Abstract
Monophosphoryl lipid A (MPL) and oligodeoxynucleotide CpG are toll-like receptor (TLR) 4 and 9 agonist, respectively. Here, we investigated the effects of MPL, CpG, and combination adjuvants on stimulating in vitro dendritic cells (DCs), in vivo innate and adaptive immune responses, and protective efficacy of influenza vaccination. Combination of MPL and CpG was found to exhibit distinct effects on stimulating DCs in vitro to secrete IL-12p70 and tumor necrosis factor (TNF)-α and proliferate allogeneic CD8 T cells. Prime immunization of mice with inactivated split influenza vaccine in the presence of low dose MPL+CpG adjuvants increased the induction of virus-specific IgG and IgG2a isotype antibodies. MPL and CpG adjuvants contribute to improving the efficacy of prime influenza vaccination against lethal influenza challenge as determined by body weight monitoring, lung function, viral titers, and histology. A combination of MPL and CpG adjuvants was effective in improving vaccine efficacy as well as in reducing inflammatory immune responses locally and in inducing cellular immune responses upon lethal influenza virus challenge. This study demonstrates unique adjuvant effects of MPL, CpG, and combination adjuvants on modulating innate and adaptive immune responses to influenza prime vaccination.
Keywords: Monophosphoryl lipid A, Oligonucleotide CpG, Adjuvant, Influenza vaccine
INTRODUCTION
Effective vaccination is the most economical measure to prevent infectious disease such as smallpox. Influenza virus causes acute respiratory disease and is responsible for approximately 250,000 deaths worldwide annually (1). Non-replicating subunit vaccines are in general safe but typically require adjuvants to enhance vaccine efficacy. For protection against seasonal influenza infection, an annual vaccination is highly recommended by World Health Organization. Inactivated split influenza vaccine (Flu-shot) is the most common platform used for vaccination but its immunogenicity and efficacy need to be improved (2,3).
The recognition of pathogens stimulates the innate immune system generating inflammatory responses and initiating adaptive immunity. This recognition is mediated by pathogen recognition receptors such as toll-like receptors (TLRs). Events of interactions between TLRs and their ligands result in the activation of transcription factors (NF-κB, AP-1, IRF 3/7) which drive the production of proinflammatory cytokines and infiltration of innate immune cells (4). The innate immune response of TLR signaling plays a critical role in dictating the quality and magnitude of the adaptive immune responses (5,6). The presence of specific TLR agonists or their combinations in vaccine formulations directs the magnitude and type of immune responses (7). The live-attenuated yellow fever vaccine, being used for 80 years, was shown to contain TLR2, TLR7/8, and TLR9 activating signals (8). Mycobacterium bovis BCG vaccine for tuberculosis appears to contain ligands for TLR2 and TLR4 (9). AS04 adjuvant which is a combination of aluminum hydroxide and monophosphoryl lipid A (MPL) has been licensed to be a part of the hepatitis B vaccine (Fendrix®; GlaxoSmithKline, Research Triangle Park, NC, USA) (10) and human papilloma virus vaccine (Cervarix®; GlaxoSmithKline) (11). These studies provide rationale for developing vaccines with adjuvants engaging in multiple signaling pathways.
MPL is an attenuated version of lipopolysaccharide (LPS), a natural endotoxin and a TLR4 ligand. LPS has been extensively studied in animal models to better understand how vaccine adjuvant works (12). Unmethylated oligodeoxynucleotides containing CpG motifs have been utilized as a potent adjuvant biasing T helper type 1 (Th1) immune responses (13,14). The inclusion of CpG in conventional oil in water emulsion adjuvants was shown to change the Th2 bias toward balanced or Th1-type response (15). CpG has been tested as an immunotherapeutic adjuvant in humans (16).
Most previous studies used relatively high doses (5 to 100 µg) of CpG or MPL adjuvant, which might cause safety concerns because of undesirable side effects. We hypothesized that low doses of MPL, CpG, or combination of MPL+CpG adjuvants would be effective in stimulating dendritic cells (DCs) in vitro, and improving immunogenicity and efficacy of inactivated split influenza virus vaccine in vivo. In this study, we tested this hypothesis and presented distinct effects of individual TLR agonists and combination adjuvants on stimulation of DCs in vitro and on the in vivo efficacy of influenza vaccination.
MATERIALS AND METHODS
Animals and reagents
Six-week old BALB/c mice were purchased from the Harlan Sprague-Dawley (Houston, TX, USA) and maintained in Georgia State University (GSU) animal facility. All mice experiments were followed by the guidelines of approved Institutional Animal Care and Use Committee (IACUC) protocol. MPL was purchased from Sigma-Aldrich and CpG (oligodeoxynucleotide 1826, 5'-tcc atg acg ttc ctg acg tt-3') was synthesized by Integrated DNA Technologies (Coralville, IA, USA). All reagents were prepared by following the manufacturer's protocol. Influenza A virus (strain A/Puerto Rico/8/1934 H1N1 [A/PR8]) was used to make inactivated split A/PR8 vaccine (sPR8). Briefly A/PR8 virus was inactivated with 1% neutral formalin for 2 h and then concentrated by ultracentrifugation (123,760 × g, 1 h). Inactivated virus pellet was resuspended in PBS and then treated with 1% Triton X-100 to disrupt virus particles. After dialysis, the protein concentration of the split vaccine was measured by DC protein assay kit (Bio-Rad, Hercules, CA, USA) and saved in −80°C.
