Abstract
Neutrophils are professional phagocytes that conduct effectors functions in the innate immune systems. They are differentiated in the bone marrow (BM) and terminally differentiated neutrophils are then released into systemic circulation. Neutrophils migrate into inflammatory foci through extravasation, reverse transmigration, and chemotaxis. As neutrophils arrive at a target site, they actively participate in eliminating pathogens. They phagocytose bacteria, and eliminate them through the generation of reactive oxygen species (ROS), release of protease-enriched granules, and formation of neutrophil extracellular traps (NETs). Since neutrophils are equipped with toxic arsenals, the activation of neutrophils is tightly controlled. Priming is the process of unlocking safety mechanisms before complete activation of neutrophils. Since the first discovery of neutrophils, they were considered as a homogeneous population with an inflammatory phenotype. However, heterogenous populations of neutrophils were discovered under physiological and pathological conditions. This review outlines the normal differentiation of neutrophils in the BM, and discusses the current understandings of neutrophil heterogeneity.
Keywords: Neutrophils, Differentiation, Low-density neutrophil, Normal-density neutrophil, Heterogeneity, Phenotype
INTRODUCTION
Neutrophils are most abundant leukocytes in humans and play key roles in innate immune system. They are professional phagocytes involved in host defense against invading pathogens. They extravasate from circulation and migrate toward inflammatory foci. As neutrophils arrive at inflammatory foci, they actively phagocytose pathogens and eliminate them via oxidative and non-oxidative mechanisms. Invading pathogens are recognized by resident macrophages, and endothelial cells are stimulated by macrophage-derived cytokines. Circulating neutrophils are captured by the adhesion molecules expressed on the endothelial cells, extravasate from blood vessels into the tissue, and finally transmigrate into inflammatory foci (1). Then, they phagocytose and eliminate pathogens. Neutrophils eliminate phagocytosed pathogens through oxidative and non-oxidative mechanisms (2,3). Neutrophils assemble NADPH oxidase complexes on the phagosomal membrane, and produce reactive oxygen species (ROS). This process of is called ‘respiratory burst’ and comprise one arm of neutrophil's main arsenal against pathogens. Another important arsenals of neutrophils are granules (4). Neutrophils induce the fusion of granules with phagosomes, and protease-enriched granules degrade pathogens. The granules of neutrophils are classified into four major types based on their contents; azurophil granule, specific granule, tertiary granule, and secretory vesicle. Neutrophils also entrap and kill bacteria extracellularly (5). Neutrophils generate the web-like structure composed of DNA, histones, and granules. The highly sticky character of this neutrophil extracellular traps (NETs) entrap pathogens and eliminate them with entangled granules.
Since the first discovery by Elie Metchnikoff (6), neutrophils are traditionally thought to have one phenotype; a phenotype which can kill bacteria. However, recent advances suggest the heterogenous populations of neutrophils in various physiological and pathological conditions (7,8,9,10,11,12). This review will address normal differentiation of neutrophils in the bone marrow (BM) and further discuss the heterogeneity of neutrophils.
DIFFERENTIATION AND TRAFFICKING OF NEUTROPHILS IN BM
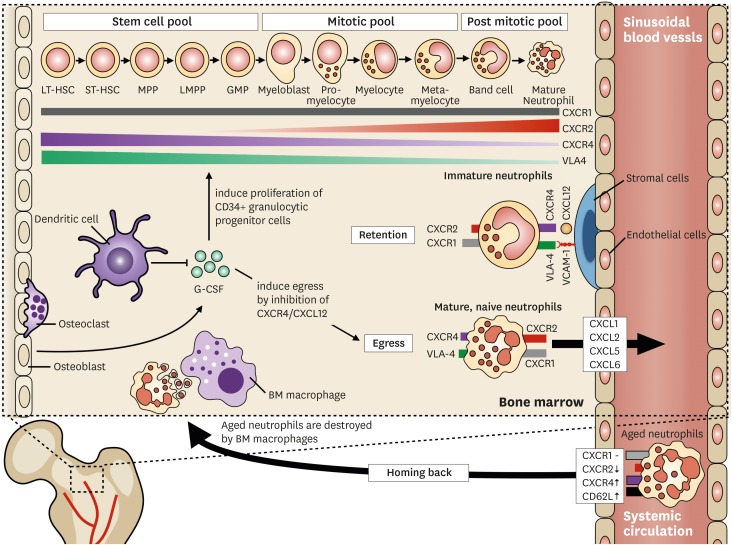
Neutrophils are produced in the BM during granulopoiesis (Fig. 1). They have been long considered to be derived from common myeloid progenitors (CMPs). However, a recent study reported that neutrophils are differentiated from lymphoid-primed multipotent progenitors (LMPPs) (13), which are derived from hematopoietic stem cells (HSCs) (14). LMPPs differentiate into granulocyte-monocyte progenitor cells (GMPs), a myeloid committed progenitor cell. Neutrophils are differentiated from GMPs through subsequent differentiation stages: myeloblasts, promyelocytes, myelocytes, metamyelocytes, and band neutrophils (14).
