Abstract
Meiotic recombination is initiated by DNA double-strand breaks (DSBs). Most DSBs are converted into nonreciprocal exchanges (gene conversions) or crossovers (COs) between sister chromatids. Only a minority of DSBs are processed toward interhomolog COs, the precursors of the chiasmata that connect homologous chromosomes. Dmc1, the meiosis-specific paralog of the universal recombination protein Rad51, is required for interhomolog COs; in its absence, univalents are primarily formed. Here, we report a ciliate-specific novel meiotic gene, BIME2, which also promotes interhomolog crossing over. In the bime2Δ mutant, DSBs are formed and repaired normally, but bivalent formation is strongly reduced. Bime2 protein forms foci on chromatin during meiotic prophase, and chromatin localization of Bime2 and Dmc1 is largely interdependent. Bime2 distantly resembles budding yeast Rdh54/Tid1 and the vertebrate Rad54B helicases and may have similar functions in promoting or stabilizing Dmc1 nucleoprotein filaments.
Electronic supplementary material
The online version of this article (doi:10.1007/s10577-017-9563-y) contains supplementary material, which is available to authorized users.
Keywords: Meiosis, Chromosome pairing, Crossover, Recombination, Double-strand break
Introduction
Meiotic recombination enables the formation of interhomolog crossovers (COs). In this process, numerous DNA double-strand breaks (DSBs) are generated to ensure proper homology searching and homologous pairing. However, only a small subset of DSBs are converted into COs and chiasmata, necessary for the orderly segregation of homologous chromosomes and genetic recombination. Most DSBs are repaired by nonreciprocal exchange (gene conversion) or recombination between sister chromatids (see Goldfarb and Lichten 2010; Chapman et al. 2012). To ensure that sufficient COs are formed between homologs, mechanisms act to suppress the more readily occurring Rad51-dependent intersister recombination events (Niu et al. 2009) or actively promote interhomolog recombination. One interhomolog-promoting factor is Dmc1, a meiosis-specific paralog of the ubiquitous Rad51 recombinase (see Brown and Bishop 2015), which performs better in exchanging homologous DNA molecules with similar but not identical DNA tracts (see Howard-Till et al. 2011; Lee et al. 2015). The strand exchange activities of Rad51 and Dmc1 are supported by numerous proteins that facilitate and stabilize their association with single-stranded DNA (ssDNA) and promote homologous strand invasion and heteroduplex formation (see Brown and Bishop 2015). One such factor is Tid1/Rdh54, which interacts with both proteins, but is believed to specifically cooperate with Dmc1 in meiosis (Nimonkar et al. 2012).
Tetrahymena thermophila is a versatile protist model organism with a history in groundbreaking discoveries, such as self-splicing introns, histone-modifying enzymes, and telomeres and telomerase (see Ruehle et al. 2016). Also, apart from fungal, animal, and plant model systems, Tetrahymena is the organism with the best-studied meiosis (see Loidl 2016). Tetrahymena meiosis is remarkable for its simplicity, the absence of a synaptonemal complex (SC), and the extreme stretching of meiotic prophase nuclei in response to Spo11-induced DSBs (Chi et al. 2014; Mochizuki et al. 2008; Loidl and Lorenz 2016). Most eukaryotes use two major pathways to form COs: The class I pathway involves SC formation and ZMM (Zip1/2/3/4, Msh4/5, and Mer3) proteins and generates interfering (i.e., mutually suppressing) COs. The class II pathway is largely ZMM-independent and produces non-interfering COs (de los Santos et al. 2003). In contrast, Tetrahymena uses a single-mixed pathway, involving the ZMM proteins Msh4, Msh5, and a protein similar to Zip3 (Shodhan et al. 2014; Shodhan et al. 2017). As in most other organisms, only a fraction of DSBs are converted into homolog-directed COs. However, unlike budding yeast and probably other SC-possessing organisms, where the chromosome axis-associated kinase Mek1 and the axial element components Red1 and Hop1 are involved in inhibiting Rad51-dependent intersister recombination (e.g., Thompson and Stahl 1999; Schwacha and Kleckner 1997; Niu et al. 2009; Chuang et al. 2012; Hollingsworth et al. 1995), Tetrahymena depends solely on the interhomolog preference of Dmc1 (Howard-Till et al. 2011). Here, we report a novel protein which, together with Dmc1, ensures interhomolog recombination in Tetrahymena.
Materials and methods
Strains and cell culture
Cells were cultured at 30 °C using standard methodology (Orias et al. 2000) and were made competent for mating by starvation in 10 mM Tris-HCl (pH 7.4) for at least 16 h. Meiosis was induced by mixing starved cultures of B2086 (mating type II) and Cu428 (mating type VII) wild-type or derivative mutant strains at equal densities (∼ 2 × 105 cells/ml).
