Abstract
In this study we investigate pyridoxamine (PM) and/or sulforaphane (SFN) as therapeutic interventions to determine whether activators of NFE2-related factor 2 (Nrf2) can be used in addition with inhibitors of advanced glycation end products (AGE) formation to attenuate oxidative stress and improve endothelial dysfunction in type 2 diabetes. Goto-kakizaki (GK) rats, an animal model of non-obese type 2 diabetes, were treated with or without PM and/or SFN during 8 weeks and compared with age-matched Wistar rats. At the end of the treatment, nitric oxide (NO)-dependent and independent vasorelaxation in isolated aorta and mesenteric arteries were evaluated. Metabolic profile, NO bioavailability and vascular oxidative stress, AGE and Nrf2 levels were also assessed. Diabetic GK rats presented significantly lower levels of Nrf2 and concomitantly exhibited higher levels of oxidative stress and endothelial dysfunction. PM and SFN as monotherapy were capable of significantly improving endothelial dysfunction in aorta and mesenteric arteries decreasing vascular oxidative damage, AGE and HbA1c levels. Furthermore, SFN + PM proved more effective reducing systemic free fatty acids levels, normalizing endothelial function, NO bioavailability and glycation in GK rats. Activators of Nrf2 can be used therapeutically in association with inhibitors of AGE and cross-linking formation to normalize endothelial dysfunction in type 2 diabetes.
Introduction
Type 2 diabetes is associated with elevated levels of oxidative stress and glycation altering vascular function and is therefore considered a major risk factor underlying development of cardiovascular disease1,2. Many of the complications in diabetes are related to hyperglycaemia and increased generation of reactive oxygen species (ROS), which lead to endothelium dysfunction. Impaired endothelium-dependent relaxation to vasodilators such as acetylcholine is a common feature in both conduit and resistance arteries from experimental models of type 1 and type 2 diabetes1,3–5. We have previously shown that diabetic GK rats have increased oxidative stress and glycation, which leads to endothelial dysfunction5,6.
Progress in the treatment of diabetes has not been effective in decreasing vascular complications associated with the disease. Given the crucial role of ROS in endothelial function, considerable efforts have been made to discover therapies to reduce ROS in the vasculature.
Nuclear factor E2(erythroid-derived 2)-Related Factor-2 (Nrf2) is a transcription factor that plays a crucial role in the cellular protection against oxidative stress. Nrf2 is referred to as the “master regulator” of the antioxidant response7,8. Induction of endogenous antioxidant enzymes by activators of the Nrf2/antioxidant response element pathway may be an interesting approach to obtain sufficient levels of antioxidants and reduce oxidative stress. Dietary Nrf2 activators, such as the isothiocyanate sulforaphane (SFN) found in cruciferous vegetables, can increase antioxidant defenses, reduce blood pressure, inhibit pro-inflammatory signalling pathways in the kidney9,10 and prevent metabolic dysfunction in endothelial cells induced by hyperglycaemia11. In diabetic models, activation of Nrf2 lowers plasma glucose levels and reduces diabetes-related nephropathy in wild type but not in Nrf2 deficient mice12. Thus, the therapeutic potential of Nrf2 inducers in vascular disease is enormous in particular associated with long-term type 2 diabetes8,13–15.
Additionally, advanced glycation is accelerated under diabetic conditions leading to vascular complications. Several authors have proved that inhibition of advanced glycation end products (AGE) formation may be a therapeutic strategy to improve vascular complications in diabetes. Pyridoxamine (PM), a naturally occurring derivative of vitamin B6, has proved to be an effective inhibitor for protein glycation and lipoxidation16. PM has been found to inhibit the formation of AGE both in vitro and in vivo with potential benefits for the treatment of diabetic nephropathy and retinopathy17.
Sulforaphane and pyridoxamine have distinct mechanisms of action. Therefore, we hypothesized that either SFN, PM alone or in association could have beneficial effects on endothelium-dependent vascular reactivity, oxidative stress and metabolic parameters in Goto–Kakizaki (GK) rats, a model of type 2 diabetes. In parallel with the development of diabetes, lipid profile, oxidative stress, glycation and nitric oxide (NO) bioavailability were evaluated. Additionally, endothelial dependent and independent vascular sensitivity to acetylcholine (ACh) and sodium nitroprusside (SNP) were assessed in aorta and mesenteric arteries. Eight months old GK rats showed a long-term diabetic phenotype with endothelial dysfunction both at conduit and resistant arteries accompanied with increased oxidative stress when compared to Wistar rats. SFN and PM alone were capable of ameliorating NO-dependent vasorelaxation in isolated arteries. In association, SFN and PM normalized endothelial function both in aorta and mesenteric arteries through a mechanism that involves an increment in NO bioavailability and a decrement in oxidative stress and AGE levels. Altogether, these studies indicate that targeting different mechanisms underlying endothelial dysfunction such as inhibiting AGE formation (with PM) and promoting an increment in antioxidant defense systems (with SFN) could be considered as a useful tool for diabetic macrovascular complications due the simultaneous action on different mechanisms that ultimately lead to vascular damage.
Results
Animal characteristics
The diabetic rats used in our experiments exhibited similar fasting plasma glucose levels at the beginning of the study. GK glycaemia evaluated before treatment (GK6m) was significantly higher when compared to age-matched non-diabetic Wistar rats (Fig. 1 supplement). Body weight was significantly lower in GK rats compared to age-matched Wistar rats and the different therapies did not significantly change this parameter (Table 1). In an intraperitoneal glucose tolerance test (IPGTT), GK exhibited marked glucose intolerance as compared with Wistar rats (Fig. 1A). Fasting glycaemia, the glucose area under the curve (AUC), HbA1c, total cholesterol and FFA were elevated in GK rats when compared to Wistar rats (Fig. 1B,C,D, Table 1). As previously6, GK rats exhibited normal non-HDL-cholesterol and triglycerides (Table 1). Treatment with SFN or PM alone did not significantly change glucose intolerance and the lipid profile evaluated (Fig. 1A,C, Table 1). Moreover PM significantly decreased fasting glucose and HbA1c levels (Fig. 1B,D). Indeed, all the therapeutic approaches were able to effectively reduce HbA1c levels in GK rats (Fig. 1D). Additionally, treatment with both SFN and PM for 8 weeks effectively reduced circulating concentrations of FFA in diabetic rats (Table 1) and also significantly ameliorated glucose intolerance (Fig. 1A,C).
Table 1.
Body weight and lipid levels in 8 months old non-diabetic Wistar (W) and diabetic Goto-Kakizaki (GK) rats and in GK rats treated with sulforaphane (GKS), pyridoxamine (GKP) or sulforaphane and pyridoxamine (GKSP).
| W | GK | GKS | GKP | GKSP | |
|---|---|---|---|---|---|
| Body weight (g) | 478.5 ± 15.2 | 390 ± 5.7*** | 374.6 ± 5.3*** | 401.8 ± 6.6*** | 375 ± 9.3*** |
| Total Cholesterol (mM) | 2.07 ± 0.07 | 2.75 ± 0.07** | 2.74 ± 0.14 | 2.53 ± 0.16*** | 2.71 ± 0.14 ** |
| Non-HDL cholesterol (mM) | 0.87 ± 0.04 | 1.1 ± 0.06 | 1.02 ± 0.09 | 0.086 ± 0.08 | 1.14 ± 0.08 |
| Triglycerides (mM) | 0.94 ± 0.07 | 1.18 ± 0.1 | 1.04 ± 1.0 | 1.29 ± 0.07 | 1.11 ± 0.06 |
| FFA (mM) | 0.58 ± 0.02 | 1.21 ± 0.08*** | 0.97 ± 0.06 ** | 0.99 ± 0.13* | 0.73 ± 0.06ϕϕϕ,§,# |
Data are expressed as mean±SE (n = 12 animals in each group).
