Abstract
Cytomegalovirus (CMV) is one of the most important opportunistic infections in transplant recipients. Tests for CMV-specific T cell responses have been proposed to change the current risk stratification strategy using CMV assays. We evaluated the usefulness of pre-transplant CMV-specific T cell assays in kidney transplant (KT) candidates for predicting the development of CMV infection after transplantation comparing the results of the overlapping peptides (OLPs)-based enzyme-linked immunospot (ELISPOT) assay and the commercial QuantiFERON-CMV assay. We prospectively enrolled all cases of KT over a 5-month period, except donor CMV-seropositive and recipient seronegative transplants that are at highest risk of CMV infection. All the patients underwent QuantiFERON-CMV, CMV OLPs-based pp65, and immediate-early 1 (IE-1)-specific ELISPOT assays before transplantation. The primary outcome was the incidence of CMV infection at 6 months after transplant. The total of 47 KT recipients consisted of 45 living-donor KTs and 2 deceased-donor KTs. There was no association between positive QuantiFERON-CMV results and CMV infection. However, 10 of 34 patients with phosphoprotein 65 (pp65)- or IE-1-specific ELISPOT results higher than cut-off value developed CMV infections compared with none of 13 patients with results lower than cut-off value developed CMV. The OLPs-based ELISPOT assays are more useful than the QuantiFERON-CMV assay for predicting CMV infection. Patients with higher CMV-specific T cell immunity at baseline appear to be more likely to develop CMV infections after KT, suggesting that the abrupt decline in CMV-specific T cell responses after immunosuppression, or high CMV-specific T cell responses due to frequent CMV activation before KT, may promote CMV infection.
Keywords: Cytomegalovirus, Cell-mediated Immunity, Enzyme-linked immunospot assay, Interferon-gamma Release Test
INTRODUCTION
The cytomegalovirus (CMV) is one of the most important opportunistic pathogens affecting morbidity in transplant recipients (1,2). Although universal prophylaxis and preemptive antiviral therapy in transplant recipients at high risk of post-transplant CMV infection have become widely used, occasional cases of tissue-invasive CMV disease occur (2). The optimal strategy for preventing CMV disease in transplant recipients with risk factors for CMV infection is therefore required but still unknown. The current risk stratification for CMV reactivation after solid organ transplantation (SOT) is largely based on the serostatus of CMV IgG before transplantation. In brief, sero-negative recipients are at highest risk of CMV infection when the donors are sero-positive. When both donor and recipient are sero-positive, the risk is modest, and sero-negative recipients receiving grafts from sero-negative donors are at least risk of CMV infection or reactivation. However, most Korean adults (>95%) are sero-positive for CMV IgG (3), so that most SOT donor and recipient combinations are in the same risk category based on serologic risk stratification.
CMV infection is mainly controlled by CMV-specific T cells that target CMV proteins such as immediate-early 1 (IE-1), IE-2, and phosphoprotein 65 (pp65) (4). Measurement of the CMV-specific T cell response has been proposed as an alternative to the current risk stratification strategy based on serology. We therefore examined the usefulness of pre-transplant CMV-specific T cell assays in kidney transplant (KT) candidates for predicting the development of CMV infections after transplantation using the commercial QuantiFERON-CMV assay and overlapping peptides (OLPs)-based enzyme-linked immunospot (ELISPOT) assays.
MATERIALS AND METHODS
Study population
All patients admitted for transplantation to a renal transplant unit in a 2,700-bed, tertiary-care hospital in Seoul, Korea, between April 2015 and August 2015, were prospectively screened. Serological tests for CMV IgG were performed in both KT recipients and donors. Sero-positive KT recipients were included in the study, regardless of donor sero-positivity. Patients who refused informed consent, and transplant candidates under 16-years-old were excluded. Universal oral valganciclovir was given only to the highest CMV risk group (D+R−). CMV antigenemia assays were performed weekly during the first month, bi-weekly during the 1st and 3rd months after KT and then monthly to 6 months after KT. CMV antigenemia of more than 50 cells per 200,000 leukocytes was an indication for preemptive therapy. Conventional-dose ganciclovir (5 mg/kg twice daily) as preemptive therapy was given daily for at least 2 weeks and until patients were negative for CMV antigenemia. All individuals were informed of the nature of the study, and all participants provided written informed consent. This investigation was approved by the Institutional Review Board of Asan Medical Center.