Somatic gene knockout and protein tagging
For somatic gene knockout, (almost) all of the ~ 50 copies of a target gene in the polyploid somatic macronucleus must be replaced with a deletion cassette carrying an antibiotic resistance marker. Moreover, to investigate the effects of gene inactivation in meiosis, the gene must be deleted in both mating partners because mating cells can share gene products (McDonald 1966). For BIME2 deletion, 1767 bp of the open reading frame was replaced with a construct carrying a neomycin resistance marker (Supplemental information S1a), by homologous recombination of flanking regions (Cassidy-Hanley et al. 1997; Mochizuki 2008). Knockout lines were selected by culture in medium with increasing concentrations of the neomycin analog paromomycin. Complete gene replacement was confirmed by Southern hybridization to a restriction fragment spanning the gene locus (Supplemental information S1b).
A Bime2-HA-tagged strain was created by fusing the HA sequence to the 3′ end of the BIME2 open reading frame (Supplemental information S1c). Construction of dmc1RNAi (Howard-Till et al. 2011) and spo11RNAi strains (Lukaszewicz et al. 2013) was done as previously reported. Mating of the Bime2-HA-tagged strain to dmc1RNAi and spo11RNAi cells led to depletion of the respective endogenous protein and HA-tagged Bime2 expression in both partners as a result of the cytoplasmatic exchange of RNA molecules and proteins between mating cells (McDonald 1966). A strain expressing mCherry-tagged histone H3 was kindly provided by Dr. Kensuke Kataoka (Natl Inst. Basic Biol., Okazaki, JP).
Cytological preparation, staining and microscopy
For 4′,6-diamidino-2-phenylindole (DAPI) staining, cells were fixed in formaldehyde and spread on a slide (Mochizuki et al. 2008). For Bime2 and Dmc1 localization studies, cells were pretreated with Triton X-100 to remove protein not bound to chromatin (Howard-Till et al. 2011), and then, primary and fluorescent secondary antibodies were applied. Dmc1 was detected using a commercial antibody (51RAD01 mouse monoclonal, NeoMarkers, Fremont, CA). Samples on slides were mounted in anti-fading solution containing DAPI as a stain for chromatin and were evaluated by fluorescence microscopy using appropriate filters. Image stacks were recorded using MetaVue software (Molecular Devices, Sunnyvale, CA) and deconvolved. A Schaudinn fixation plus Giemsa staining method (Bruns and Brussard 1981; Shodhan et al. 2014) was used to release nuclei from cells, and the resulting flattened, well-separated chromosomes were inspected under bright-field microscopy.
DSB detection
To analyze DSB-dependent DNA fragmentation, DNA was isolated from cells at different time points after induction of meiosis. DNA fragments were separated by pulsed-field gel electrophoresis and visualized by Southern hybridization to a radiolabeled probe specific to the germline nucleus (for details, see Lukaszewicz et al. 2010).
Protein co-immunoprecipitation
For co-immunoprecipitation (co-IP) experiments, cells were harvested at ~ 3.5-h post-meiotic induction (at the stage with maximum nuclear elongation), washed, resuspended in ice-cold Tris lysis buffer (100 mM Tris-Base, Tris-HCl, 1 M KCl, 1 M MgCl, 1% Triton X-100, 0.01 M PMSF, pH 7.5), and ground in a Dounce homogenizer. The cell lysate was clarified, filtered, and incubated with anti-HA magnetic beads (Thermo Fisher Scientific, Waltham, MA) for 2 h at 4 °C. (For details of the procedure, see Shodhan et al. 2017.) After washing, two thirds of the protein-loaded beads were analyzed by mass spectrometry and protein eluted from the remaining third was analyzed by Western blotting.
Results and discussion
Bime2 is important for bivalent formation
We identified BIME2 (BIvalents in MEiosis 2; TTHERM_00530659—see http://ciliate.org/) in a reverse genetic screen, in which genes exclusively expressed during sexual reproduction (conjugation) were knocked out. BIME2 expression is highest at around 2–4 h after induction of meiosis (Xiong et al. 2012); http://tfgd.ihb.ac.cn/), i.e., when homologous pairing and recombination occur (see Loidl and Lorenz 2016). BIME2 expression is controlled by the conjugation-specific cyclin Cyc2, and CYC2 deletion led to the strongest repression of BIME2 transcription compared with all other meiotic genes (Xu et al. 2016).
Cytological analysis showed that bime2Δ cells undergo all stages of meiosis (Fig. 1). However, after anaphase II, all four haploid nuclei are degraded in about half (52%, n = 100) of bime2Δ meiotic cells, and none of the mating pairs produced viable sexual progeny (compared with 72% of wild-type mating pairs; n = 150 each).
