.Steinbach G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med. 1999;131:88–95. doi: 10.7326/0003-4819-131-2-199907200-00003. [DOI] [PubMed] [Google Scholar]
- 6.Ruskone-Fourmestraux A, et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut. 2001;48:297–303. doi: 10.1136/gut.48.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye H, et al. High incidence of t(11;18)(q21; q21) in Helicobacter pylori-negative gastric MALT lymphoma. Blood. 2003;101:2547–2550. doi: 10.1182/blood-2002-10-3167. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura S, Matsumoto T. Treatment strategy for gastric mucosa-associated lymphoid tissue lymphoma. Gastroenterol Clin North Am. 2015;44:649–660. doi: 10.1016/j.gtc.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Park HS, Kim YJ, Yang WI, Suh CO, Lee YC. Treatment outcome of localized Helicobacter pylori-negative low-grade gastric MALT lymphoma. World J Gastroenterol. 2010;16:2158–2162. doi: 10.3748/wjg.v16.i17.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zullo A, et al. Eradication therapy in Helicobacter pylori-negative, gastric low-grade mucosa-associated lymphoid tissue lymphoma patients: a systematic review. J Clin Gastroenterol. 2013;47:824–827. doi: 10.1097/MCG.0b013e318286ff72. [DOI] [PubMed] [Google Scholar]
- 11.Asano N, Iijima K, Koike T, Imatani A, Shimosegawa T. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphomas: A review. World J Gastroenterol. 2015;21:8014–8020. doi: 10.3748/wjg.v21.i26.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laine L, Estrada R, Trujillo M, Knigge K, Fennerty MB. Effect of proton-pump inhibitor therapy on diagnostic testing for Helicobacter pylori. Ann Intern Med. 1998;129:547–550. doi: 10.7326/0003-4819-129-7-199810010-00007. [DOI] [PubMed] [Google Scholar]
- 13.Murakami K, et al. Influence of anti-ulcer drugs used in Japan on the result of (13)C-urea breath test for the diagnosis of Helicobacter pylori infection. J Gastroenterol. 2003;38:937–941. doi: 10.1007/s00535-003-1176-x. [DOI] [PubMed] [Google Scholar]
- 14.Miftahussurur M, Yamaoka Y. Diagnostic methods of Helicobacter pylori infection for epidemiological studies: critical importance of indirect test validation. Biomed Res Int. 2016;2016:4819423. doi: 10.1155/2016/4819423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura S, et al. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: a clinicopathologic and molecular study with reference to antibiotic treatment. Cancer. 2006;107:2770–2778. doi: 10.1002/cncr.22326. [DOI] [PubMed] [Google Scholar]
- 16.Akamatsu T, et al. Comparison of localized gastric mucosa-associated lymphoid tissue (MALT) lymphoma with and without Helicobacter pylori infection. Helicobacter. 2006;11:86–95. doi: 10.1111/j.1523-5378.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 17.Azevedo NF, et al. Coccoid form of Helicobacter pylori as a morphological manifestation of cell adaptation to the environment. Appl Environ Microbiol. 2007;73:3423–3427. doi: 10.1128/AEM.00047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal N, Snyder P, Owens SR. Unusual Helicobacter pylori in gastric resection specimens: an old friend with a new look. Int J Surg Pathol. 2011;19:297–302. doi: 10.1177/1066896911398654. [DOI] [PubMed] [Google Scholar]
- 19.Lee JY, Kim N. Diagnosis of Helicobacter pylori by invasive test: histology. Ann Transl Med. 2015;3:10. doi: 10.3978/j.issn.2305-5839.2014.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willis TG, et al. Bcl10 is involved in t(1;14)(p22; q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell. 1999;96:35–45. doi: 10.1016/S0092-8674(00)80957-5. [DOI] [PubMed] [Google Scholar]
- 21.Du MQ. MALT lymphoma: many roads lead to nuclear factor-κb activation. Histopathology. 2011;58:26–38. doi: 10.1111/j.1365-2559.2010.03699.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet. 2001;357:39–40. doi: 10.1016/S0140-6736(00)03571-6. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama T, et al. API2-MALT1 chimeric transcript is a predictive marker for the responsiveness of H. pylori eradication treatment in low-grade gastric MALT lymphoma. Gastroenterology. 2001;120:1884–1885. doi: 10.1053/gast.2001.25305. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, et al. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 2002;122:1286–1294. doi: 10.1053/gast.2002.33047. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Nakamura S, Yonezumi M, Seto M, Yokoi T. The t(11;18)(q21; q21) translocation in H. pylori-negative low-grade gastric MALT lymphoma. AmJ Gastroenterol. 2000;95:3314–3315. doi: 10.1111/j.1572-0241.2000.03314.x. [DOI] [PubMed] [Google Scholar]