OLPs-based ELISPOT
Peripheral blood was collected from each patient before transplantation, and peripheral blood mononuclear cells (PBMCs) were isolated using Lymphocyte Separation Medium (Corning, New York, NY, USA). The CMV ELISPOT assay was performed with frozen PBMC samples and OLPs (JPT Peptide Technologies, Berlin, Germany) dissolved in 4% dimethyl sulfoxide (DMSO). Ninety-six-well plates were pre-coated with anti-human interferon (IFN)-γ antibody (2 μg/ml) (Thermo Fisher Scientific, Waltham, MA, USA). PBMCs were suspended at 2.0 ×106 cells/ml in RPMI 1640+5% FBS, and samples of 2.0×105 cells were placed in wells. The samples were stimulated with CMV pp65 OLPs, IE-1 OLPs, phytohemagglutinin (Thermo Fisher Scientific), or medium containing solvent, then incubated for 27 h. Spots were detected with anti-human IFN-γ antibody (Thermo Fisher Scientific) and streptavidin-alkaline phosphatase (Invitrogen, Carlsbad, CA, USA). Spots were counted and analyzed with an automated ELISPOT plate reader (ImmunoSpot Analyzer; Cellular Technology Limited, Cleveland, OH, USA), and the results were expressed as spot-forming units/2.0×105 cells.
QuantiFERON-CMV
Peripheral blood was placed in QuantiFERON-CMV (Qiagen, Hilden, Germany) collection tubes consisting of Nil control tubes, a CMV antigen tube and a mitogen tube. The tubes were mixed repeatedly, incubated for 24 hours, and centrifuged, and plasma samples were assayed by IFN-γ ELISA in accordance with the manufacturer's instruction. Acceptable control results were: CMV-Nil <0.2 IU/ml, and Mitogen-Nil ≥0.5 IU/ml (non-reactive, CMV-Nil ≥ 0.2 IU/ml; reactive, CMV-Nil <0.2 IU/ml; and intermediate, Mitogen-Nil < 0.5 IU/ml).
Assessment of outcomes
The primary outcome was the development of CMV infection 6 months after KT. Patients with CMV antigenemia or CMV disease were considered to have a CMV infection. CMV antigenemia was defined as pp65 antigenemia, and CMV disease was defined as CMV syndrome or tissue-invasive CMV disease. CMV syndrome was defined as CMV antigenemia and at least one of the following: fever >38°C; new onset severe malaise; leucopenia in 2 successive measurements (WBC count of <3,500 cells/μl); atypical lymphocytes of >5%; thrombocytopenia of <100,000/mm. Tissue-invasive CMV was defined as evidence of localized CMV infection (cells with CMV inclusions, or in situ detection of CMV antigen by immunohistochemistry or DNA) in a biopsy or other appropriate specimen (e.g., bronchoalveolar lavage, cerebral spinal fluid), and symptoms of organ dysfunction.