Figure 1.
Trafficking of neutrophils in the BM. The granulopoietic compartments of neutrophils are divided into 3 different pools. Neutrophils express and shed various receptors according to their different developmental stages. CXCR2 is upregulated whereas CXCR4 and VLA-4 are downregulated. CXCR1 is constitutively expressed during neutrophil maturation. Retention of neutrophils is mediated by CXCR4/CXCL12 and VLA-4/VCAM-1 pathways. Immature neutrophils adhere to the marrow endothelial cells and stromal cells via the CXCR4/CXCL12 and VLA-4/VCAM-1 axes. The CXCR2/CXCL2-ligands induces neutrophil egress from the BM. Mature CXCR2hi CXCR4low neutrophils preferentially egress from the BM depending on CXCR2-ligands. G-CSF orchestrate s the trafficking of neutrophils in the BM. G-CSF induces the proliferation of granulocytic progenitors and enhances the egress of neutrophils by inhibiting CXCR4/CXCL12. DCs regulate G-CSF production in the BM. Aged neutrophils are cleared by the BM. Senescent neutrophils show decreased expressions of CXCR2 and CD62L with increased expressions of CXCR4 and CCR5. This surface marker profile of aged neutrophils ensures homing back to the BM where BM macrophages destroy the aged neutrophils.
The granulopoietic compartments of neutrophils within the BM are divided into 3 different pools: stem cell pool, mitotic pool, and post-mitotic pool (15,16,17). The stem cell pool consists of undifferentiated progenitor cells, including CD34+ HSCs, LMPPs, and GMPs. The mitotic pool refers to committed granulocyte progenitor cells such as myeloblasts, promyelocytes, metamyelocytes, and myelocytes. The post-mitotic pool consists of metamyelocytes and band neutrophils. Finally, completely mature neutrophils are fully differentiated neutrophils that are ready for release into systemic circulation. Detailed descriptions of neutrophil differentiation have been extensively reviewed elsewhere (14,18).
The stroma of the BM is comprised of osteoblasts, osteoclasts, macrophages, fibroblasts, and endothelial cells (19). The sinusoid of the BM is mainly comprised of endothelial cells, and is further wrapped by mesenchymal stromal cells (17,19). These structural characteristics of the BM separate the hematopoietic compartment from the circulation (17).
Neutrophil trafficking within the BM is dependent on 2 major factors, namely, retention signals, and egress signals. The CXCR4/CXCL12 (stromal derived factor-1 [SDF-1]) and VLA-4/VCAM-1 pathways are important regulators of neutrophil retention within the BM (20,21). Neutrophils within the BM adhere to the marrow endothelial cells and stromal cells via VLA-4/VCAM-1 signaling, and the CXCR4/CXCL12 pathway augments this binding (20). Since the expression of both VLA-4 and CXCR4 on neutrophils decreases during maturation (20,22), downregulation of these signaling pathways ensures neutrophil egress from the BM. Egress of neutrophils from the BM is mediated by CXCR2, the ligands of which include CXCL1 (growth-regulated protein α [GROα]), CXCL2 (macrophage inflammatory protein-2α [MIP-2α]), CXCL5 (epithelial-derived neutrophil-activating peptide 78 [ENA78]), and CXCL6 (granulocyte chemotactic protein-2 [GCP-2]) (1,23). CXCL1 and CXCL2 are constitutively expressed on endothelial cells of the BM (21,23), whereas osteoblasts are the major source of CXCL12 in this tissue (24,25). Reciprocal interactions between CXCR2/CXCR2-ligands and CXCR4/CXCL12 signaling are considered to play a major role in the trafficking of neutrophils from the BM to the systemic circulation (21,23).