- 26.Choi YJ, et al. Characteristics of Helicobacter pylori-positive and Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma and their influence on clinical outcome. Helicobacter. 2013;18:197–205. doi: 10.1111/hel.12033. [DOI] [PubMed] [Google Scholar]
- 27.Yeh KH, et al. Nuclear expression of BCL10 or nuclear factor kappa B helps predict Helicobacter pylori-independent status of low-grade gastric mucosa-associated lymphoid tissue lymphomas with or without t(11;18)(q21;q21) Blood. 2005;106:1037–1041. doi: 10.1182/blood-2005-01-0004. [DOI] [PubMed] [Google Scholar]
- 28.Ghoshal UC, et al. Frequency of Helicobacter pylori and CagA antibody in patients with gastric neoplasms and controls: the Indian enigma. Dig Dis Sci. 2008;53:1215–1222. doi: 10.1007/s10620-008-0229-7. [DOI] [PubMed] [Google Scholar]
- 29.Achyut BR, Moorchung N, Srivastava AN, Gupta NK, Mittal B. Risk of lymphoid follicle development in patients with chronic antral gastritis: role of endoscopic features, histopathological parameters, CagA status and interleukin-1 gene polymorphisms. Inflamm Res. 2008;57:51–56. doi: 10.1007/s00011-007-7033-2. [DOI] [PubMed] [Google Scholar]
- 30.Eck M, et al. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology. 1997;112:1482–1486. doi: 10.1016/S0016-5085(97)70028-3. [DOI] [PubMed] [Google Scholar]
- 31.Fischbach W, Jung T, Goebeler-Kolve M, Eck M. Comparative analysis of the Helicobacter pylori status in patients with gastric MALT-type lymphoma and their respective spouses. Z Gastroenterol. 2000;38:627–630. doi: 10.1055/s-2000-7513. [DOI] [PubMed] [Google Scholar]
- 32.Lehours P, Ruskone-Fourmestraux A, Lavergne A, Cantet F, Mégraud F. Which test to use to detect Helicobacter pylori infection in patients with low-grade gastric mucosa-associated lymphoid tissue lymphoma? Am J Gastroenterol. 2003;98:291–295. doi: 10.1111/j.1572-0241.2003.t01-1-07264.x. [DOI] [PubMed] [Google Scholar]
- 33.Sumida T, et al. Antibodies to Helicobacter pylori and CagA protein are associated with the response to antibacterial therapy in patients with H. pylori-positive API2-MALT1-negative gastric MALT lymphoma. Cancer Sci. 2009;100:1075–1081. doi: 10.1111/j.1349-7006.2009.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo SH, et al. Expressions of the CagA protein and CagA-signaling molecules predict Helicobacter pylori dependence of early-stage gastric DLBCL. Blood. 2017;129:188–198. doi: 10.1182/blood-2016-04-713719. [DOI] [PubMed] [Google Scholar]
- 35.Lin WC, et al. Translocation of Helicobacter pylori CagA into Human B lymphocytes, the origin of mucosa associated lymphoid tissue lymphoma. Cancer Res. 2010;70:5740–5748. doi: 10.1158/0008-5472.CAN-09-4690. [DOI] [PubMed] [Google Scholar]
- 36.Umehara S, Higashi H, Ohnishi N, Asaka M, Hatakeyama M. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene. 2003;22:8337–8342. doi: 10.1038/sj.onc.1207028. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, et al. The Helicobacter pylori virulence factor CagA promotes Erk1/2-mediated Bad phosphorylation in lymphocytes: a mechanism of CagA-inhibited lymphocyte apoptosis. Cell Microbiol. 2007;9:952–561. doi: 10.1111/j.1462-5822.2006.00843.x. [DOI] [PubMed] [Google Scholar]
- 38.Ohnishi N, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA. 2008;105:1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo SH, et al. Detection of the Helicobacter pylori CagA protein in gastric mucosa-associated lymphoid Tissue lymphoma cells — clinical and biological significance. Blood Cancer J. 2013;3:e125. doi: 10.1038/bcj.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuo SH, et al. Helicobacter pylori CagA translocation is closely associated with the expression of CagA signaling molecules in low-grade gastric mucosa-associated lymphoid tissue lymphoma. Am J Surg Pathol. 2015;39:761–766. doi: 10.1097/PAS.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 41.Copie-Bergman C, et al. Proposal for a new histological grading system for post-treatment evaluation of gastric MALT lymphoma. Gut. 2003;52:1656. doi: 10.1136/gut.52.11.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruskoné-Fourmestraux A, et al. EGILS group. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747–758. doi: 10.1136/gut.2010.224949. [DOI] [PubMed] [Google Scholar]