Statistical analysis
Since this study was a proof-of-concept study, no required sample size was calculated. We planned to enroll over a 5-month period and monitor the development of CMV infection for an additional 6 months. The optimal cut-off values for ELISPOT results were obtained from receiver operating characteristic (ROC) curves. Sensitivity and specificity were used to express diagnostic performance. Fisher's exact test was used to test differences between positive and negative groups. Differences between continuous variables were compared using the Mann-Whitney U test. The p values of less than 0.05 in 2-tailed tests were considered to be statistically significant. All statistical analysis was performed with the SPSS for Windows software package, version 23 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
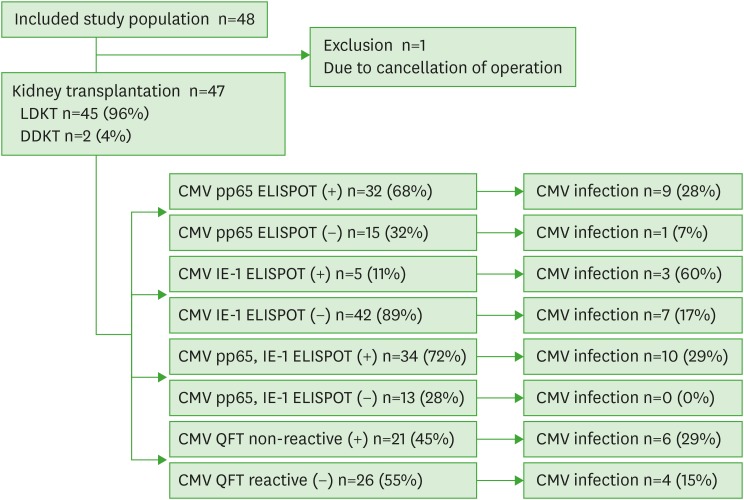
Fig. 1 is a flow chart of the study. A total 48 candidates for KT were enrolled between April 2015 and August 2015. However, one patient was excluded due to cancellation of the operation. Finally, 47 patients undergoing 45 living-donor KTs (96%) and 2 deceased-donor KTs (4%) were enrolled. The development of CMV infections after KT was observed between April 2015 and February 2016. The baseline clinical characteristics of the study patients are shown in Table 1.
Figure 1.
Flow chart of the study. The chart indicates the number and percentage of patients with CMV episode within the group of patients with positive or negative results of assay that defined by the cut-off value obtained from ROC curve.
CMV, cytomegalovirus; ROC, receiver operating characteristic; LDKT, living-donor kidney transplant; DDKT, deceased-donor kidney transplant; pp65, phosphoprotein 65; IE-1, immediate-early 1; ELISPOT, enzyme-linked immunospot.
Table 1. Characteristics of transplant recipients.
| Patient characteristic | Value | |
|---|---|---|
| Mean age, years | 43±12 | |
| Male gender | 25 (52) | |
| Primary reason for transplant | ||
| Glomerulonephritis | 16 (34) | |
| Hypertension | 12 (26) | |
| Diabetes mellitus | 4 (9) | |
| Unknown | 10 (21) | |
| Polycystic kidney disease | 1 (2) | |
| Others | 4 (9) | |
| Transplant type | ||
| Living donor kidney | 45 (96) | |
| Deceased donor kidney | 2 (4) | |
| ABO-mismatch transplantation | 14 (30) | |
| Primary transplant induction therapy at transplantation | ||
| Anti-IL2 receptor antibodies | 45 (96) | |
| Rituximab | 15 (32) | |
| CMV serostatus | ||
| D+/R+ | 46 (98) | |
| D−/R+ | 1 (2) | |
| CMV infection | ||
| CMV antigenemia | 10 (21) | |
| CMV antigenemia > 50 CMV-positive cell/200,000 leukocytes | 3 (7) | |
| CMV syndrome | 0 | |
| Tissue-invasive CMV | 1 (2) | |
Values are presented as number of patients (%) or mean±standard deviation.
CMV, cytomegalovirus; D, donor; R, recipient
Development of CMV infection and interferon-γ release assay (IGRA) assays
After KT, 10 of the 47 patients (21%) developed CMV infections. Of these 10, 3 (7%) had significant CMV antigenemia (>50 CMV positive cells/200,000 leukocytes) and 1 (2%) had a tissue-invasive CMV infection. To assess the diagnostic performance of the OLPs-based ELISPOT assay, we obtained optimal cut-off values for each OLP using ROC curves. The cut-off values for predicting CMV development after KT were 134 spots and 128 spots for the CMV pp65 ELISPOT and IE-1 ELISPOT, respectively. When we applied the cut-off value for the CMV pp65 ELISPOT, 9 of the 32 patients (28%) with positive results and 1 of the 15 patients (7%) with negative results developed CMV (p=0.14). Using the cut-off value for the CMV IE-1 ELISPOT, 3 of the 5 patients (60%) with positive results and 7 of the 42 patients (17%) with negative results had CMV infection (p=0.057). In addition, when we used the criterion of positive CMV pp65 or IE-1 ELISPOT (>134 spots), 10 of 34 patients (29%) with positive CMV pp65 or IE-1 ELISPOT results and none of the patients with negative results developed CMV (p=0.04). The results of pp65 and IE-1 ELISPOT were significantly correlated (p=0.04). However, there were no significant correlation between the results of ELISPOT and QuantiFERON-CMV (Supplementary Fig. 1).