*P < 0.05, **P < 0.01, ***P < 0.001 vs W rats.
ϕϕϕP < 0.001 vs GK rats.
§P < 0.05 vs GKS rats.
#P < 0.05 vs GKP.
Figure 1.
Effects of sulforaphane and pyridoxamine treatment on intraperitoneal glucose tolerance test (IPGTT; A), fasting glycaemia (B), the glucose area under the curve (AUC; C) and HbA1c levels (D) in 8 months old diabetic Goto-Kakizaki (GK) rats compared with nondiabetic Wistar (W) rats. C) The AUC of IPGTT curves was calculated to measure the degree of the glucose tolerance impairment. Data are expressed as mean ± SE (n = 12). ***P < 0.001 vs Wistar group; ϕP < 0.05, ϕϕP < 0.01, ϕϕϕP < 0.001 vs GK group.
NO dependent vascular relaxation
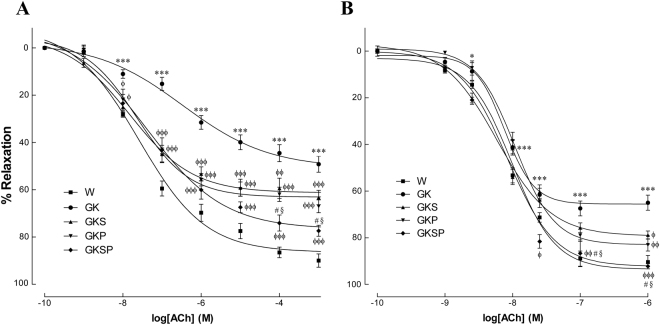
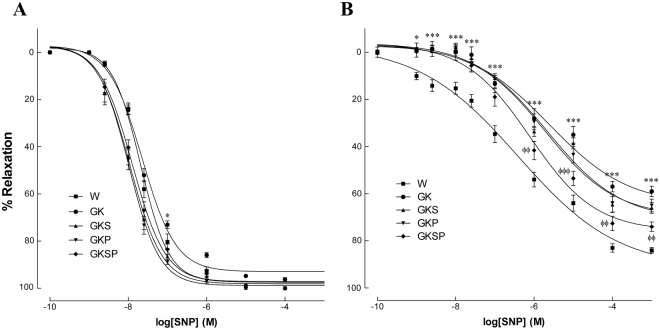
In 8 months old GK rats endothelium-mediated vascular relaxation of phenylephrine-precontracted aorta arterial rings in response to ACh was impaired compared with age-matched Wistar rats (Fig. 2A), but the endothelium-independent relaxations to sodium nitroprusside (SNP) were similar in both strains (Fig. 3A). Indeed, maximal endothelium-mediated relaxation of phenylephrine-precontracted aorta rings in response to ACh declined by 49% in diabetic rats (Fig. 2A). Preincubation of the arterial rings with the NOS inhibitor N-nitro-L-arginine- methyl ester (L-NAME) and the cyclooxygenase inhibitor indomethacin almost completely abolished relaxation by ACh in GK rats and Wistar rats (Fig. 2 supplement). The residual component due to other vasodilators is around 15% in our experimental conditions. In aorta, no differences on maximal relaxation were observed in the concentration-effect curves for SNP between the different groups (Fig. 3A). Maximal relaxation significantly improved in all GK treated groups with an improvement in vascular reactivity in response to ACh (Fig. 2A, Table 2). Treatment with SFN, PM or SFN + PM significantly improved endothelium-dependent vascular relaxation in aorta of diabetic rats (Fig. 2A, Table 2) This vasodilation is not sensitive to indomethacin and is blocked by L-NAME indicating that the mechanism is dependent on endogenous NO release (Fig. 2 supplement). Furthermore, an increment in SNP sensitivity was observed in all GK treated groups (Fig. 3A, Table 2). In mesenteric arteries, maximal endothelium-mediated relaxation of phenylephrine-precontracted rings in response to ACh declined by 36% in diabetic rats (Fig. 2B). Additionally, maximal relaxation in response to SNP declined by 39% in diabetic rats (Fig. 3B). Treatment with SFN, PM or SFN + PM was able to improve endothelial-dependent vasodilation (Fig. 2B, Table 3). Similarly, this effect is not sensitive to indomethacin and is inhibited by L-NAME (Fig. 2 supplement). Detailed data on maximal relaxations and EC50 values are summarized in Tables 2 and 3. These results indicated that treatment with SFN or PM alone improved endothelium-dependent vascular relaxation in both aorta and mesenteric arteries of diabetic GK rats. In association these therapies were able to normalize endothelium-mediated relaxation and improve SNP sensitivity in aorta and mesenteric arteries (Figs 2, 3).
Figure 2.
Effects of sulforaphane and pyridoxamine treatment on vasodilatory responses to acetylcholine in aorta (A) and mesenteric arteries (B) of GK rats compared with nondiabetic Wistar (W) rats. Data are expressed as mean ± SE (n = 12). *P < 0.05, ***P < 0.001 vs Wistar group; ϕP < 0.05, ϕϕP < 0.01, ϕϕϕP < 0.001 vs GK group; §P < 0.05 vs GKS group; #P < 0.05 vs GKP group.
Figure 3.
Effects of sulforaphane and pyridoxamine treatment on vasodilatory responses to sodium nitroprusside in aorta (A) and mesenteric arteries (B) of GK rats compared with nondiabetic Wistar (W) rats. Data are expressed as mean ± SE (n = 12). *P < 0.05, ****P < 0.001 vs Wistar group; ϕϕP < 0.01, ϕϕϕP < 0.001 vs GK group.
Table 2.
Maximal relaxation responses (%) and −logEC50 in isolated aorta arteries of 8 month-old variously treated spontaneously diabetic Goto-Kakizaki (GK) rats and age-matched non-diabetic Wistar rats.
| W | GK | GKS | GKP | GKSP | |
|---|---|---|---|---|---|
| ACh | |||||
| pEC50 | 7.59 ± 0.1 | 6.47 ± 0.18*** | 7.8 ± 0.24ϕϕϕ | 7.64 ± 0.13ϕϕϕ | 7.4 ± 0.18ϕϕ |
| Maximal relaxation (%) | 86.5 ± 3.8 | 50.7 ± 3.7*** | 63 ± 3.6***,ϕϕϕ | 63.1 ± 2.7***,ϕϕϕ | 74.3 ± 3.1 **,ϕϕϕ,§,# |
| SNP | |||||
| pEC50 | 7.7 ± 0.03 | 7.6 ± 0.03 | 7.96 ± 0.05 ***,ϕϕϕ | 7.98 ± 0.04*,ϕϕϕ | 7.9 ± 0.04*,ϕϕϕ |
| Maximal relaxation (%) | 97.1 ± 1.2 | 92.9 ± 1.4 | 98.1 ± 1.6 | 98.8 ± 1.4 | 97.5 ± 2.2 |
Data are expressed as mean±SE (n = 12 animals in each group). pEC50 values are presented as the negative logarithm (−logEC50) of concentration of the agonist.
*P < 0.05, **P < 0.01, ***P < 0.001 vs W rats.
ϕϕP < 0.01, ϕϕϕP < 0.001 vs GK rats.
§P < 0.05 vs GKS.
#P < 0.05 vs GKP.
Table 3.