- 43.Copie-Bergman C, et al. Gela histological scoring system for post-treatment biopsies of patients with gastric MALT lymphoma is feasible and reliable in routine practice. Br J Haematol. 2013;160:47–52. doi: 10.1111/bjh.12078. [DOI] [PubMed] [Google Scholar]
- 44.Fischbach W, et al. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut. 2007;56:1685e7. doi: 10.1136/gut.2006.096420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hancock BW, et al. Chlorambucil versus observation after anti-Helicobacter therapy in gastric MALT lymphomas: results of the international randomised LY03 trial. Br J Haematol. 2009;144:367–375. doi: 10.1111/j.1365-2141.2008.07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raderer M, Streubel B, Wöhrer S, Häfner M, Chott A. Successful antibiotic treatment of Helicobacter pylori negative gastric mucosa associated lymphoid tissue lymphomas. Gut. 2006;55:616–618. doi: 10.1136/gut.2005.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nozaki K, et al. Helicobacter pylori negative/API2-MALT1 translocation-negative low-grade MALT lymphoma. Gastric Cancer. 2006;9:229–234. doi: 10.1007/s10120-006-0367-6. [DOI] [PubMed] [Google Scholar]
- 48.Terai S, et al. Long-term outcomes of gastric mucosa-associated lymphoid tissue lymphomas after Helicobacter pylori eradication therapy. Tohoku J Exp Med. 2008;214:79–87. doi: 10.1620/tjem.214.79. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura T, et al. Clinical features and prognosis of gastric MALT lymphoma with special reference to responsiveness to H. pylori eradication and API2-MALT1 status. Am J Gastroenterol. 2008;103:62–70. doi: 10.1111/j.1572-0241.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 50.Dong G, et al. BCL10 nuclear expression and t(11;18)(q21; q21) indicate nonresponsiveness to Helicobacter pylori eradication of Chinese primary gastric MALT lymphoma. Int J Hematol. 2008;88:516–523. doi: 10.1007/s12185-008-0187-z. [DOI] [PubMed] [Google Scholar]
- 51.Stathis A, et al. Long-term outcome following Helicobacter pylori eradication in a retrospective study of 105 patients with localized gastric marginal zone B-cell lymphoma of MALT type. Ann Oncol. 2009;20:1086–1093. doi: 10.1093/annonc/mdn760. [DOI] [PubMed] [Google Scholar]
- 52.Asano N, et al. Eradication therapy is effective for Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma. Tohoku J Exp Med. 2012;228:223–227. doi: 10.1620/tjem.228.223. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura S, et al. JAPAN GAST Study Group. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61:507–513. doi: 10.1136/gutjnl-2011-300495. [DOI] [PubMed] [Google Scholar]
- 54.Ryu KD, et al. Treatment outcome for gastric mucosa-associated lymphoid tissue lymphoma according to Helicobacter pylori infection status: a single-center experience. Gut Liver. 2014;8:408–414. doi: 10.5009/gnl.2014.8.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raderer M, et al. Antibiotic treatment as sole management of Helicobacter pylori-negative gastric MALT lymphoma: a single center experience with prolonged follow-up. Ann Hematol. 2015;94:969–973. doi: 10.1007/s00277-014-2298-3. [DOI] [PubMed] [Google Scholar]
- 56.Li X, et al. Evaluation of the clinical characteristics, management, and prognosis of 103 patients with gastric mucosa-associated lymphoid tissue lymphoma. Oncol Lett. 2016;11:1713–1718. doi: 10.3892/ol.2016.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim JS, Kang SH, Moon HS, Sung JK, Jeong HY. Clinical outcome of eradication therapy for gastric mucosa-associated lymphoid tissue lymphoma according to H. pylori infection status. Gastroenterol Res Pract. 2016;2016:6794848. doi: 10.1155/2016/6794848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong EJ, et al. Helicobacter pylori eradication therapy Is effective as the initial treatment for patients with H. pylori-negative and disseminated gastric mucosa-associated lymphoid tissue lymphoma. Gut Liver. 2016;10:706–713. doi: 10.5009/gnl15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zullo A, et al. Primary low-grade and high-grade gastric MALT-lymphoma presentation. J Clin Gastroenterol. 2010;44:340–344. doi: 10.1097/MCG.0b013e3181d6b543. [DOI] [PubMed] [Google Scholar]