When we evaluated the diagnostic performance of the QuantiFERON-CMV assay according to the manufacturer's recommendation, 6 of the 21 patients (29%) with non-reactive results had CMV infections after transplantation and 4 of the 26 patients (15%) with reactive QuantiFERON-CMV results did not (p=0.31). The diagnostic performance of each assay is shown in Table 2 and the responses to CMV according to the presence of CMV infection after KT are shown in Fig. 2.
Table 2. Performance of each test in predicting CMV infection after kidney transplantation.
| Assays | Sensitivity | Specificity | PPV, % (95% CI) | NPV, % (95% CI) | LR+ (95% CI) | LR− (95% CI) | AUC (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| Proportion | Percentage (95% CI) | Proportion | Percentage (95% CI) | ||||||
| CMV pp65 PBMC ELISPOT >134 spots/2×105 PBMCs | 9/10 | 90 (56–100) | 14/37 | 39 (24–57) | 28 (14–47) | 94 (70–100) | 1.49 (1.09–2.07) | 0.25 (0.04–1.69) | 0.64 (0.49–0.77) |
| CMV IE-1 PBMC ELISPOT >128 spots/2×105 PBMCs | 3/10 | 30 (7–65) | 35/37 | 95 (82–99) | 60 (15–95) | 83 (69–93) | 5.55 (1.07–28.82) | 0.74 (0.49–1.12) | 0.51 (0.36–0.66) |
| CMV pp65 or IE-1 PBMC ELISPOT >134 spots/2×105 PBMCs | 10/10 | 100 (69–100) | 13/37 | 35 (20–53) | 29 (15–48) | 100 (75–100) | 1.54 (1.22–1.95) | Not applicable | 0.68 (0.52–0.80) |
| QuantiFERON-CMV <0.2 IU/ml | 6/10 | 60 (26–88) | 22/37 | 59 (42–75) | 29 (11–52) | 85 (65–96) | 1.48 (0.78–2.80) | 0.67 (0.30–1.50) | 0.55 (0.36–0.73) |
CMV, cytomegalovirus; PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio; AUC, area under curve; CI, confidence interval; pp65, phosphoprotein 65; PBMC, peripheral blood mononuclear cell; ELISPOT, enzyme-linked immunospot; IE-1, immediate-early 1.
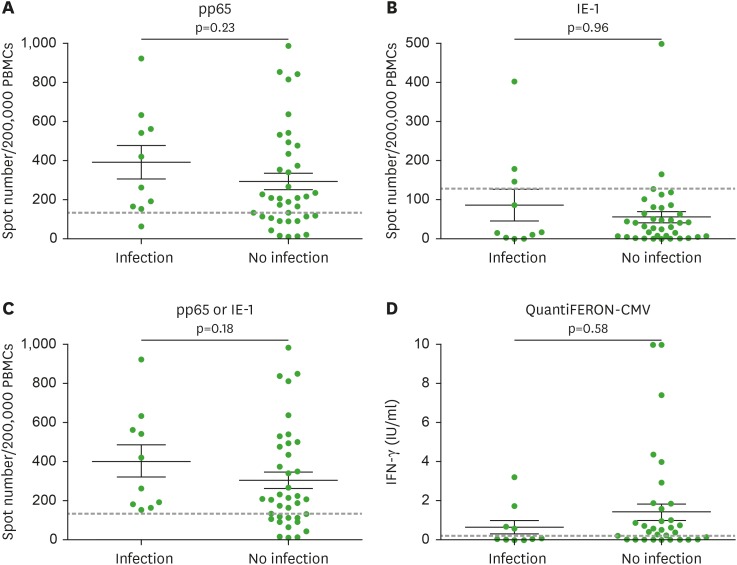
Figure 2.