Maximal relaxation responses (%) and −logEC50 in isolated mesenteric arteries of 8 month-old variously treated spontaneously diabetic Goto-Kakizaki (GK) rats and matched non-diabetic Wistar rats.
| W | GK | GKS | GKP | GKSP | |
|---|---|---|---|---|---|
| ACh | |||||
| pEC50 | 8.1 ± 0.04 | 8.1 ± 0.05 | 8.2 ± 0.04 | 7.9 ± 0.04 | 8.0 ± 0.04 |
| Maximal relaxation (%) | 92.3 ± 2.5 | 65.6 ± 2.5*** | 79.2 ± 2.4 ***,ϕ | 83 ± 2.0***,ϕϕ | 93.5 ± 2.4 ***,ϕϕϕ,§,# |
| SNP | |||||
| pEC50 | 6.4 ± 0.1 | 5.6 ± 0.2*** | 5.66 ± 0.14*** | 5.7 ± 0.14*** | 6.1 ± 0.07*** |
| Maximal relaxation (%) | 84 ± 1.2 | 60.8 ± 2.2 | 66 ± 2.3 | 65.3 ± 2.7 | 74 ± 2 |
Data are expressed as mean±SE (n = 12 animals in each group). pEC50 values are presented as the negative logarithm (−logEC50) of concentration of the agonist.
***P < 0.001 vs W rats.
ϕP < 0.05, ϕϕP < 0.01, ϕϕϕP < 0.01 vs GK rats.
§P < 0.05 vs GKS.
#P < 0.05 vs GKP.
Oxidative stress in the vascular wall
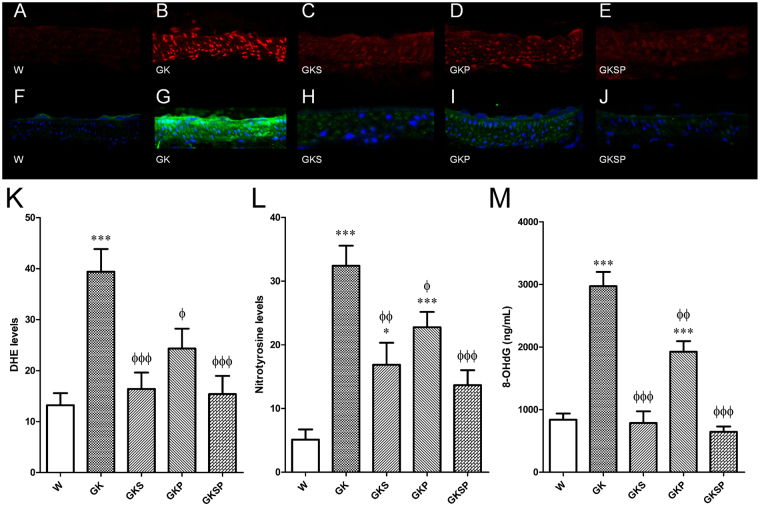
We determined the potential impact of SFN, PM or both on the oxidative stress in the diabetic vasculature. Interestingly, diabetes induced a 3-fold increase in superoxide production in diabetic aorta (p < 0.001; Fig. 4B,K). The density of dihydroethidium (DHE) was significantly decreased in the aorta of diabetic rats treated with SFN, PM and SFN + PM compared to GK rats (Fig. 4C,D,E,K). Additionally, diabetic GK rats also had increased immunoreactive nitrotyrosine levels in their mesenteric arteries (p < 0.001; Fig. 4G,L) and treatment with SFN, PM or both significantly decreased these levels (Fig. 4H,I,J,L). Thus, all therapies significantly reduced vascular oxidative damage in diabetic GK rats both in aorta and mesenteric arteries. Accordingly, urinary levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG) were significantly higher in GK rats, when compared to age-matched Wistar rats (Fig. 4M). Treatment with SFN, PM or both for 8 weeks to GK rats significantly reduced 8-OHdG levels (Fig. 4M).
Figure 4.
Effects of sulforaphane and pyridoxamine treatment on systemic and vascular oxidative stress in GK rats compared with nondiabetic Wistar (W) rats. Representative DHE-stained aorta artery sections reflect O2·– production with the different treatments (A–E). The endothelium is facing up in all layers. At identical settings, fluorescence in diabetic GK (B) was markedly increased compared with normal vessel (Wistar, A). Note the increased fluorescence reflecting O2·− levels in the endothelium, intima, and media of GK aorta. DHE fluorescence decreased in the diabetic GK rats treated with SFN (GKS, C), PM (GKP, D) and SFN + PM (GKSP, E). Panel (K) contains quantification of the fluorescence ethidium signal in the different groups of arteries. Representative mesenteric sections showing nitrotyrosine staining in nondiabetic Wistar (F), diabetic GK (G), and diabetic GK rats treated with SFN (GKS, H), PM (GKP, I) and SFN + PM (GKSP, J). Panel (L) contains quantification of the green fluorescence in the different groups of arteries. (M) Urinary 8-hydroxydeoxyguanosine (8-OHdG) levels in the different groups of rats. Data are expressed as mean ± SE (n = 12). *P < 0.05, ***P < 0.001 vs Wistar group; ϕP < 0.05, ϕϕP < 0.01, ϕϕϕP < 0.001 vs GK group.
pVASP and total VASP in aorta
The phosphorylation of vasodilator-stimulated phosphoprotein (VASP) at Ser239 has been shown to be an indicator of bioactive NO and the activity of the NO-cGMP-protein kinase G signalling pathway18. To establish NO bioavailability, we measured phosphorylated vasodilator-stimulated phosphoprotein (pVASP) and total VASP in the aorta. The ratio of pVASP/tVASP in GK rats was significantly decreased compared to Wistar rats in aorta (Fig. 5A,B). Treatment with SFN, PM or both significantly increased pVASP/tVASP ratio in diabetic GK rats (GKS, GKP; GKSP; Fig. 5A,B). A similar profile was observed in mesenteric arteries (data not shown).
Figure 5.
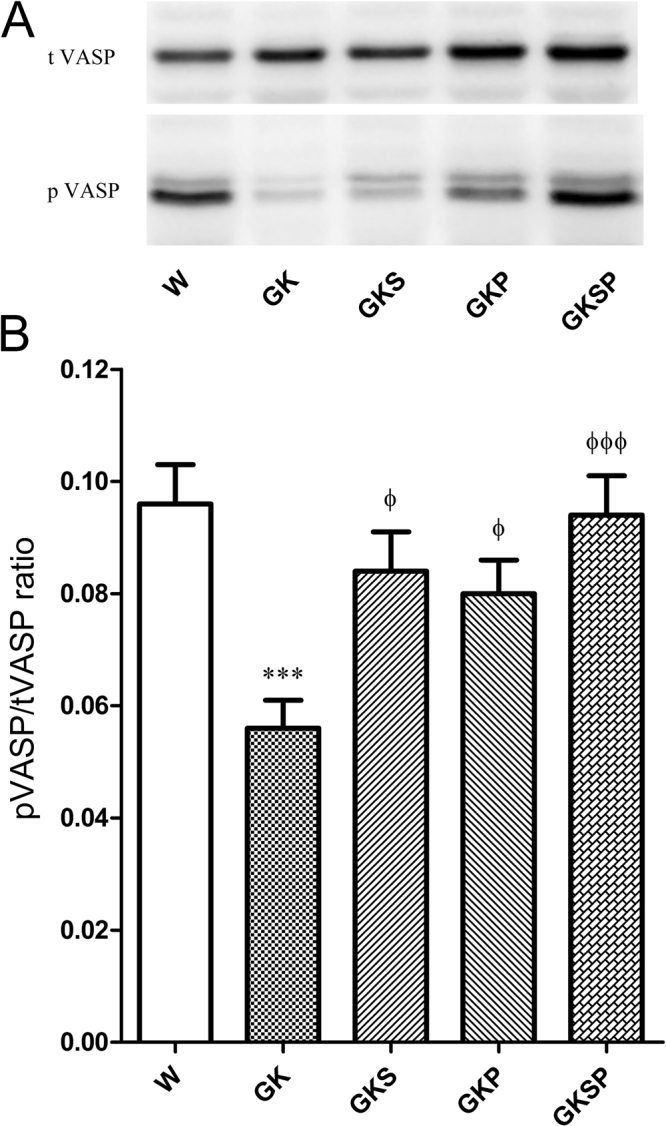
Effects of sulforaphane and pyridoxamine treatment on pVASP and tVASP expression levels. To examine NO-cGMP signal activation, total vasodilator-stimulated phosphoprotein (t VASP) and phosphorylated (Ser239) VASP (pVASP) expression were assessed in aorta. Aortic lysates were resolved by SDS-PAGE. (A) Representative cropped western blot of total VASP and pVASP protein levels in aortas of the different groups of arteries. (B) Average densitometric data for pVASP/total VASP ratio. Data are expressed as mean ± SE (n = 12). ***P < 0.001 vs Wistar group; ϕP < 0.05, ϕϕϕP < 0.001 vs GK group.