- 60.Luminari S, et al. Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas. Ann Oncol. 2009;21:855–859. doi: 10.1093/annonc/mdp402. [DOI] [PubMed] [Google Scholar]
- 61.Sena Teixeira Mendes L, Attygalle AD, Wotherspoon AC. Helicobacter pylori infection in gastric extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma: a re-evaluation. Gut. 2014;63:1526–1527. doi: 10.1136/gutjnl-2014-307389. [DOI] [PubMed] [Google Scholar]
- 62.Camargo MC, et al. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am J Gastroenterol. 2014;109:485–495. doi: 10.1038/ajg.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malfertheiner P, et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 64.Liou JM, et al. Levofloxacin-based and clarithromycin-based triple therapies as first-line and second-line treatments for Helicobacter pylori infection: a randomized comparative trial with crossover design. Gut. 2010;59:572–578. doi: 10.1136/gut.2009.198309. [DOI] [PubMed] [Google Scholar]
- 65.Megraud F, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 66.Liou JM, et al. Taiwan Gastrointestinal Disease and Helicobacter Consortium. The Primary Resistance of Helicobacter pylori in Taiwan after the National Policy to Restrict Antibiotic Consumption and Its Relation to Virulence Factors-A Nationwide Study. PLoS One. 2015;10:e0124199. doi: 10.1371/journal.pone.0124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao W, et al. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15:460–466. doi: 10.1111/j.1523-5378.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 68.Okamura T, et al. Antimicrobial resistance and characteristics of eradication therapy of Helicobacter pylori in Japan: a multi-generational comparison. Helicobacter. 2014;19:214–220. doi: 10.1111/hel.12124. [DOI] [PubMed] [Google Scholar]
- 69.Lee JW, et al. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 2013;18:206–214. doi: 10.1111/hel.12031. [DOI] [PubMed] [Google Scholar]
- 70.Bilgilier C, et al. Prevalence of clarithromycin-resistant Helicobacter pylori strains in gastric mucosa-associated lymphoid tissue lymphoma patients. Ann Hematol. 2016;95:1115–1120. doi: 10.1007/s00277-016-2672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakamura S, et al. Helicobacter pylori and primary gastric lymphoma: a histopathologic and immunohisotochemical analysis of 237 patients. Cancer. 1997;79:3–11. doi: 10.1002/(SICI)1097-0142(19970101)79:1<3::AID-CNCR2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 72.Morgner A, et al. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821–828. doi: 10.1016/S0016-5085(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 73.Okamura T, et al. A case of Helicobacter heilmannii-associated primary gastric mucosa-associated lymphoid tissuelymphoma achieving complete remission after eradication. Clin J Gastroenterol. 2013;6:38–45. doi: 10.1007/s12328-012-0355-9. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto ML, Schiestl RH. Lymphoma caused by intestinal microbiota. Int J Environ Res Public Health. 2014;11:9038–9049. doi: 10.3390/ijerph110909038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Govi S, et al. Six-month oral clarithromycin regimen is safe and active in extranodal marginal zone B-cell lymphomas: final results of a single-centre phase II trial. Br J Haematol. 2010;150:226–229. doi: 10.1111/j.1365-2141.2010.08179.x. [DOI] [PubMed] [Google Scholar]
- 76.Ohara T, et al. Antibiotics directly induce apoptosis in B cell lymphoma cells derived from BALB/c mice. Anticancer Res. 2004;24:3723–3730. [PubMed] [Google Scholar]
- 77.Mizunoe S, et al. Clarithromycin and azithromycin induce apoptosis of activated lymphocytes via down-regulation of Bcl-xL. Int Immunopharmacol. 2004;4:1201–1207. doi: 10.1016/j.intimp.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 78.Wu L, et al. Immunomodulatory effects of erythromycin and its derivatives on human T-lymphocyte in vitro. Immunopharmacol Immunotoxicol. 2007;29:587–596. doi: 10.1080/08923970701692841. [DOI] [PubMed] [Google Scholar]
- 79.Ratzinger F, et al. Azithromycin suppresses CD4(+) T-cell activation by direct modulation of mTOR activity. Sci Rep. 2014;4:7438. doi: 10.1038/srep07438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishimatsu Y, et al. Two cases with pulmonary mucosa-associated lymphoid tissue lymphoma successfully treated with clarithromycin. Chest. 2010;138:730–733. doi: 10.1378/chest.09-2358. [DOI] [PubMed] [Google Scholar]