Fig. 1.
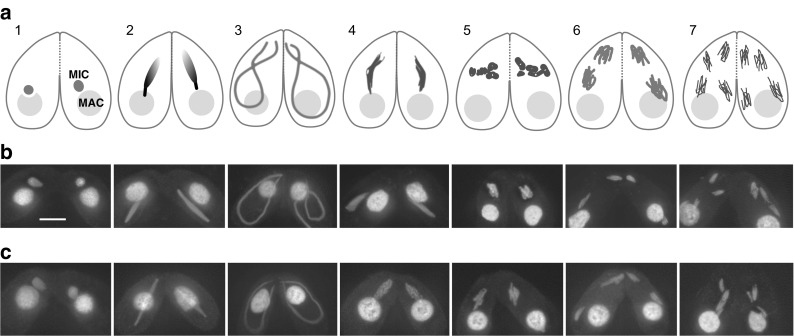
Stages of Tetrahymena meiosis. a Two cells of different mating types mate upon starvation. Each cell has a polyploid somatic macronucleus (MAC) and a diploid germline micronucleus (MIC). Only the germline nucleus undergoes meiosis. The somatic nucleus is degraded after meiosis, whereas the products of micronuclear meiosis undergo reciprocal fertilization, and progeny germline and somatic nuclei are formed from the zygotic nuclei. 1. Initiation of synchronous closed meioses in the two mating partners. 2. Early prophase. DSBs are formed, meiotic nuclei begin to elongate. 3. Mid-prophase. Nuclei elongate to about twice the cell length. Chromosomes are arranged in parallel bundles within the elongated nuclei, with centromeres assembled near one tip and telomeres at the opposite tip. Homologous pairing takes place. 4. Late prophase. Nuclei shorten, DSBs are repaired and COs are formed. 5. Five condensed bivalents appear. 6. First meiotic division. 7. Second meiotic division. (For detailed descriptions of cytological stages and the corresponding events of molecular recombination, see e.g. Loidl and Lorenz 2016; Shodhan et al. 2014; Loidl et al. 2012.) b DAPI-stained wild-type meiosis. c DAPI-stained bime2Δ meiosis. Scale bar: 10 μm
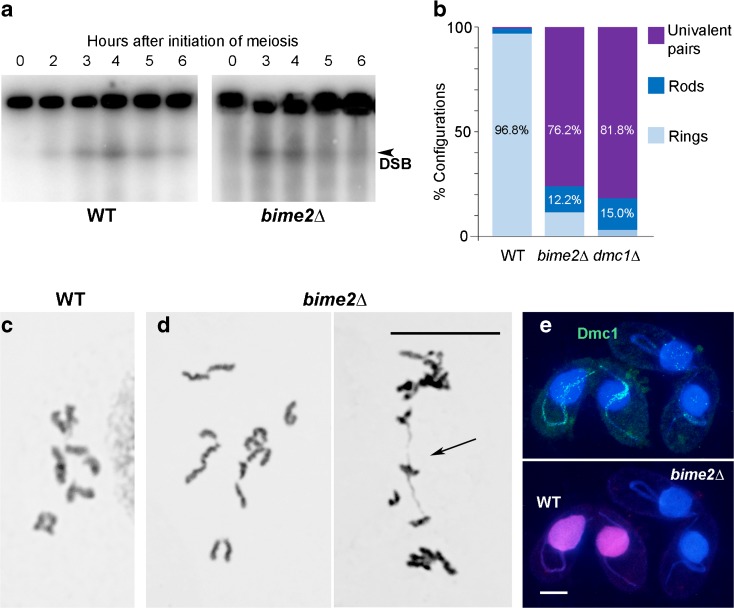
In Tetrahymena, DSBs trigger the extreme elongation of meiotic prophase nuclei (Mochizuki et al. 2008). In bime2Δ cells, nuclear elongation is normal, suggesting that DSBs are formed during meiosis. DSB formation in bime2Δ cells was confirmed by the detection of transient germline chromosome fragments by pulsed-field gel electrophoresis, similar to wild-type cells (Fig. 2a).
Fig. 2.