Nrf2 levels in aorta and mesenteric arteries
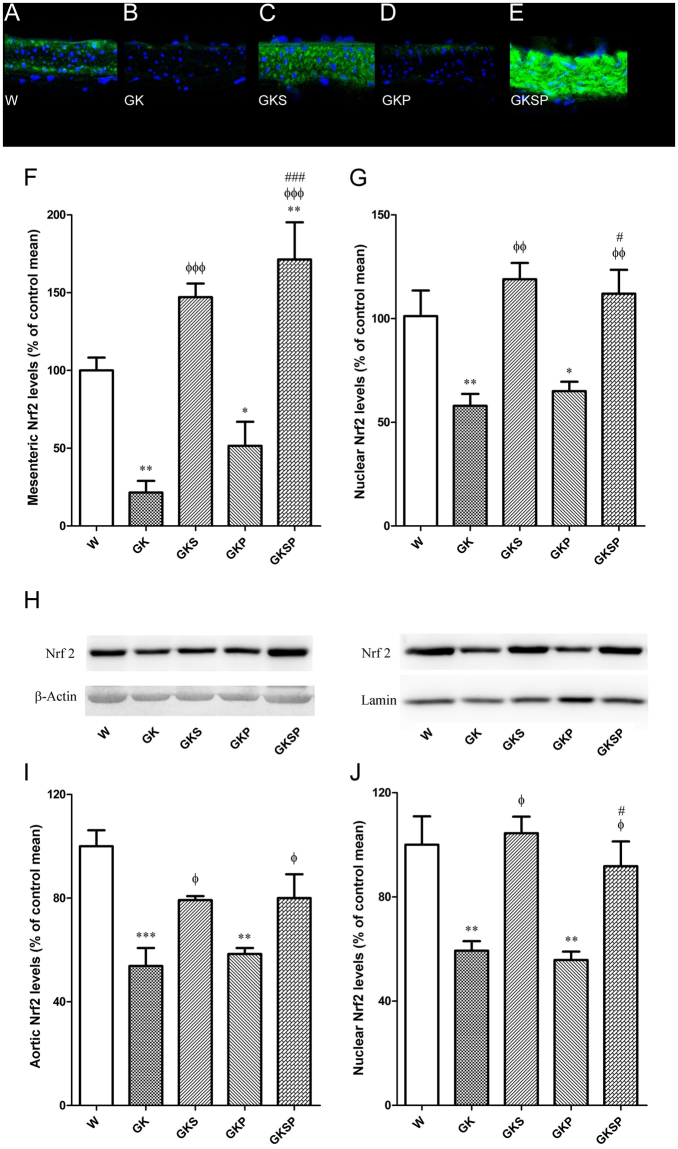
We next quantified Nrf2 levels in the arteries of the different groups of rats. Nrf2 levels were assessed by western blot in aorta and by immunofluorescence with confocal microscopy in mesenteric arteries. Nrf2 levels were significantly down-regulated in vessels from the diabetic rats compared with age-matched Wistar rats in both aorta and mesenteric arteries (Fig. 6I,F). Sulforaphane treated groups (GKS and GKSP) dramatically increased the nuclear Nrf2 levels in aorta and mesenteric arteries (Fig. 6J,G). The Nrf2-activator SFN increases nuclear localization of Nrf2 in diabetic GK rats which probably leads to an increment in antioxidant defense promoting a decrement in vascular oxidative stress. SFN produced a significant increase in Nrf2 expression in both aorta and mesenteric arteries of diabetic GK rats (Fig. 6), suggesting that it promotes both increased expression and nuclear translocation, consistent with its documented effects in other tissues.
Figure 6.
Effects of sulforaphane and pyridoxamine treatment on Nrf2 levels in aorta and mesenteric arteries of GK rats compared with nondiabetic Wistar rats. Representative mesenteric sections showing Nrf2 staining in nondiabetic Wistar (A), diabetic GK (B), and diabetic GK rats treated with SFN (GKS, C), PM (GKP, D) and SFN + PM (GKSP, E). Panel (F) contains quantification of the green fluorescence in the different groups of arteries. (G) The fluorescence intensity of Nrf2 label (green) localized to nuclear regions, as defined by DAPI staining (blue), was quantified and expressed as % of control mean of Wistar rats pooled from multiple mesenteric artery sections. (H) Cropped western blot of total Nrf2 (left panel) and nuclear Nrf2 protein levels in aortas of the different groups of arteries. Average densitometric data for total Nrf2 levels (I) and nuclear Nrf2 levels (J) in aortas normalized to the respective loading control. Data are mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 vs Wistar group; ϕP < 0.05, ϕϕP < 0.01, ϕϕϕP < 0.001 vs GK group; #P < 0.05, ###P < 0.001 vs GKP group.
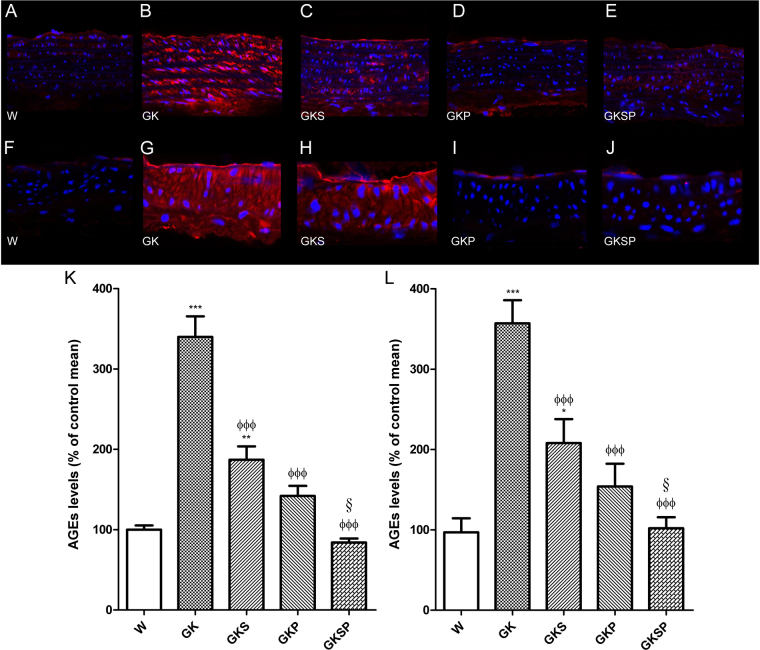
AGE levels in aorta and mesenteric arteries
We next quantified AGE levels in the arteries of the different groups of rats. AGE levels were assessed by immunofluorescence with confocal microscopy in aorta and mesenteric arteries. AGE levels were significantly increased in vessels from the diabetic rats compared with age-matched Wistar rats in both aorta and mesenteric arteries (Fig. 7B,K,G,L, respectively). GK treated groups (GKS, GKP and GKSP) significantly decreased the vascular AGE levels in aorta and mesenteric arteries (Fig. 7K,L). Noteworthy the groups treated with PM (GKP, GKSP) had normalized AGE levels in the vascular wall.