Pre-transplant CMV-specific T cell responses in the patients with CMV episode. CMV-specific T cell responses were measured by stimulating PBMCs with OLPs pools of (A) pp65, (B) IE-1 in ELISPOT or (D) combination of immunodominant peptides of CMV in QuantiFERON-CMV. The combined result of (A) and (B) is represented in (C) pp65 or IE-1. In (A-C), pre-transplant CMV-specific T cell responses of the patients with post-transplant CMV episode were likely to be higher than the patients without post-transplant CMV episode. In (D), however, IFN-γ concentration was tended to lower in the patients with CMV infection. Each result was obtained by 2 repeated experiments.
CMV, cytomegalovirus; PBMC, peripheral blood mononuclear cell; OLP, overlapping peptide; pp65, phosphoprotein 65; IE-1, immediate-early 1; ELISPOT, enzyme-linked immunospot; IFN, interferon.
DISCUSSION
In this study, we evaluated pre-transplant CMV-specific cell-mediated immunity using the QuantiFERON-CMV and OLP-based IFN-γ ELISPOT assays to predict CMV the development after KT in KT recipients at moderate risk of CMV infection. We found that the in-house OLP-based CMV ELISPOT assay predicted infection after operation better than the commercial IFN-γ-releasing assay. Our data thus indicate that patients who have high CMV-specific ELISPOT responses before transplantation are prone to develop CMV infection after transplantation.
Previous studies that monitored CMV-specific pre-transplantation T cell responses in order to predict CMV infection consistently reported that strong responses to CMV antigen were inversely correlated with viral loads in the blood, or the extent of virus replication in solid organ transplant recipients (5,6,7). However, studies of congenital CMV infection (8,9) and our previous examination of CMV infections in hematopoietic stem cell transplant recipients (4) found that stronger IFN-γ responses to viral antigen were instead positively correlated with CMV infection. Furthermore, the same positive correlation was observed for infections with other viruses such as varicella-zoster virus and BK virus (10,11). Hence, our findings are consistent with these previous studies (4,8,9,10,11). There are a few possible explanations for the discrepancy between the results our study and those of previous studies. First, a single cytokine result of IFN-γ may not thoroughly represent CMV-specific cell-mediated immunity. Because a large population of effector T cells against CMV is polyfunctional, information about the responses of multiple cytokines could provide a more reliable prediction of CMV infection than a single cytokine result of IFN-γ (12). Second, we evaluated cell-mediated immune response at a single time point during the pre-transplant period, when immunosuppressive therapy was not used. In our previous study (4), we have observed that aggressive change of CMV immunity in allogeneic stem cell transplantation can be useful for identifying patients who are at risk of CMV development or relapse. In terms of methods, our hospital routinely diagnosed CMV infection by positive CMV antigenemia assay, whereas previous studies (5,6,7,8) performed CMV surveillance by quantitative real-time PCR. Quantitative real-time PCR in plasma specimen assay were proved to be more sensitive than CMV antigenemia in detecting CMV viremia in allogenic stem cell transplantation (13). Thus, the serostatus of patients might affect the results of IFN-γ releasing assay, and only seropositive recipients were enrolled in our study. It may be possible that the difference in serostatus of patients in other studies and that of our R+ patients caused the discrepancy.