Deletion of BIME2 prevents chromosome pairing and inhibits Dmc1 chromatin localization. a Southern hybridization of DSB-dependent chromosome fragments separated by pulsed-field gel electrophoresis using a probe specific to the germline nucleus. DSB formation is similar in wild-type and bime2Δ cells. b Ring bivalents are mainly formed in the wild type, whereas univalents and rare bivalents are formed in bime2Δ and dmc1Δ meiosis. In wild type and bime2Δ, 500 configurations (bivalents or pairs of univalents) were counted; in dmc1Δ, 400 configurations were counted. c, d Examples of Giemsa-stained diakinesis-metaphase I wild-type (c) and bime2Δ (d) nuclei (arrow indicates a rod bivalent). e Chromatin-associated Dmc1 foci are present in elongated prophase nuclei in wild-type mating cells (distinguished by mCherry-tagged histone—magenta), but foci numbers are greatly reduced in bime2Δ mating cells. (Foci in somatic nuclei represent Rad51, which is also recognized by the anti-Dmc1 antibody). Scale bars: 10 μm
To investigate the cause of infertility, we analyzed chromosome pairing by Schaudinn fixation followed by Giemsa staining, which releases diakinesis-metaphase I chromosomes from cells. We found that bivalent formation was strongly reduced in bime2Δ compared to wild type. In the wild type, 0.6% of chromosome pairs formed univalents, 2.6% formed rod bivalents (in which one chromosome arm is connected), and 96.8% formed ring bivalents (in which both arms are connected). However, in bime2Δ meiosis, we found that 76.2% of chromosome pairs formed univalents, 12.2% formed rod bivalents, and only 11.6% formed ring bivalents (Fig. 2b, c). If we assume that ring bivalents have at least two COs (one in each arm) and rod bivalents have one, the number of COs in bime2Δ is estimated at 18% of the wild-type number. This strong reduction in COs is reminiscent of dmc1Δ meiosis (Fig. 2b and Howard-Till et al. 2011).
After DSB resection, the association of Dmc1 with single-stranded 3′ overhangs at DSB ends enables homology searching. More than 150 Dmc1 foci are visible in elongated nuclei in wild-type strains (Howard-Till et al. 2011; Lukaszewicz et al. 2015). In contrast, in the absence of Bime2, 80% (n = 250) of elongated nuclei completely lacked a Dmc1 signal, while the rest showed a faint or diffuse Dmc1 signal (Fig. 2e). To enable a direct side-by-side comparison, bime2Δ and wild-type mating cells were mixed just before fixation and then stained together on the same slide. To allow their discrimination, cells expressing mCherry-tagged histone were used as wild type (Fig. 2e). Together, the reduced chromatin localization of Dmc1 in the absence of Bime2 and the similar degree of reduction in bivalent formation in bime2 and dmc1 mutants suggest that Bime2 and Dmc1 cooperate in homologous CO formation.
Bime2 localizes to meiotic nuclei in a DSB-dependent manner
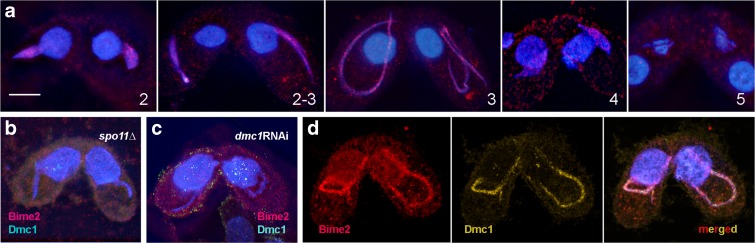
The subcellular localization of HA-tagged Bime2 was determined. Mating the Bime2-HA strain to a bime2Δ strain rescued the mutant phenotype (~ 85% ring bivalents were observed instead of ~ 10% in bime2Δ; n = 200 cells). Thus, the tagged protein was considered functional. Bime2 could only be seen during meiotic prophase: Bime2-HA foci first became visible in slightly elongated nuclei and disappeared by metaphase I (Fig. 3a). As these foci were seen in detergent-treated preparations in which non-chromatin-bound proteins are removed (Howard-Till et al. 2011), we conclude that they represent chromatin-associated Bime2. Bime2 localization was dependent on DSBs, since foci were absent in Bime2-HA × spo11RNAi mating cells (Fig. 3b). Next, dmc1RNAi cells were mated with Bime2-HA cells to test whether Bime2 localization is Dmc1 dependent. We found that Bime2 was always absent in mating pairs lacking Dmc1 (Fig. 3c). Because Bime2 localization is dependent on Dmc1 and Dmc1 localization is partially dependent on Bime2 (see above), it might be reasonable to assume that these two proteins colocalize in meiotic nuclei. However, double immunostaining of Bime2-HA and Dmc1 failed to show a complete overlap of Bime2 and Dmc1 foci (Figs. 3d and S2). It is possible that the two proteins occupy adjacent positions, but the large number of foci precluded a detailed analysis of spatial relationships.
Fig. 3.