Figure 7.
Effects of sulforaphane and pyridoxamine treatment on AGE levels in aorta and mesenteric arteries of GK rats compared with nondiabetic Wistar rats. Representative aortic sections showing AGE staining in nondiabetic Wistar (A), diabetic GK (B), and diabetic GK rats treated with SFN (GKS, C), PM (GKP, D) and SFN + PM (GKSP, E). Panel (K) contains quantification of the red fluorescence in the different groups of arteries. Representative mesenteric sections showing AGE staining in nondiabetic Wistar (F), diabetic GK (G), and diabetic GK rats treated with SFN (GKS, H), PM (GKP, I) and SFN + PM (GKSP, J). Panel (L) contains quantification of the red fluorescence in the different groups of arteries. Data are mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001 vs Wistar group; ϕP < 0.05, ϕϕP < 0.01, ϕϕϕP < 0.001 vs GK group; §P < 0.05 vs GKS group.
Discussion
In the current study, the therapeutic potential of sulforaphane, pyridoxamine and its association is clearly demonstrated in diabetic endothelial dysfunction of GK rats. Treatment of GK rats with SFN, PM or both in association significantly reduced endothelial dysfunction in aorta and mesenteric arteries. In these vessels, SFN, PM or SFN + PM treatment retarded pathological characteristics of diabetic endothelial dysfunction including oxidative damage. Additionally, both therapies in association were able to reduce FFA levels and also increased NO bioavailability which can partially explain the normalization of endothelial function achieved in this long-term model of type 2 diabetes.
GK rats are an animal model of non-obese type 2 diabetes with mild hyperglycaemia, hyperinsulinaemia and insulin resistance5,6,19. Long-term diabetes is frequently associated with loss of pancreatic function in these animals, characteristics that also resemble features associated with human disease progression. This model shares many cardiovascular phenotypes with human type 2 diabetes including abnormal vascular reactivity5,6 justifying its use as a model to study diabetic complications.
Herein, we show that 8 months old GK rats are hyperglycaemic, with significantly increased total cholesterol and circulating levels of FFA. Endothelial dysfunction is evident in both mesenteric and aorta arteries accompanied by increased oxidative stress, glycation, decreased NO bioavailability and depletion of Nrf2 levels.
Nrf2 antioxidant functions may be important in vascular disease20–22. SFN, an activator of Nrf2 found in broccoli, increases the expression of protective enzymes under antioxidant response element-linked transcriptional control and prevents metabolic dysfunction in endothelial cells induced by hyperglycaemia11. The concentration of SFN used was determined based on other studies23,24 and on the bioavailability of this compound in broccoli sprouts. We estimate that SFN could have potential therapeutically benefits for the treatment of endothelial dysfunction at physiological doses feasible to be obtained in human diet25. On the other hand, we have recently examined the AGE levels in type 2 diabetes animal models and found that AGE levels are increased in plasma as well as in the vascular wall26. These high levels of AGE can be reduced with compounds such as PM and this approach has potential beneficial effects in endothelial function17. The therapeutic effect of both therapies at the macrovascular disease has not been previously studied. Herein, we study the effect of SFN with or without the concomitant inhibition of AGE formation with PM at the vascular level.
Treatment with SFN or PM alone for 8 weeks did not significantly improve the triglyceride, cholesterol and FFA levels. PM has been shown to correct hyperlipidaemia and nephropathy in both diabetic and obese rats, probably by interfering with various reactive carbonyl intermediates of AGE/advanced lipoxidation end products (ALE) formation from lipid oxidation27,28. This lipid-lowering effect was described for higher concentrations of PM (400 mg/Kg/day) in different animal models. Additionally, the Nrf2 pathway was identified as having regulatory functions in liver lipid metabolism. Nrf2 negatively regulates many genes encoding enzymes involved in lipid biosynthesis, fatty acid desaturation, and fatty acid transport29. In contrast, 0.5 mg/Kg of SFN impaired hepatic function and aggravated cholesterol levels in streptozotocin-induced diabetic rats24. Moreover in our experimental conditions SFN treatment alone did not change the lipid profile.
SFN + PM treatment had no effects on total cholesterol and triglycerides levels but it did significantly lower FFA and HbA1c levels. Both therapies in association were able to improve glucose intolerance, significantly reducing the glucose area under the curve. Targeting glycation and antioxidant defense systems with PM and SFN is highly effective in reducing FFA and HbA1c levels. Importantly, SFN and PM treatment had no effects on insulin levels, explaining the hyperglycaemia observed in the treated diabetic rats. Indeed, in all the GK treated groups, the plasma glucose levels were significantly higher than those of Wistar rats. However, the vasculoprotective actions of SFN + PM were evident under these conditions suggesting that the complete restoration of normoglycaemia is not a prerequisite for vasculoprotection.
Others have previously reported that activation of Nrf2 lowers plasma glucose levels and reduces diabetes-related nephropathy in wild type but not Nrf2 deficient mice12. In this study, SFN treated group (GKS) did not promote a decrement in blood glucose levels. Different animal models and experimental conditions may explain these differences.
The role of oxidative stress in the pathogenesis of endothelial dysfunction is well established. Diabetes increases ROS in the vasculature, compromises the antioxidant defense enzymes, and attenuates the levels of intracellular antioxidants30, creating an environment with increased oxidative stress2,31,32. In vascular models, activators of the Nrf2 pathway have been shown to restore redox homeostasis by increasing antioxidant/electrophilic response element-mediated expression of phase II and antioxidant enzymes, including NAD(P)H:quinone oxidoreductase-1, heme oxygenase-1 and γ-glutamate cysteine ligase catalytic subunit33,34. In this study, we present evidence that treatment with SFN and PM exerts anti-oxidative effects in vivo: the amounts of urinary 8-OHdG and tissue O2 • − anion and nitrotyrosine accumulation were diminished by SFN, PM or SFN + PM in diabetic GK rats. This anti-oxidative effect is an important factor behind the normalization mechanism of endothelial dysfunction observed. Accordingly, other studies have reported the inhibitory effect of SFN on oxidative stress under various conditions10,35–37. Targeting the Nrf2 pathway with SFN effectively restores normal redox balance and myogenic responsiveness to resistance arterioles in the diabetic db/db microvasculature38. In addition, SFN downregulates the expression of vascular cell adhesion molecule-1 in aortas of mice fed with a high fat diet mediated by an upregulation of Nrf2 activity39. Our data support a model in which oxidative stress is increased in aorta and mesenteric arteries by a mechanism involving an Nrf2-dependent reduction in the antioxidant capacity. We demonstrate, in vivo, that targeting the Nrf2 pathway with SFN effectively restores normal redox balance and ameliorates endothelial responsiveness to acetylcholine in aorta and mesenteric arteries in the diabetic vasculature. These beneficial effects are potentiated with PM, an inhibitor of AGE/ALE and cross-linking formation.
In diabetic animals, oxidative stress is elevated in proportion to the accumulation of AGE40,41 leading to the progression of vascular complications42. PM has been shown to inhibit AGE formation and the formation of lipid derived Maillard products; ALE43. It does not directly interact with Amadori products, but interferes with the post-Amadori oxidative reactions. Furthermore, PM traps reactive carbonyl compounds, inhibiting AGE and ALE adducts. PM inhibits lipaemia and the development of renal and vascular complications in obese rats28. The reduction of oxidative stress by PM may partly be due to inhibition of glycation - a process that directly causes free-radical production.