Granulocyte colony stimulating factor (G-CSF) is not only a primary regulator of granulopoiesis but also a disruptor of neutrophil retention (14). Under physiologic conditions, G-CSF induces the proliferation and differentiation of CD34+ granulocytic progenitors and reduces the transmit time through each differentiation stage (17). It also enhances the egress of mature neutrophils from the BM (15,17,26). The effect of G-CSF on the egress of neutrophils from the BM is mediated by its inhibitory effect on the CXCR4/CXCL12 axis (17). G-CSF inhibits CXCL12 generation from BM stromal cells (27), and decreases the surface expression of CXCR4 on myeloid cells (28) resulting in the egress of neutrophils from the BM.
Recently, dendritic cells (DCs) have been suggested to have a role in neutrophil trafficking (1,29). DCs control the production of G-CSF and chemokines (e.g., CXCL1, CXCL2, and CXCL10), and both are important for the egress of neutrophils from the BM (1). Mice with defects in DC development show increased numbers of peripheral neutrophils, and the depletion of conventional DCs (cDCs) results in a systemic increase in neutrophil numbers (29). Since increased plasma G-CSF level was observed in cDC-depleted mice, the regulation of neutrophil trafficking via cDC-mediated G-CSF production was proposed as a possible mechanism (29).
BM is a site for not only neutrophil production but also the site for clearance of aged neutrophils. As neutrophils age in the systemic circulation, the aged neutrophils exhibit increased surface expression of CXCR4 and CCR5, with decreased expression of CXCR2 and CD62L (22). This surface marker profile of senescent neutrophils ensures homing back to the BM where aged neutrophils are destroyed by BM macrophages (22). Recently, an important function has been suggested for BM-homing neutrophils. After intradermal injection of a modified Ankara virus, neutrophils delivered the virus from the dermis to the BM, and induced the generation of virus-specific CD8+ T cells (30). Although the precise mechanism of BM-homing in virus-exposed neutrophils is not fully understood, increased expression of CXCR4 in virus-exposed neutrophils might be involved in this process (30).
HETEROGENOUS POPULATIONS OF NEUTROPHILS
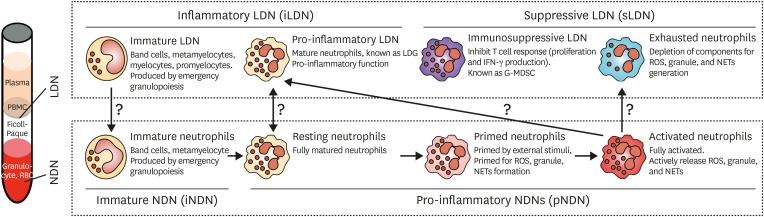
Terminally differentiated neutrophils are released into circulation and exert their primary function in host innate defenses by transmigration, phagocytosis, and bactericidal activity. Neutrophils are traditionally thought to be a homogenous population with an inflammatory phenotype. However, recent studies suggest heterogenous populations of neutrophils (7,8,9,10,11,12). The concept of heterogenous neutrophil phenotypes has been proposed by Gallin (31), based on studies in the twentieth century. However, this idea has received little attention; hence, few studies investigated this phenomenon. Only recently, researchers have gained interest in the heterogeneity of neutrophils, and have characterized the heterogenous phenotypes of neutrophils (9,12,32). Neutrophils are usually obtained from blood by density gradient centrifugation. Cells heavier than Ficoll-Paque are collected from whole blood, red blood cells are removed, and the remaining cells are considered to be neutrophils. However, neutrophils might also be found in the peripheral blood mononuclear cells (PBMCs) fractions. These neutrophils are lighter than Ficoll-Paque, and are known as ‘low density neutrophils’ (LDNs) (33). Hence, the classical neutrophils that are heavier than Ficoll-Paque are named as ‘normal density neutrophils’ (NDNs) (9).