There are two possible explanations for this observation. First, patients with high latent virus loads or experiencing frequent virus reactivation pre-transplantation might have strong cell-mediated immune responses to CMV, and the high CMV-specific IFN-γ response could be a surrogate for this increased CMV burden. Second, it is possible that the abrupt change of CMV-specific T cell response upon receipt of an immunosuppressive drug might increase vulnerability to CMV reactivation, compared with a certain absolute value of CMV-specific IFN-γ response. That is, a dynamic change of T cell response during a certain period may provide important information concerning the future development of CMV infection. Although we did not evaluate this dynamic change between 2 time points, we assume that an initial high CMV-specific T cell response before KT might result in a greater dynamic change, based on our previous data (4).
There are several limitations to our study. First, most of the KT recipients enrolled in this study had D+R+ serostatus. Thus, further studies are needed of KT recipients of different CMV serostatus. Second, some may argue that evaluation of the IFN-γ-releasing T cell response gives limited information about CMV infection. Recent studies revealed that polyfunctional T cell responses predicted risk of CMV after lung transplant (12). Hence, evaluation of polyfunctional CMV-specific T cell responses might provide additional useful information for predicting CMV infection after KT. Further studies are needed in this area. Second, some may argue that immunosuppressive regimen could affect the results of ELISPOT and QuantiFERON assays in KT recipients. The blood samples were drawn 2–5 days before the scheduled transplant surgery and the immunosuppressive therapy in living-donor KT recipients, so the results of ELISPOT and QuantiFERON could not have been affected by the immunosuppressive regimen in the 45 living-donor KT recipients. However, the blood samples from 2 deceased-donor KT were obtained 1–2 days after emergency transplant surgery and the induction of immunosuppressive drugs. Therefore, the results of ELISPOT and QuantiFERON might have been affected by the immunosuppressive regimen in deceased-donor KT recipients. Nevertheless, our previous studies on ELISPOT assays for TB in KT recipients (20) revealed that the subgroup analysis limited to living-donor KT recipients yielded similar results from the total patients cohort including living-donor and deceased-donor KT recipients; therefore, the immunosuppressive therapy would not have significantly affected our study results. Third, we measured CMV-specific T cell responses by pp65 and IE-1 in individual wells, and some may argue that simultaneous stimulation of both antigens is better than individual antigen stimulation. Further studies are needed to determine which type of antigenic stimulation may better predict the development of CMV infection after the transplantation. Fourth, the present sample size is not sufficient to measure true difference between groups. However, previous studies (5,6,7,14,15,16,17,18,19) also enrolled small number of patients ranging from 10 to 80 (average 35 patients). Therefore, this preliminary study may be useful for calculating the sample size for further confirmatory studies. Finally, we measured T cell responses only in the pre-transplant period. To identify whether CMV-specific T cell response is dynamically changed as shown in our previous study (4) and affect the development of CMV infection after transplantation, multiple time point observations are needed in future studies.
In conclusion, a higher CMV-specific T cell response at baseline appears to increase the probability of a CMV infection after KT. This suggests that the recipients with abrupt change of CMV-specific T cell response due to immunosuppression, or a high CMV-specific T cell response due to frequent CMV activation before KT might be prone to CMV infection.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HI15C1763).
Abbreviations
- CMV
cytomegalovirus
- ELISPOT
enzyme-linked immunospot
- IE-1
immediate-early 1
- IFN
interferon
- KT
kidney transplant
- OLP
overlapping peptide
- PBMC
peripheral blood mononuclear cell
- pp65
phosphoprotein 65
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Shin EC, Kim SH, Han DJ.
- Data curation: Kwon JS, Sung H.
- Formal analysis: Kwon JS, Kim T, Shin EC, Kim SH.
- Funding acquisition: Kim SH.
- Investigation: Kwon JS, Kim T, Kim SM, Sung H.
- Methodology: Kwon JS, Sung H, Shin EC, Kim SH.
- Project administration: Shin EC, Kim SH, Han DJ.
- Resources: Sung H, Shin S, Kim YH, Han DJ.
- Supervision: Shin EC, Kim SH, Han DJ.
- Validation: Kwon JS, Kim T, Kim SM, Sung H.
- Visualization: Shin EC, Kim SH.
- Writing - original draft: Kwon JS, Kim SH.