Moreover, treatment with SFN, PM or both significantly decreased vascular oxidative stress, and concomitantly increased NO bioavailability which can partially explain the beneficial effects on vascular function. AGE directly block NO activity and produce ROS in vascular endothelium44. The diabetic milieu is characterized by low levels of NO at the vascular level due to oxidative stress and increased AGE levels2. SFN and PM are highly effective in decrementing oxidative stress normalizing NO bioavailability. Probably only when AGE and ALE levels and cross-linking formation are lowered by PM together with the increment in antioxidant defense enzymes (SFN action) it is possible to promote higher levels of NO in the vasculature.
The effects of SFN on Nrf2-dependent gene expression are well documented45. Importantly, GK rats show a depletion of Nrf2 at the vascular level. In accordance, reduced levels of Nrf2 have been observed in mice diabetic arteries and human cardiac tissues38,46–48. Additionally, previous studies have suggested that aging is associated with Nrf2 dysfunction in the vasculature, which likely exacerbates age-related cellular oxidative stress and increases sensitivity of aged vessels to oxidative stress-induced cellular damage49. In agreement with these findings, we also found that Nrf2 expression in the aorta and mesenteric arteries were significantly down-regulated in the diabetic GK rats with 8 months old along with a significant nuclear decrement. The mechanism of Nrf2 reduction is not known and its elucidation is beyond the scope of the current study; nevertheless, our data demonstrate that disruption of Nrf2 signalling occurs in the diabetic vasculature and it probably leads to GSH depletion and decrement in other antioxidant defense mechanisms. In addition, previous studies have shown that Nrf2 induces the expression of glyoxalase 1 which protects against AGE induced protein and DNA damage and preserves cell function50.
Exploiting Nrf2 activators in association with inhibition of AGE/ALE levels and cross-linking formation and characterizing their beneficial effects may provide a novel therapeutic strategy for restoring cellular homeostasis in diabetes and its vascular complications. Presumably these findings lead to the discovery and evaluation of new antioxidant molecules, such as Nrf2 activators and AGE inhibitors, which may, at an early stage, hopefully inhibit the mechanism leading to diabetic complications.
Conclusions
Collectively, our findings indicate that the therapeutic benefit of SFN + PM in endothelial dysfunction is multifactorial. In addition to its antioxidant function reducing oxidative stress, Nrf2 positively regulates NO bioavailability. These results provide convincing experimental evidence that SFN can be used with or without association with PM therapeutically to improve endothelial dysfunction and relieve vascular damage induced by diabetes. Decreasing glycation concomitantly with the activation of Nrf2 normalized endothelial function in an animal model with long-term diabetes highlighting the importance to target different mechanisms to achieve major beneficial outcomes.
Putting this together, one could envision a future strategy consisting not only in an early aggressive treatment of hyperglycaemia, but with the simultaneous use of compounds active on AGE formation, together with compounds capable of specifically targeting reactive species or capable of enhancing our antioxidant defense systems. Potential therapeutic effects of the simultaneous activation of Nrf2 and inhibition of AGE/ALE levels and cross-linking formation could be a potential strategy.
Methods
Drugs
PM, phenylephrine, acetylcholine, N-nitro-L-arginine- methyl ester (L-NAME), and polyethylene glycol-superoxide dismutase were obtained from SIGMA (St. Louis, MO, USA). Vasodilator-stimulated phosphoprotein (VASP), pVASP, β-actin, Nrf2, AGE (clone No. 6D12) and nitrotyrosine were obtained from Cell Signaling Technology (Danvers, MA, USA), Abcam plc (Cambridge, UK), Santa Cruz Biotechnology (Santa Cruz, CA, USA), Upstate Biotechnology (Lake placid, NY, USA) and Transgenic Inc (Kobe, Japan), respectively. Sulforaphane (SFN, L-isomer) was obtained from LKT laboratories (St. Paul, MN) whereas DHE was obtained from Invitrogen (Barcelona, Spain). All other chemicals and reagents used in the study were of high grade.
Experimental animals
Wistar and GK rats were obtained from our local breeding colony (Faculty of Medicine, University of Coimbra, Portugal). Wistar and GK diabetic rats were divided into four experimental groups each. (1) Groups (W; GK) maintained with vehicle (corn oil) for 8 weeks; (2) Groups treated with sulforaphane (1 mg/Kg/day, intraperitoneal) for 8 weeks (WS; GKS); (3) Groups treated with pyridoxamine (100 mg/Kg/day, in the drinking water as previous51) for 8 weeks (WP; GKP); (4) Groups treated with sulforaphane and pyridoxamine for 8 weeks (WSP; GKSP). The animals were maintained with a standard commercial pellet chow and used with 8 months old. A pilot study (data not shown) with 3 different concentrations of SFN (0.5, 1 and 2 mg/Kg) has shown that 1 mg/Kg has a better outcome in terms of metabolic and endothelial function and it approaches the physiologic concentrations achieved by broccoli sprout extract ingestion in human and mice25,52.
All animals received care in accordance with the Portuguese Law on Experimentation with Laboratory Animals (last amendment, 2004), which is based on the principles of laboratory animal care as adopted by the EU Directive 2010/63/EU for animal experiments. The experimental protocols were approved by ORBEA – IBILI, Faculty of Medicine University of Coimbra.
Determination of metabolic and oxidative stress parameters
After a 15 h fast, animals were anesthetized with ketamine/chlorpromazine [Ketamine chloride (75 mg/kg, im, Parke-Davis, Ann Arbor, MI, USA) and chlorpromazine chloride (2.65 mg/kg, im, Lab. Vitória, Portugal)] and killed by decapitation. Blood was taken by heart puncture for determination of lipids. Fasting serum lipids (total and HDL cholesterol and triglycerides) were quantified using commercially available kits Olympus-Diagnóstica Portugal, Produtos de Diagnóstico SA, Portugal). Plasma free fatty acids (FFA) levels were evaluated using enzymatic assay kits (Roche Applied Science, Portugal). Rats were placed in metabolic cages for 24 h and urine collected. Urinary 8-hydroxy-2-deoxyguanosine was measured using EIA kits (Cayman Chemical, Europe).
For glucose tolerance tests, rats were previously fasted overnight and were given an intraperitoneal injection of glucose (1.75 g kg−1 body weight). Blood glucose was determined by sampling from the tail vein at 0, 30, 60 and 120 min after glucose injection by a glucose-oxidase method using a glucometer (Glucometer-Elite-Bayer, Portugal S.A.) and compatible reactive test strips. The AUC of IPGTT curves was calculated with standard computer programs (GraphPad Prism PC Software version 3.0). Glycated haemoglobin (HbA1c) was determined using the A1C NOW + system (Bayer, Portugal S.A.).
Isometric tension studies
Aorta were rapidly excised and freed of connective tissue. The aorta was divided into two segments (4-mm width). Ring segments were mounted between stainless steel triangles into individual organ chambers filled with oxygenated (95% O2, 5% CO2) modified Krebs-Henseleit buffer (37 °C, pH 7.4) (composition in mM: NaCl 119; KCl 4.7; CaCl2 1.6; MgSO4 1.2; NaHCO3 25; KH2PO4 1.2; Glucose 11.0). Indomethacin in a concentration of 10 µM was present in the experiments to inhibit prostaglandin synthesis. Aortic rings were subject to a resting tension of 9.8 mN. After equilibration for 60 min all vessels were preconstricted with phenylephrine. Ligand stimulated receptor-mediated NO bioavailability was assessed by a concentration-dependent relaxation to acetylcholine (ACh, 10−9 to 10−3 M), whereas sodium nitroprusside (SNP, 10−9 to 10−4 M) was used as an endothelium-independent agonist. Relaxation responses to ACh and SNP were expressed as percentage of relaxation from a submaximal phenylephrine-induced constriction and dose-response curves were obtained as previously described5,6.