NDNs normally include both terminally differentiated and immature neutrophils (1). In steady-state conditions, terminally differentiated neutrophils are not stimulated by any external stimuli; hence, these cells are considered to be in the resting state (resting neutrophils). Immature neutrophils only comprise small percentages of peripheral neutrophils (34). However, systemic inflammation increases the de novo production of neutrophils and also enhances the mobilization of immature neutrophils into circulation, a phenomenon known as ‘emergency granulopoiesis’ (34,35). Most of these mobilized immature cells are metamyelocytes and band cells. Since neutrophils acquire granules during differentiation, the density of these cells is considered to be similar to that of mature neutrophils. Therefore, most of the mobilized immature neutrophils during emergency granulopoiesis are usually found within the NDN fraction, but some of the immature neutrophils (myelocytes and metamyelocytes) can be found in the LDN fraction (12).
Other phenotypes of neutrophils include primed, activated, and exhausted phenotypes. Priming refers to a process of augmentation of neutrophils in response to an activating stimulation (2,36). As neutrophils migrate into inflammatory foci, priming agents such as various chemokines, cytokines, pathogen-associated molecular patterns (PAMPs), and damage-associated molecular patterns (DAMPs) primes the neutrophils (15,17). Primed neutrophils show enhanced ROS generation, granule release, and NETs formation in response to activating stimulations compared to unprimed resting neutrophils, whereas priming agents alone do not induce effector functions in neutrophils (2,37). Priming induces assembly of the NADPH oxidase complex, depolymerization of actin filaments, and enhanced phosphorylation of intracellular signaling molecules; hence, primed neutrophils show more enhanced responses to subsequent activating stimuli. When neutrophils are excessively stimulated, they undergo exhaustion. Because they have already secreted their stored granules and NETs, they show diminished granule and NETs release in response to activating stimuli. ROS generation is also greatly decreased by the desensitization of intracellular signaling molecules due to excessive stimulations. This phenomenon is previously known as immune paralysis of neutrophils (38,39,40). The resting, primed, and activated neutrophils are found in the NDN fraction. However, it is still unclear whether exhausted neutrophils are found in the NDN fraction or LDL fraction. Theoretically, exhausted neutrophils already empty their granules and DNA into external spaces and their densities might be decreased compared to that of resting neutrophils. However, the density changes in neutrophils after neutrophil activation have not been clearly studied.
Interestingly, a subset of LDNs shows immunosuppressive functions contrary to the normal effector functions of neutrophils. Increased numbers of LDNs are found in various diseases such as solid cancer, hematologic malignancies, human immunodeficiency virus (HIV)-1 infection, and sepsis (12,14,32,41,42). Since these LDNs suppress T cell responses such as proliferation and interferon-γ production, they are defined as immunosuppressive LDNs. These immunosuppressive LDNs are also regarded as granulocytic-myeloid derived suppressor cells (G-MDSCs) because of their immature phenotype (10). Moreover, recent study showed that immunosuppressive G-MDSCs from cancer patients have relatively lower density compared to NDNs isolated from the same cancer patients (43). They further identified distinct differences in gene profiles between low-density G-MDSCs and normal-density NDNs (43). Although these studies suggest the possible link between LDNs and G-MDSCs, it is still unclear whether immunosuppressive LDNs are equal to G-MDSCs.
Another interesting subset of LDNs is the pro-inflammatory phenotype. Pro-inflammatory LDNs are found in several autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) (33,44,45,46). They share the patterns of surface marker of activated neutrophils and also show effector functions similar to activated neutrophils. Therefore, it is probable that pro-inflammatory LDNs are merely activated neutrophils before exhaustion rather than a distinct subset of LDNs.