Bime2 localization. a HA-tagged Bime2 (red) localizes to the chromatin of meiotic nuclei from the start of nuclear elongation to the end of prophase. It is undetectable in metaphase I. (Numbers refer to the stages in Fig. 1a.) b Similar to Dmc1, Bime2 localization is Spo11 dependent. c Bime2 foci are not formed in Dmc1-depleted cells. Preparations were co-stainined for Dmc1—the absence of Dmc1 in meiotic germline nuclei confirms the efficiency of RNAi-mediated Dmc1 knockdown. Foci seen in somatic nuclei represent antibody cross-reactivity with Rad51. For Dmc1 localization in wild-type cells, see Fig. 2e. d Co-staining of Bime2-HA and Dmc1. Although both proteins form foci on the chromatin of meiotic prophase nuclei, they do not colocalize. Chromatin was stained with DAPI (blue). The bloated appearance of nuclei is caused by detergent treatment to remove non-chromatin-bound proteins. Scale bar: 10 μm
To test for a possible interaction between Bime2 and Dmc1, Bime2-HA immunoprecipitation (IP) was performed and co-precipitating proteins were analyzed by Western blotting. However, Dmc1 was not detected (data not shown). Mass spectrometry showed that Bime2 was enriched in the Bime2-HA pulldown (log2 LFQ ratio Bime2-HA/Bime2-untagged = 12), but neither Dmc1 nor any other protein known to be involved in DSB repair or meiotic recombination was a significant hit (data not shown). Similarly, mass spectrometry analysis of a reciprocal Dmc1 IP did not identify Bime2 as a co-precipitating protein (Miao Tian, unpublished). We, therefore, conclude that chromatin localization of Dmc1 and Bime2 is mutually promoted but does not involve strong direct interaction.
Bime2 is distantly related to Rad54B and Rdh54/Tid1 proteins
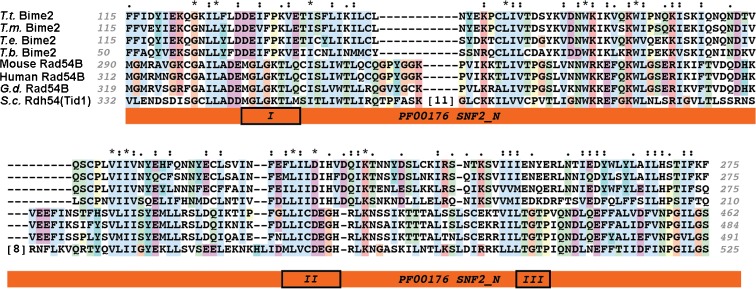
Tetrahymena Bime2 proteins were predicted to contain a PF00176.22/SNF2_N domain (E = 2.2e-04, HHpred using Bime2 orthologs from four Tetrahymena species as input). Further sequence analysis revealed significant similarity between Bime2 and the PANTHER Rad54B/PTHR10799:SF918 subfamily in the Rad54-like subgroup of SNF2 helicase-related proteins (E = 3.7e-09. PANTHER db v11.1 hmm Score) (Mi et al. 2017) (Fig. 4). Bime2 was also a significant hit (E = 0.001) in a reciprocal search of the Tetrahymena proteome using the Rad54B/PTHR10799:SF918 profile. However, in the same search, more than 20 other Tetrahymena proteins had greater similarity to the profile; the top hit TTHERM_00237490p is annotated as Rad54 in the Tetrahymena genome database (http://ciliate.org/). The Bime2 ATPase domain shows divergence from canonical helicase motifs that are typically conserved in the SNF2 helicase-like family, suggesting that it might lack ATPase activity, and resulting in the weaker support in the reciprocal search. However, none of the closer family members in Tetrahymena are expected to have a meiosis-specific function in interhomolog recombination because all are ubiquitously expressed (http://tfgd.ihb.ac.cn/).
Fig. 4.
Multiple partial sequence alignment showing the region of the highest sequence similarity between Tetrahymena thermophila (T.t.) Bime2 proteins and representatives of the Rad54B family: mouse, human and chicken (Gallus domesticus (G.d.)) Rad54B and budding yeast (Saccharomyces cerevisiae (S.c.)) Rdh54/Tid1. The aligned Bime2 sequence segment was selected to include the region with significant similarity to the PANTHER family Rad54B/PTHR10799:SF918 (HMMscore versus PANTHER v11.1—E = 3.7e-09) and Pfam family PF00176/SNF2_N. Characteristic helicase sequence motifs, Motifs I (Walker A), II (DExx), and III, reported to form the primary ATP binding site in the active site cleft (Dürr et al. 2005) are marked, but appear not to be functionally conserved in Bime2. Bime2 has clear homologs only in other Tetrahymena species (T. malaccensis (T.m.), T. elliotti (T.e.), and T. borealis (T.b.)). The alignment was generated using MUSCLE v3.8.31 (Edgar 2004) and visualized using Clustalx v2.1 (Thompson et al. 1997)
Rad54 has important functions in mitotic and meiotic DSB repair (Nimonkar et al. 2012; Arbel et al. 1999; Shinohara et al. 2000; Schmuckli-Maurer and Heyer 2000): It is involved in ATP-dependent chromatin remodeling during homology searching and D-loop formation (Petukhova et al. 1998; Jaskelioff et al. 2003; Solinger et al. 2001), heteroduplex extension (Bugreev et al. 2006), and Rad51 turnover or removal from dsDNA (Solinger et al. 2002; Agarwal et al. 2011). However, Rad54 also has ATP-independent functions, such as the stabilization of Rad51 nucleofilaments on ssDNA during DNA repair (Mazin et al. 2003; Agarwal et al. 2011). The budding yeast and mammalian Rad54 paralogs, Rdh54/Tid1, and Rad54B, respectively, support both Rad51 and Dmc1 nucleofilament formation in mitosis and meiosis, but in meiosis, they preferentially promote Dmc1-mediated interhomolog recombination (Brown and Bishop 2015; Sarai et al. 2006; Shinohara et al. 1997). Bime2 shows considerable sequence divergence from other Rad54 family members with a functional ATPase domain, which makes it difficult to prove orthology of Bime2 to Rad54 or Rdh54/Tid1. Nevertheless, its meiosis-specific expression and pro-CO activity, along with the mutual promotion of chromatin localization by Bime2 and Dmc1, suggest that these two proteins cooperate to promote interhomolog vs. intersister COs.