Segments of second order superior mesenteric artery (approx. 200 μm external diameter) were trimmed free of fat and adhering connective tissue and mounted in a myograph according to the technique of Mulvany and Halpern53. Isometric tension was recorded and collected by a PowerLab data acquisition system (ADInstruments, UK) and recorded on a computer using the LabChart 7 data acquisition and analysis software (ADInstruments, UK). Segments were allowed to equilibrate for 30 min in modified Krebs-Henseleit solution, maintained at 37 °C and gassed with 5% CO2. Each vessel was placed under a stretch equivalent to the in vivo arterial blood pressure in each individual rat according to the technique of Mulvany and Halpern53. The tissue contractility was assessed by exposure to KCl (125 mM); subsequently cumulative concentration-response curves were obtained to phenylephrine (0.1–30 μM). Tissues were allowed to re-equilibrate for 30 min. The mesenteric rings were then used to study the response to one of the following agents: relaxation was assessed in response to either acetylcholine (ACh, 10−9 to 10−6 M) [both in the presence and absence of indomethacin (10 μM)] or sodium nitroprusside (SNP, 10−9 to 10−3 M); relaxation responses were obtained following preconstruction with phenylephrine to approximately 50% of the maximum as determined from the phenylephrine response curve as previous54. In a second series of experiments using mesenteric artery segments the acute effects of nitric oxide synthase inhibition were assessed using L-NAME. Responses to acetylcholine were obtained as above and subsequently repeated in the presence of L-NAME (300 μM). Relaxation responses to ACh and SNP were expressed as percentage of relaxation from a submaximal phenylephrine-induced constriction and concentration–response curves were obtained as previously described54.
Detection of Superoxide
Unfixed frozen, 30-µm-thick sections of proximal aorta were incubated with DHE (2 × 10−6 M) in PBS for 30 minutes at 37 °C in a humidified chamber protected from light. DHE is oxidized on reaction with O2·– to ethidium bromide, which binds to DNA in the nucleus and fluoresces red6. For ethidium bromide detection, images were obtained with a fluorescence microscope (Leica DMIRE200, Wetzlar, Germany). Fluorescence was detected with a 568-nm filter. Normal and diabetic tissues were processed and imaged in parallel with identical settings. Microscope and camera settings were kept constant for all preparations. Fluorescence was quantified using ImageJ (1.40 g, NIH).
Assessment of mesenteric immunofluorescence
Sections (6 μm) of mesenteric arteries were washed with PBS and fixed in ice cold acetone, for 10 min. Sections were then permeabilized for 10 min in 1% Triton X-100 in PBS, pH 7.4, and blocked with 10% goat serum, for 30 min. Primary antibodies were diluted in PBS containing 0.02% BSA (PBS/BSA). The primary antibodies were added and the sections were incubated overnight at 4 °C. After incubation, the sections were extensively washed with PBS/BSA solution. After, sections were incubated with the secondary antibodies, diluted in PBS/BSA for 1 h. The coverslips were washed before mounting with Glycergel Dako mounting medium (Dako, Carpinteria, CA, USA). Immunostained mesenteric sections were visualized with a Leica DMIRE200 fluorescence microscope. Immunostained mesenteric sections were counterstained with DAPI and examined, photographed and quantified as described above for DHE fluorescence54.
Western blot analysis
Segments of endothelium-intact vessels were washed with cold PBS and chilled in buffer containing in mM: Tris–HCl 50, NaCl 150, ethylenediamine tetraacetic acid (EDTA) 1, ethylene glycol tetraacetic acid (EGTA) 0.1, as well as NP-40, 0.1%, SDS 0.1% and deoxycholate 0.5%. Phenylmethylsulfonyl fluoride (1 mM), aprotonin (10 μg ml−1), leupeptin (10 μg ml−1), and pepstatin (10 μg ml−1) all from Sigma Chemicals (St. Louis, MO, USA) were added as the protease inhibitors. Tissues were homogenized in a standard fashion, followed by centrifugation at 14,000 × g for 20 min at 4 °C. The supernatants were collected and total protein concentration was determined. Samples containing 40 μg of protein were loaded on to a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, run and electroblotted onto polyvinylidene difluoride membrane. Prestained molecular weight marker proteins were used as standards for the SDS-PAGE. A ponceau staining was performed to verify the quality of the transfer and to ensure equal protein loading. Blots were blocked in 5% skimmed nonfat milk in PBS for 1 h, treated overnight with primary antibody against VASP, pVASP, β-actin or Nrf2 and then incubated with alkaline phosphatase secondary antibodies for 1 h. Anti-VASP phosphoserine 239 antibody was used for the analysis of the phosphorylation state of VASP at Ser239 (pVASP), which is a reliable biochemical marker of vascular cGMP-dependent protein kinase-1 activity. Activation of VASP was indicated by the intensity ratio pVASP/tVASP. Immunoblots were developed with an ECF Western blotting detection system (Amersham Biosciences).
Protein content was determined using a Bio-Rad protein assay kit.
Statistical analysis
All data were analysed by standard computer programs (GraphPad Prism PC Software version 3.0, ANOVA) and are expressed as mean ± SEM (n = 12 individual animals per group). Significant differences were evaluated using one-way ANOVA followed by the Bonferroni post-hoc test for individual comparisons. P < 0.05 was considered significant. Dose response curves were fitted by nonlinear regression with simplex algorithm. Relaxation responses were given as the percentage of phenylephrine-preconstriction. Comparisons of dose–response curves were evaluated by 2-way ANOVA for repeated measures followed by the Bonferroni post-hoc test for individual comparisons.
Electronic supplementary material
Acknowledgements
The present work was supported by: PEst FCT: UID/NEU/04539/2013; COMPETE: POCI-01-0145-FEDER-007440. PTDC/BIM-MET/4447/2014; COMPETE: POCI-01-0145-FEDER-016784.