CONCLUSION
Based on recent advances in studies on neutrophil heterogeneity, a schematic summarization of neutrophil heterogeneity is illustrated in Fig. 2. However, the determination of neutrophil heterogeneity should be assessed carefully because these subsets might merely be a reflection of the physiological changes in neutrophils under pathological conditions rather than distinct subsets. Immature neutrophils can be found both in NDN and LDN fractions together with their maturation stages. Pro-inflammatory LDNs share the same functional and phenotypic characteristics of activated neutrophils. Because priming, activation, and exhaustion of neutrophils have been studied for a long time, these phenotypes are not classically defined as distinct subtypes of neutrophils. The phenotype characterization of immunosuppressive LDNs is not fully understood. Moreover, it is still unclear whether neutrophils actively change their phenotypes under pathological conditions. Therefore, the characterization of each phenotype of neutrophils, based on their surface marker expressions, gene expressions, and functional properties, is needed to develop this interim classification.
Figure 2.
Different phenotypes of circulating neutrophils. Based on their weight, neutrophils are divided into either NDNs and LDNs. NDNs include immature neutrophils, resting neutrophils, primed neutrophils, and activated neutrophils. LDNs include immature LDNs, pro-inflammatory LDNs, immunosuppressive LDNs, and exhausted neutrophils. Immature LDNs and proinflammatory LDNs are further classified as inflammatory LDNs, whereas immunosuppressive LDNs and exhausted neutrophils are classified as suppressive LDNs. Although this illustration depicts a schematic classification of neutrophils, it is still unclear whether these are distinct neutrophil subsets.
ACKNOWLEDGEMENTS
This study was supported by grant NRF-2012R1A6A3A04040639 and NRF-2017R1C1B2009015 from National Research Foundation of Korea (NRF).
Abbreviations
- BM
bone marrow
- cDC
conventional dendritic cell
- DC
dendritic cell
- G-CSF
granulocyte colony stimulating factor
- G-MDSC
granulocytic-myeloid derived suppressor cell
- GMP
granulocyte-monocyte progenitor cell
- LDN
low density neutrophil
- LMPP
lymphoid-primed multipotent progenitor
- NDN
normal density neutrophil
- NET
neutrophil extracellular trap
- ROS
reactive oxygen species
Footnotes
Conflict of Interest: The author declares no potential conflicts of interest.
Author Contributions: Conceptualization: Hong CW. Visualization: Hong CW. Writing - original draft: Hong CW. Writing - review & editing: Hong CW.
References
- 1.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 2.El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016;273:180–193. doi: 10.1111/imr.12447. [DOI] [PubMed] [Google Scholar]
- 3.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 4.Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev. 2016;273:11–28. doi: 10.1111/imr.12440. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 6.Metchnikoff E. Immunity in Infective Diseases. Cambridge: Cambridge University Press; 1905. [Google Scholar]
- 7.Beyrau M, Bodkin JV, Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2012;2:120134. doi: 10.1098/rsob.120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmona-Rivera C, Kaplan MJ. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol. 2013;35:455–463. doi: 10.1007/s00281-013-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124:710–719. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 10.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Reports. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127:2173–2181. doi: 10.1182/blood-2016-01-688887. [DOI] [PubMed] [Google Scholar]
- 12.Scapini P, Marini O, Tecchio C, Cassatella MA. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev. 2016;273:48–60. doi: 10.1111/imr.12448. [DOI] [PubMed] [Google Scholar]
- 13.Görgens A, Radtke S, Möllmann M, Cross M, Dürig J, Horn PA, Giebel B. Revision of the human hematopoietic tree: granulocyte subtypes derive from distinct hematopoietic lineages. Cell Reports. 2013;3:1539–1552. doi: 10.1016/j.celrep.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 15.Pruchniak MP, Arazna M, Demkow U. Life of neutrophil: from stem cell to neutrophil extracellular trap. Respir Physiol Neurobiol. 2013;187:68–73. doi: 10.1016/j.resp.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Futosi K, Fodor S, Mócsai A. Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17:1185–1197. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elghetany MT. Surface antigen changes during normal neutrophilic development: a critical review. Blood Cells Mol Dis. 2002;28:260–274. doi: 10.1006/bcmd.2002.0513. [DOI] [PubMed] [Google Scholar]