Electronic supplementary material
(PDF 146 kb).
.
(PDF 174 kb).
.
Acknowledgments
Open access funding provided by the Austrian Science Fund (FWF). Wild-type Tetrahymena thermophila strains were obtained from the Tetrahymena Stock Center at Cornell University. We are grateful to Miao Tian (MFPL Vienna) for sharing his Dmc1-co-IP results and to Kensuke Kataoka (Natl Inst. Basic Biol., Okazaki, JP) for providing us with a Tetrahymena strain expressing mCherry-tagged histone H3.
Abbreviations
- DSB
Double-strand break
- CO
Crossover
- SC
Synaptonemal complex
- FISH
Fluorescence in situ hybridization
- co-IP
Co-immunoprecipitation
Compliance with Ethical Standards
Funding information
This study was supported by grants W1238-B20 and P27313-B20 from the Austrian Science Fund (FWF).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10577-017-9563-y) contains supplementary material, which is available to authorized users.
References
- Agarwal S, van Cappellen WA, Guénolé A, Eppink B, Linsen SEV, Meijering E, Houtsmuller A, Kanaar R, Essers J. ATP-dependent and independent functions of Rad54 in genome maintenance. J Cell Biol. 2011;192:735–750. doi: 10.1083/jcb.201011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbel A, Zenvirth D, Simchen G. Sister chromatid-based DNA repair is mediated by RAD54, not by DMC1 or TID1. EMBO J. 1999;18:2648–2658. doi: 10.1093/emboj/18.9.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Bishop DK. DNA strand exchange and RecA homologs in meiosis. CSH Perspect Biol. 2015;7:a016659. doi: 10.1101/cshperspect.a016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns PJ, Brussard TEB. Nullisomic Tetrahymena: eliminating germinal chromosomes. Science. 1981;213:549–551. doi: 10.1126/science.213.4507.549. [DOI] [PubMed] [Google Scholar]
- Bugreev DV, Mazina OM, Mazin AV. Rad54 protein promotes branch migration of Holliday junctions. Nature. 2006;442:590–593. doi: 10.1038/nature04889. [DOI] [PubMed] [Google Scholar]
- Cassidy-Hanley D, Bowen J, Lee JH, Cole E, VerPlank LA, Gaertig J, Gorovsky MA, Bruns PJ. Germline and somatic transformation of mating Tetrahymena thermophila by particle bombardment. Genetics. 1997;146:135–147. doi: 10.1093/genetics/146.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JR, Taylor MRG, Boulton SJ. Playing the end game: DNA double-strand break repair pathway choice. Mol Cell. 2012;47:497–510. doi: 10.1016/j.molcel.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Chi J, Mahé F, Loidl J, Logsdon J, Dunthorn M. Meiosis gene inventory of four ciliates reveals the prevalence of a synaptonemal complex-independent crossover pathway. Mol Biol Evol. 2014;31:660–672. doi: 10.1093/molbev/mst258. [DOI] [PubMed] [Google Scholar]
- Chuang C-N, Cheng Y-H, Wang T-F. Mek1 stabilizes Hop1-Thr318 phosphorylation to promote interhomolog recombination and checkpoint responses during yeast meiosis. Nucleic Acids Res. 2012;40:11416–11427. doi: 10.1093/nar/gks920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Santos T, Hunter N, Lee C, Larkin B, Loidl J, Hollingsworth NM. The Mus81/Mms4 endonuclease acts independently of double-Holliday junction resolution to promote a distinct subset of crossovers during meiosis in budding yeast. Genetics. 2003;164:81–94. doi: 10.1093/genetics/164.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr H, Körner C, Müller M, Hickmann V, Hopfner KP. X-ray structures of the Sulfolobus solfataricus SWI2/SNF2 ATPase core and its complex with DNA. Cell. 2005;121:363–373. doi: 10.1016/j.cell.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb T, Lichten M. Frequent and efficient use of the sister chromatid for DNA double-strand break repair during budding yeast meiosis. PLoS Biol. 2010;8:e1000520. doi: 10.1371/journal.pbio.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth NM, Ponte L, Halsey C. MSH5, a novel MutS homolog, facilitates meiotic reciprocal recombination between homologs in Saccharomyces cerevisiae but not mismatch repair. Genes Dev. 1995;9:1728–1739. doi: 10.1101/gad.9.14.1728. [DOI] [PubMed] [Google Scholar]