Author Contributions
A. Pereira, R. Fernandes, J. Crisóstomo and C.M. Sena performed the research. C.M. Sena designed the research study. R.M. Seiça contributed with essential reagents or tools. A. Pereira and C.M. Sena analysed the data. C.M. Sena wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14733-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sena CM, Pereira AM, Seiça R. Endothelial dysfunction – a major mediator of diabetic vascular disease. Biochim. Biophys. Acta. 2014;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Cosentino F, Luscher TF. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res. 1999;43:274–278. doi: 10.1016/S0008-6363(99)00134-0. [DOI] [PubMed] [Google Scholar]
- 4.Ding H, et al. Endothelial dysfunction in the streptozotocin-induced diabetic apoE deficient mouse. Br. J. Pharmacol. 2005;146:1110–1118. doi: 10.1038/sj.bjp.0706417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sena CM, et al. Effects of alpha-lipoic acid on endothelial function in aged diabetic and high-fat fed rats. Br. J. Pharmacol. 2008;153:894–906. doi: 10.1038/sj.bjp.0707474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sena CM, et al. Metformin restores endothelial function in aorta of diabetic rats. Br. J. Pharmacol. 2011;163:424–437. doi: 10.1111/j.1476-5381.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sykiotis GP, Habeos IG, Samuelson AV, Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Cur. Opin. Clin. Nutr. Metab. Care. 2011;14:41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juurlink BH. Dietary Nrf2 activators inhibit atherogenic processes. Atherosclerosis. 2012;225:29–33. doi: 10.1016/j.atherosclerosis.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 9.Noyan-Ashraf MH, Wu L, Wang R, Juurlink BH. Dietary approaches to positively influence fetal determinants of adult health. Faseb. J. 2006;20:371–373. doi: 10.1096/fj.05-4889fje. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–1041. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue M, et al. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57:2809–2817. doi: 10.2337/db06-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng H, et al. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–3066. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapple SJ, Siow RC, Mann GE. Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging. Int. J. Biochem. Cell Biol. 2012;44:1315–1320. doi: 10.1016/j.biocel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Mann GE. Nrf2-mediated redox signalling in vascular health and disease. Free Radic. Biol. Med. 2014;75(Suppl. 1):S1. doi: 10.1016/j.freeradbiomed.2014.10.595. [DOI] [PubMed] [Google Scholar]
- 15.Tan SM, de Haan JB. Combating oxidative stress in diabetic complications with nrf2 activators: how much is too much? Redox Rep. 2014;19:107–117. doi: 10.1179/1351000214Y.0000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voziyan PA, Hudson BG. Pyridoxamine as a multifunctional pharmaceutical: targeting pathogenic glycation and oxidative damage. Cell Mol. Life Sci. 2005;62:1671–1681. doi: 10.1007/s00018-005-5082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monnier VM, et al. The role of the amadori product in the complications of diabetes. Ann. N. Y. Acad. Sci. 2008;1126:81–88. doi: 10.1196/annals.1433.052. [DOI] [PubMed] [Google Scholar]
- 18.Oelze M, et al. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circ. Res. 2000;87:999–1005. doi: 10.1161/01.RES.87.11.999. [DOI] [PubMed] [Google Scholar]
- 19.Sena CM, et al. Antioxidant and vascular effects of gliclazide in type 2 diabetic rats fed high-fat diet. Physiol. Res. 2009;58:203–209. doi: 10.33549/physiolres.931480. [DOI] [PubMed] [Google Scholar]
- 20.Levonen AL, et al. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler. Thromb. Vasc. Biol. 2007;27:741–747. doi: 10.1161/01.ATV.0000258868.80079.4d. [DOI] [PubMed] [Google Scholar]
- 21.Jyrkkanen HK, et al. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circ. Res. 2008;103:e1–e9. doi: 10.1161/CIRCRESAHA.108.176883. [DOI] [PubMed] [Google Scholar]
- 22.Bai Y, et al. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxid. Med. Cell Longev. 2015;2015:407580. doi: 10.1155/2015/407580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, et al. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid. Med. Cell Longev. 2014;2014:123963. doi: 10.1155/2014/123963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Souza CG, et al. Metabolic effects of sulforaphane oral treatment in streptozotocin-diabetic rats. J. Med. Food. 2012;15:795–801. doi: 10.1089/jmf.2012.0016. [DOI] [PubMed] [Google Scholar]
- 25.Fahey JW, et al. Sulforaphane Bioavailability from Glucoraphanin-Rich Broccoli: Control by Active Endogenous Myrosinase. PLoS ONE. 2015;10:e0140963. doi: 10.1371/journal.pone.0140963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sena CM, et al. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol. Res. 2012;65:497–506. doi: 10.1016/j.phrs.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Degenhardt TP, et al. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002;61:939–950. doi: 10.1046/j.1523-1755.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- 28.Alderson NL, et al. The AGE inhibitor pyridoxamine inhibits lipemia and development of renal and vascular disease in Zucker obese rats. Kidney Int. 2003;63:2123–2133. doi: 10.1046/j.1523-1755.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 29.Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. TrendS Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida K, et al. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: regulation of glutathione synthesis and efflux. Diabetologia. 1995;38:201–210. doi: 10.1007/BF00400095. [DOI] [PubMed] [Google Scholar]
- 31.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: key lessons from epidemiologic studies. Am. J. Cardiol. 2008;101:75D–86D. doi: 10.1016/j.amjcard.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Fahey JW, Talalay P. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem. Toxicol. 1999;37:973–979. doi: 10.1016/S0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 34.Hur KY, et al. Protective effects of magnesium lithospermate B against diabetic atherosclerosis via Nrf2-ARE-NQO1 transcriptional pathway. Atherosclerosis. 2010;211:69–76. doi: 10.1016/j.atherosclerosis.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 35.Danilov CA, et al. Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia. 2009;57:645–656. doi: 10.1002/glia.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soane L, Li Dai W, Fiskum G, Bambrick LL. Sulforaphane protects immature hippocampal neurons against death caused by exposure to hemin or to oxygen and glucose deprivation. J. Neurosci. Res. 2010;88:1355–1363. doi: 10.1002/jnr.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramprasath T, et al. Regression of oxidative stress by targeting eNOS and Nrf2/ARE signaling: a guided drug target for cardiovascular diseases. Curr. Top. Med. Chem. 2015;15:857–871. doi: 10.2174/1568026615666150220114417. [DOI] [PubMed] [Google Scholar]
- 38.Velmurugan GV, Sundaresan NR, Gupta MP, White C. Defective Nrf2-dependent redox signalling contributes to microvascular dysfunction in type 2 diabetes. Cardiovasc. Res. 2013;100:143–150. doi: 10.1093/cvr/cvt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, Y. et al. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid. Med. Cell Longev. 123963 (2014). [DOI] [PMC free article] [PubMed]
- 40.Forbes JM, et al. Reduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic nephropathy. Diabetes. 2002;51:3274–3282. doi: 10.2337/diabetes.51.11.3274. [DOI] [PubMed] [Google Scholar]
- 41.Forbes JM, et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004;53:1813–1823. doi: 10.2337/diabetes.53.7.1813. [DOI] [PubMed] [Google Scholar]
- 42.Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 43.Metz TO, Alderson NL, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: a novel therapy for treatment of diabetic complications. Arch. Biochem. Biophys. 2003;419:41–49. doi: 10.1016/j.abb.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 44.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products. Sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 45.Guerrero-Beltrán CE, Calderón-Oliver M, Pedraza-Chaverri J, Chirino YI. Protective effect of sulforaphane against oxidative stress: recent advances. Exp. Toxicol. Pathol. 2012;64:503–508. doi: 10.1016/j.etp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Alves-Lopes R, et al. Internal pudental artery dysfunction in diabetes mellitus is mediated by NOX1-derived ROS-, Nrf2-, and Rho kinase-dependent mechanisms. Hypertension. 2016;68:1056–1064. doi: 10.1161/HYPERTENSIONAHA.116.07518. [DOI] [PubMed] [Google Scholar]
- 47.Sharma A, et al. The nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activator dh404 protects against diabetes-induced endothelial dysfunction. Cardiovasc. Diabetol. 2017;16:33. doi: 10.1186/s12933-017-0513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan Y, et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60:625–633. doi: 10.2337/db10-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ungvari Z, et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue M, et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012;443:213–222. doi: 10.1042/BJ20111648. [DOI] [PubMed] [Google Scholar]
- 51.Almeida F, et al. Pyridoxamine reverts methylglyoxal-induced impairment of survival pathways during heart ischemia. Cardiovasc. Ther. 2013;31:e79–85. doi: 10.1111/1755-5922.12039. [DOI] [PubMed] [Google Scholar]
- 52.Xu Z, et al. Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Sci. Rep. 2016;6:30252. doi: 10.1038/srep30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circulation. 1977;41:19–26. doi: 10.1161/01.RES.41.1.19. [DOI] [PubMed] [Google Scholar]
- 54.Sena, C. M., Pereira, A., Fernandes, R., Letra, L. & Seiça, R. M. Adiponectin improves endothelial function in mesenteric arteries of rats fed a high-fat diet: role of perivascular adipose tissue. Br J Pharmacol. Feb25, 10.1111/bph.13756 (2017). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.