- 19.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petty JM, Lenox CC, Weiss DJ, Poynter ME, Suratt BT. Crosstalk between CXCR4/stromal derived factor-1 and VLA-4/VCAM-1 pathways regulates neutrophil retention in the bone marrow. J Immunol. 2009;182:604–612. doi: 10.4049/jimmunol.182.1.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rankin SM. The bone marrow: a site of neutrophil clearance. J Leukoc Biol. 2010;88:241–251. doi: 10.1189/jlb.0210112. [DOI] [PubMed] [Google Scholar]
- 23.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, Levesque JP, Chappel J, Ross FP, Link DC. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lord BI, Bronchud MH, Owens S, Chang J, Howell A, Souza L, Dexter TM. The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo . Proc Natl Acad Sci USA. 1989;86:9499–9503. doi: 10.1073/pnas.86.23.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 28.Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108:812–820. doi: 10.1182/blood-2005-10-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiao J, Dragomir AC, Kocabayoglu P, Rahman AH, Chow A, Hashimoto D, Leboeuf M, Kraus T, Moran T, Carrasco-Avino G, et al. Central role of conventional dendritic cells in regulation of bone marrow release and survival of neutrophils. J Immunol. 2014;192:3374–3382. doi: 10.4049/jimmunol.1300237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duffy D, Perrin H, Abadie V, Benhabiles N, Boissonnas A, Liard C, Descours B, Reboulleau D, Bonduelle O, Verrier B, et al. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity. 2012;37:917–929. doi: 10.1016/j.immuni.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Gallin JI. Human neutrophil heterogeneity exists, but is it meaningful? Blood. 1984;63:977–983. [PubMed] [Google Scholar]
- 32.Mishalian I, Granot Z, Fridlender ZG. The diversity of circulating neutrophils in cancer. Immunobiology. 2017;222:82–88. doi: 10.1016/j.imbio.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986;29:1334–1342. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- 34.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 35.Hirai H, Zhang P, Dayaram T, Hetherington CJ, Mizuno S, Imanishi J, Akashi K, Tenen DG. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat Immunol. 2006;7:732–739. doi: 10.1038/ni1354. [DOI] [PubMed] [Google Scholar]
- 36.Levin R, Grinstein S, Canton J. The life cycle of phagosomes: formation, maturation, and resolution. Immunol Rev. 2016;273:156–179. doi: 10.1111/imr.12439. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Latif D, Steward M, Macdonald DL, Francis GA, Dinauer MC, Lacy P. Rac2 is critical for neutrophil primary granule exocytosis. Blood. 2004;104:832–839. doi: 10.1182/blood-2003-07-2624. [DOI] [PubMed] [Google Scholar]
- 38.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 39.Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol. 2006;2:98–108. doi: 10.1186/1710-1492-2-3-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alves-Filho JC, Spiller F, Cunha FQ. Neutrophil paralysis in sepsis. Shock. 2010;34(Suppl 1):15–21. doi: 10.1097/SHK.0b013e3181e7e61b. [DOI] [PubMed] [Google Scholar]
- 41.Darcy CJ, Minigo G, Piera KA, Davis JS, McNeil YR, Chen Y, Volkheimer AD, Weinberg JB, Anstey NM, Woodberry T. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit Care. 2014;18:R163. doi: 10.1186/cc14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janols H, Bergenfelz C, Allaoui R, Larsson AM, Rydén L, Björnsson S, Janciauskiene S, Wullt M, Bredberg A, Leandersson K. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J Leukoc Biol. 2014;96:685–693. doi: 10.1189/jlb.5HI0214-074R. [DOI] [PubMed] [Google Scholar]
- 43.Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1:aaf8943. doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, McCune WJ, Kaplan MJ. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, Malech HL, Ledbetter JA, Elkon KB, Kaplan MJ. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]