- Writing - review & editing: Shin EC, Kim SH, Han DJ.
Supplementary Material
Correlation between ELISPOT results and QuantiFERON-CMV. (A) CMV pp65 ELISPOT result and IE-1 ELISPOT results show significant correlation. (B-D) Results of QuantiFERON-CMV (represented by the amount of IFN-γ) and those of ELISPOT had no significant correlation.
References
- 1.Kim SH, Lee HJ, Kim SM, Jung JH, Shin S, Kim YH, Sung H, Lee SO, Choi SH, Kim YS, et al. Diagnostic usefulness of cytomegalovirus (CMV)-specific T cell immunity in predicting CMV infection after a Pilot Proof-of-Concept Study. Infect Chemother. 2015;47:105–110. doi: 10.3947/ic.2015.47.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim T, Lee YM, Lee SO, Choi SH, Kim YS, Woo JH, Sung H, Jung JH, Shin S, Kim YH, et al. Differences of cytomegalovirus diseases between kidney and hematopoietic stem cell transplant recipients during preemptive therapy. Korean J Intern Med. 2016;31:961–970. doi: 10.3904/kjim.2015.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han JS, Lee SJ, Park WK, Ko YW, Kim HO, Lee SY. A survey on the cytomegalovirus antibodies in blood donors and the diseased. Korean J Blood Transfus. 1990;1:21–34. [Google Scholar]
- 4.Jung J, Lee HJ, Kim SM, Kang YA, Lee YS, Chong YP, Sung H, Lee SO, Choi SH, Kim YS, et al. Diagnostic usefulness of dynamic changes of CMV-specific T-cell responses in predicting CMV infections in HCT recipients. J Clin Virol. 2017;87:5–11. doi: 10.1016/j.jcv.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Lochmanova A, Lochman I, Tomaskova H, Marsalkova P, Raszka J, Mrazek J, Dedochova J, Martinek A, Brozmanova H, Grundmann M. Quantiferon-CMV test in prediction of cytomegalovirus infection after kidney transplantation. Transplant Proc. 2010;42:3574–3577. doi: 10.1016/j.transproceed.2010.07.101. [DOI] [PubMed] [Google Scholar]
- 6.Cantisán S, Lara R, Montejo M, Redel J, Rodríguez-Benot A, Gutiérrez-Aroca J, González-Padilla M, Bueno L, Rivero A, Solana R, et al. Pretransplant interferon-γ secretion by CMV-specific CD8+ T cells informs the risk of CMV replication after transplantation. Am J Transplant. 2013;13:738–745. doi: 10.1111/ajt.12049. [DOI] [PubMed] [Google Scholar]
- 7.Rittà M, Costa C, Sidoti F, Ballocco C, Ranghino A, Messina M, Biancone L, Cavallo R. Pre-transplant assessment of CMV-specific immune response by Elispot assay in kidney transplant recipients. New Microbiol. 2015;38:329–335. [PubMed] [Google Scholar]
- 8.Forner G, Saldan A, Mengoli C, Gussetti N, Palù G, Abate D. Cytomegalovirus (CMV) enzyme-linked immunosorbent spot assay but not CMV QuantiFERON assay is a novel biomarker to determine risk of congenital CMV infection in pregnant women. J Clin Microbiol. 2016;54:2149–2154. doi: 10.1128/JCM.00561-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saldan A, Forner G, Mengoli C, Gussetti N, Palù G, Abate D. Strong cell-mediated immune response to human cytomegalovirus is associated with increased risk of fetal infection in primarily infected pregnant women. Clin Infect Dis. 2015;61:1228–1234. doi: 10.1093/cid/civ561. [DOI] [PubMed] [Google Scholar]
- 10.Distler E, Schnürer E, Wagner E, von Auer C, Plachter B, Wehler D, Huber C, Kolbe K, Meyer RG, Herr W. Recovery of varicella-zoster virus-specific T cell immunity after T cell-depleted allogeneic transplantation requires symptomatic virus reactivation. Biol Blood Marrow Transplant. 2008;14:1417–1424. doi: 10.1016/j.bbmt.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Schachtner T, Stein M, Babel N, Reinke P. The loss of BKV-specific Immunity from pretransplantation to posttransplantation identifies kidney transplant recipients at increased risk of BKV replication. Am J Transplant. 2015;15:2159–2169. doi: 10.1111/ajt.13252. [DOI] [PubMed] [Google Scholar]