- 81.Kiesewetter B, et al. Clarithromycin Leading to Complete Remission in the First-Line Treatment of Ocular Adnexal Mucosa-Associated Lymphoid Tissue Lymphoma. J Clin Oncol. 2015;33:e130–2. doi: 10.1200/JCO.2013.49.8006. [DOI] [PubMed] [Google Scholar]
- 82.Ferreri AJ, et al. High-dose clarithromycin is an active monotherapy for patients with relapsed/refractory extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT): the HD-K phase II trial. Ann Oncol. 2015;26:1760–1765. doi: 10.1093/annonc/mdv214. [DOI] [PubMed] [Google Scholar]
- 83.Lucas PC, et al. A dual role for the API2 moiety in API2-MALT1-dependent NF-κB activation: heterotypic oligomerization and TRAF2 recruitment. Oncogene. 2007;26:5643–5654. doi: 10.1038/sj.onc.1210342. [DOI] [PubMed] [Google Scholar]
- 84.Rosebeck S, Lucas PC, McAllister-Lucas LM. Protease activity of the API2-MALT1 fusion oncoprotein in MALT lymphoma development and treatment. Future Oncol. 2011;7:613–617. doi: 10.2217/fon.11.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du MQ. MALT lymphoma: A paradigm of NF-κB dysregulation. Semin Cancer Biol. 2016;39:49–60. doi: 10.1016/j.semcancer.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 86.Ye H, et al. Strong BCL10 nuclear expression identifies gastric MALT lymphomas that do not respond to H pylori eradication. Gut. 2006;55:137–138. doi: 10.1136/gut.2005.081117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuo SH, et al. B cell-activating factor signalling pathway is associated with H. pylori independence in gastric MALT lymphoma without t(11;18)(q21; q21) J Pathol. 2017;241:420–433. doi: 10.1002/path.4852. [DOI] [PubMed] [Google Scholar]
- 88.Nakamura S, et al. Clinical impact of genetic aberrations in gastric MALT lymphoma: a comprehensive analysis using interphase fluorescence in situ hybridisation. Gut. 2007;56:1358–1363. doi: 10.1136/gut.2007.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uren GA, et al. Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell. 2000;6:961–967. doi: 10.1016/s1097-2765(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 90.Lucas PC, et al. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-kappa B signaling pathway. J Biol Chem. 2001;276:19012–19019. doi: 10.1074/jbc.M009984200. [DOI] [PubMed] [Google Scholar]
- 91.Dzierzanowska-Fangrat K, Lehours P, Mégraud F, Dzierzanowska D. Diagnosis of Helicobacter pylori infection. Helicobacter. 2006;11(Suppl 1):6–13. doi: 10.1111/j.1478-405X.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 92.Hirschl AM, Makristathis A. Methods to detect Helicobacter pylori: from culture to molecular biology. Helicobacter. 2007;12(Suppl 2):6–11. doi: 10.1111/j.1523-5378.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 93.Wang YK, et al. Diagnosis of Helicobacter pylori infection: Current options and developments. World J Gastroenterol. 2015;21:11221–11235. doi: 10.3748/wjg.v21.i40.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Isaacson PG, Du MQ. Gastrointestinal lymphoma: where morphology meets molecular biology. J Pathol. 2005;205:255–274. doi: 10.1002/path.1703. [DOI] [PubMed] [Google Scholar]
- 95.Inagaki H, et al. API2-MALT1 fusion transcripts involved in mucosa-associated lymphoid tissue lymphoma: multiplex RT-PCR detection using formalin-fixed paraffin-embedded specimens. Am J Pathol. 2001;158:699–706. doi: 10.1016/S0002-9440(10)64012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuo SH, et al. Nuclear expression of BCL10 or nuclear factor kappa B predicts Helicobacter pylori-independent status of early-stage, high-grade gastric mucosa-associated lymphoid tissue lymphomas. J Clin Oncol. 2004;22:3491–3497. doi: 10.1200/JCO.2004.10.087. [DOI] [PubMed] [Google Scholar]
- 97.Ye H, et al. BCL10 expression in normal and neoplastic lymphoid tissue. Nuclear localization in MALT lymphoma. Am J Pathol. 2000;157:1147–1154. doi: 10.1016/S0002-9440(10)64630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohshima K, et al. Bcl10 expression, rearrangement and mutation in MALT lymphoma: correlation with expression of nuclear factor-kappaB. Int J Oncol. 2001;19:283–189. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods and Supplementary Figure 1