- Howard-Till RA, Lukaszewicz A, Loidl J. The recombinases Rad51 and Dmc1 play distinct roles in DNA break repair and recombination partner choice in the meiosis of Tetrahymena. PLoS Genet. 2011;7:e1001359. doi: 10.1371/journal.pgen.1001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskelioff M, Van Komen S, Krebs JE, Sung P, Peterson CL. Rad54p is a chromatin remodeling enzyme required for heteroduplex DNA joint formation with chromatin. J Biol Chem. 2003;278:9212–9218. doi: 10.1074/jbc.M211545200. [DOI] [PubMed] [Google Scholar]
- Lee JY, Terakawa T, Qi Z, Steinfeld JB, Redding S, Kwon Y, Gaines WA, Zhao W, Sung P, Greene EC. Base triplet stepping by the Rad51/RecA family of recombinases. Science. 2015;349:977–981. doi: 10.1126/science.aab2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl J. Conservation and variability of meiosis across the eukaryotes. Annu Rev Genet. 2016;50:293–316. doi: 10.1146/annurev-genet-120215-035100. [DOI] [PubMed] [Google Scholar]
- Loidl J, Lorenz A. DNA double-strand break formation and repair in Tetrahymena meiosis. Sem Cell Dev Biol. 2016;54:126–134. doi: 10.1016/j.semcdb.2016.02.021. [DOI] [PubMed] [Google Scholar]
- Loidl J, Lukaszewicz A, Howard-Till RA, Koestler T. The Tetrahymena meiotic chromosome bouquet is organized by centromeres and promotes interhomolog recombination. J Cell Sci. 2012;125:5873–5880. doi: 10.1242/jcs.112664. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A, Howard-Till RA, Loidl J. Mus81 nuclease and Sgs1 helicase are essential for meiotic recombination in a protist lacking a synaptonemal complex. Nucleic Acids Res. 2013;41:9296–9309. doi: 10.1093/nar/gkt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A, Howard-Till RA, Novatchkova M, Mochizuki K, Loidl J. MRE11 and COM1/SAE2 are required for double-strand break repair and efficient chromosome pairing during meiosis of the protist Tetrahymena. Chromosoma. 2010;119:505–518. doi: 10.1007/s00412-010-0274-9. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A, Shodhan A, Loidl J. Exo1 and Mre11 execute meiotic DSB end resection in the protist Tetrahymena. DNA Repair. 2015;35:137–143. doi: 10.1016/j.dnarep.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Mazin AV, Alexeev AA, Kowalczykowski SC. A novel function of Rad54 protein—stabilization of the Rad51 nucleoprotein filament. J Biol Chem. 2003;278:14029–14036. doi: 10.1074/jbc.M212779200. [DOI] [PubMed] [Google Scholar]
- McDonald BB. The exchange of RNA and protein during conjugation in Tetrahymena. J Protozool. 1966;13:277–285. doi: 10.1111/j.1550-7408.1966.tb01908.x. [DOI] [PubMed] [Google Scholar]
- Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017;45D:D183–D189. doi: 10.1093/nar/gkw1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki K. High efficiency transformation of Tetrahymena using a codon-optimized neomycin resistance gene. Gene. 2008;425:79–83. doi: 10.1016/j.gene.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Mochizuki K, Novatchkova M, Loidl J. DNA double-strand breaks, but not crossovers, are required for the reorganization of meiotic nuclei in Tetrahymena. J Cell Sci. 2008;121:2148–2158. doi: 10.1242/jcs.031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimonkar AV, Dombrowski CC, Siino JS, Stasiak AZ, Stasiak A, Kowalczykowski SC. Saccharomyces cerevisiae Dmc1 and Rad51 proteins preferentially function with Tid1 and Rad54 proteins, respectively, to promote DNA strand invasion during genetic recombination. J Biol Chem. 2012;287:28727–28737. doi: 10.1074/jbc.M112.373290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Wan L, Busygina V, Kwon Y, Allen JA, Li X, Kunz RC, Kubota K, Wang B, Sung P, Shokat KM, Gygi SP, Hollingsworth NM. Regulation of meiotic recombination via Mek1-mediated Rad54 phosphorylation. Mol Cell. 2009;36:393–404. doi: 10.1016/j.molcel.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias E, Hamilton EP, Orias JD. Tetrahymena as a laboratory organism: useful strains, cell culture, and cell line maintenance. In: Asai DJ, Forney JD, editors. Tetrahymena thermophila. San Diego: Academic Press; 2000. pp. 189–211. [DOI] [PubMed] [Google Scholar]