- 12.Snyder LD, Chan C, Kwon D, Yi JS, Martissa JA, Copeland CA, Osborne RJ, Sparks SD, Palmer SM, Weinhold KJ. Polyfunctional T-cell signatures to predict protection from cytomegalovirus after lung transplantation. Am J Respir Crit Care Med. 2016;193:78–85. doi: 10.1164/rccm.201504-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solano C, Muñoz I, Gutiérrez A, Farga A, Prósper F, García-Conde J, Navarro D, Gimeno C. Qualitative plasma PCR assay (AMPLICOR CMV test) versus pp65 antigenemia assay for monitoring cytomegalovirus viremia and guiding preemptive ganciclovir therapy in allogeneic stem cell transplantation. J Clin Microbiol. 2001;39:3938–3941. doi: 10.1128/JCM.39.11.3938-3941.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiereghin A, Gabrielli L, Zanfi C, Petrisli E, Lauro A, Piccirilli G, Baccolini F, Dazzi A, Cescon M, Morelli MC, et al. Monitoring cytomegalovirus T-cell immunity in small bowel/multivisceral transplant recipients. Transplant Proc. 2010;42:69–73. doi: 10.1016/j.transproceed.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Costa C, Astegiano S, Terlizzi ME, Sidoti F, Curtoni A, Solidoro P, Baldi S, Bergallo M, Cavallo R. Evaluation and significance of cytomegalovirus-specific cellular immune response in lung transplant recipients. Transplant Proc. 2011;43:1159–1161. doi: 10.1016/j.transproceed.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Abate D, Fiscon M, Saldan A, Cofano S, Mengoli C, Sgarabotto D, d'Agostino C, Barzon L, Cusinato R, Toscano G, et al. Human cytomegalovirus-specific T-cell immune reconstitution in preemptively treated heart transplant recipients identifies subjects at critical risk for infection. J Clin Microbiol. 2012;50:1974–1980. doi: 10.1128/JCM.06406-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel M, Stefanidou M, Long CB, Fazzari MJ, Tesfa L, Del Rio M, Lamour J, Ricafort R, Madan RP, Herold BC. Dynamics of cell-mediated immune responses to cytomegalovirus in pediatric transplantation recipients. Pediatr Transplant. 2012;16:18–28. doi: 10.1111/j.1399-3046.2011.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westall GP, Mifsud NA, Kotsimbos T. Linking CMV serostatus to episodes of CMV reactivation following lung transplantation by measuring CMV-specific CD8+ T-cell immunity. Am J Transplant. 2008;8:1749–1754. doi: 10.1111/j.1600-6143.2008.02294.x. [DOI] [PubMed] [Google Scholar]
- 19.Lisboa LF, Kumar D, Wilson LE, Humar A. Clinical utility of cytomegalovirus cell-mediated immunity in transplant recipients with cytomegalovirus viremia. Transplantation. 2012;93:195–200. doi: 10.1097/TP.0b013e31823c1cd4. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Lee SO, Park JB, Park IA, Park SJ, Yun SC, Jung JH, Kim YH, Kim SC, Choi SH, et al. A prospective longitudinal study evaluating the usefulness of a T-cell-based assay for latent tuberculosis infection in kidney transplant recipients. Am J Transplant. 2011;11:1927–1935. doi: 10.1111/j.1600-6143.2011.03625.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between ELISPOT results and QuantiFERON-CMV. (A) CMV pp65 ELISPOT result and IE-1 ELISPOT results show significant correlation. (B-D) Results of QuantiFERON-CMV (represented by the amount of IFN-γ) and those of ELISPOT had no significant correlation.