- Petukhova G, Stratton S, Sung P. Catalysis of homologous DNA pairing by yeast Rad51 and Rad54 proteins. Nature. 1998;393:91–94. doi: 10.1038/30037. [DOI] [PubMed] [Google Scholar]
- Ruehle MD, Orias E, Pearson CG. Tetrahymena as a unicellular model eukaryote: genetic and genomic tools. Genetics. 2016;203:649–665. doi: 10.1534/genetics.114.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarai N, Kagawa W, Kinebuchi T, Kagawa A, Tanaka K, Miyagawa K, Ikawa S, Shibata T, Kurumizaka H, Yokoyama S. Stimulation of Dmc1-mediated DNA strand exchange by the human Rad54B protein. Nucleic Acids Res. 2006;34:4429–4437. doi: 10.1093/nar/gkl562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuckli-Maurer J, Heyer WD. Meiotic recombination in RAD54 mutants of Saccharomyces cerevisiae. Chromosoma. 2000;109:86–93. doi: 10.1007/s004120050415. [DOI] [PubMed] [Google Scholar]
- Schwacha A, Kleckner N. Interhomolog bias during meiotic recombination: meiotic functions promote a highly differentiated interhomolog-only pathway. Cell. 1997;90:1123–1135. doi: 10.1016/S0092-8674(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Gasior SL, Bishop DK, Shinohara A. Tid1/Rdh54 promotes colocalization of Rad51 and Dmc1 during meiotic recombination. Proc Natl Acad Sci U S A. 2000;97:10814–10819. doi: 10.1073/pnas.97.20.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Shita-Yamaguchi E, Buerstedde J-M, Shinagawa H, Ogawa H, Shinohara A. Characterization of the roles of the Saccharomyces cerevisiae RAD54 gene and a homologue of RAD54, RDH54/TID1, in mitosis and meiosis. Genetics. 1997;147:1545–1556. doi: 10.1093/genetics/147.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shodhan A, Kataoka K, Mochizuki K, Novatchkova M, Loidl J. A Zip3-like protein plays a role in crossover formation in the SC-less meiosis of the protist Tetrahymena. Mol Biol Cell. 2017;28:825–833. doi: 10.1091/mbc.E16-09-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shodhan A, Lukaszewicz A, Novatchkova M, Loidl J. Msh4 and Msh5 function in SC-independent chiasma formation during the streamlined meiosis of Tetrahymena. Genetics. 2014;198:983–993. doi: 10.1534/genetics.114.169698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinger JA, Kiianitsa K, Heyer W-D. Rad54, a Swi2/Snf2-like recombinational repair protein, disassembles Rad51:dsDNA filaments. Mol Cell. 2002;10:1175–1188. doi: 10.1016/S1097-2765(02)00743-8. [DOI] [PubMed] [Google Scholar]
- Solinger JA, Lutz G, Sugiyama T, Kowalczykowski SC, Heyer W-D. Rad54 protein stimulates heteroduplex DNA formation in the synaptic phase of DNA strand exchange via specific interactions with the presynaptic Rad51 nucleoprotein filament. J Mol Biol. 2001;307:1207–1221. doi: 10.1006/jmbi.2001.4555. [DOI] [PubMed] [Google Scholar]
- Thompson DA, Stahl FW. Genetic control of recombination partner preference in yeast meiosis: isolation and characterization of mutants elevated for meiotic unequal sister-chromatid recombination. Genetics. 1999;153:621–641. doi: 10.1093/genetics/153.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Lu X, Zhou Z, Chang Y, Yuan D, Tian M, Zhou Z, Wang L, Fu C, Orias E, Miao W. Transcriptome analysis of the model protozoan, Tetrahymena thermophila, using deep RNA sequencing. PLoS One. 2012;7:e30630. doi: 10.1371/journal.pone.0030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang R, Ghanam AR, Yan G, Miao W, Song X. The key role of CYC2 during meiosis in Tetrahymena thermophila. Protein Cell. 2016;7:236–249. doi: 10.1007/s13238-016-0254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 146 kb).
(PDF 174 kb).