In vitro stimulation assays of DCs
DCs were generated in vitro from femur bone marrow cells of BALB/c mice. The bone marrow cells were cultured in complete RPMI1640 (containing 10% FBS, L-glutamine and penicillin/streptomycin) with 20 ng/ml of mouse granulocyte-macrophage colony stimulating factor (mGM-CSF) supplementation to enrich DCs. Floating cells were removed and fresh complete media with GM-CSF were replaced every 2 days. After 6–10 days, the immature DCs were collected and seeded at 2.5×105 cells/ml in 6-well (2 ml/well) or 96-well plates (200 µl/well). Enriched immature DCs were cultured for 2 days in the presence of MPL, CpG, or MPL+CpG. Activation markers (CD40, CD80, and CD86) and cytokines (IL-12p70, IL-6, and tumor necrosis factor [TNF]-α) were measured by flow cytometry and ELISA, respectively.
Allogeneic naïve lymphocytes were harvested from spleen cells of C57BL/6 mice, labeled with CFSE and co-cultured with DCs that were pre-treated with the adjuvants (MPL, CpG, or MPL+CpG). The ratio of DC and lymphocytes was 1:20. After 5-day culture, the cells were harvested, stained with antibodies for surface markers and intracellular cytokines and analyzed by flow cytometry.
Immunization and virus infection
BALB/c mice (n=5) were immunized intramuscularly with sPR8 vaccine (0.3 µg) only or adjuvated with MPL (1 µg), CpG (4 µg), or MPL+CpG (1 µg+4 µg, respectively). The immunization was one time (prime) only and sera were collected 2-wk later. At 6 wk after immunization, naïve and immunized mice were challenged intranasally with a lethal dose (3×LD50) of A/PR8 virus. Body weight (BW) changes and enhanced pause (PenH) of respiration using whole body plethysmography were monitored for 7 days. According to the IACUC guideline, the mice displaying a range of 20% to 25% BW loss are considered to have reached the endpoint and humanely euthanized to avoid severe pain.
ELISA for antibody and cytokine levels
Immune sera were collected 2 weeks after immunization and antigen-specific antibody levels were determined by ELISA. Briefly, ELISA plates were coated with inactivated A/PR8 virus (200 ng/well) and blocked with 1% bovine serum albumin and 0.05% tween20 in PBS. Immune sera were serially diluted and added to the ELISA plates. After washing, horseradish peroxidase-labeled secondary antibody was incubated to detect antigen-specific IgG, IgG1, and IgG2a antibodies. Tetramethylbenzidine (TMB) was used as a substrate and OD measured at 450 nm by an ELISA reader (Bio-Rad).
To measure cytokine and chemokine levels in lung extracts and cell culture supernatants, IL-1β, IL-6, IL-10 IL-12p70, TNF-α, interferon (IFN)-γ ready-set-go kits (eBioscience, Sand Diego, CA, USA) and monocyte chemoattractant protein 1 (MCP-1), regulated on activation, normal T cell expressed and secreted (RANTES), keratinocyte-derived chemokine (KC), IFN gamma-induced protein 10 (IP-10) chemokine kits (R&D systems, Minneapolis, MN, USA) were used by following the manufacturer's manual.
Lung virus titration
The harvested lungs at day 7 post infection were minced mechanically with 1.5 ml of PBS per each lung and extracts collected after centrifugation. Embryonated chicken eggs were inoculated with diluted lung extracts and virus titers determined by hemagglutination assay of the allantoic fluids collected after 3-day incubation. Virus titers as 50% egg infection dose (EID50)/ml were evaluated according to the Reed and Muench method (17).
Flow cytometry
To investigate inflammatory cell infiltration in the respiratory tract, lung samples were harvested at day 7 post infection and then single cells were stained with antibodies specific for CD45 (30-F11; BD Biosciences, San Jose, CA, USA), CD11b (M1/70; eBiosciences), CD11c (N418; eBiosciences), F4/80 (BM8; eBiosciences), Ly6c (HK1.4; Biolegend, San Diego, CA, USA), major histocompatibility complex (MHC) II (M5/114.15.2; eBiosciences), B220 (RA3-6B2; BD Biosciences), and CD103 (2E7; Biolegend). Briefly, the harvested lung cells were washed with PBS and blocked Fc receptors on their surface by anti-CD16/32 antibodies (eBiosciences). The antibody cocktails were added to the cells and incubated for 30 min in 4°C. After washing, the stained cells were acquired by BD LSRFortessa and BD FACS Diva program (BD Biosciences). The data analysis was performed by FlowJo (FlowJo, LLC, Ashland, OR, USA).
Intracellular cytokine staining of bronchoalveolar lavage (BAL) and lung cells was performed after 6-hours stimulation with a mixture of 2 MHCI peptides (IYSTVASSL and LYEKVKSQL) or a pool of 5 MHCII peptides (SFERFEIFPKE, HNTNGVTAACSH, CPKYVRSAKLRM, KLKNSYVNKKGK, and NAYVSVVTSNYNRRF) (18). Golgi-stop (monensin, a protein transport inhibitor) was added to each sample during the stimulation. For intracellular cytokine staining, Fixation/Permeabilization solution kit (BD biosciences) was used by following the manufacturer's protocol.
Statistical analysis
All results were presented as means±standard error of mean (SEM). The statistical significance was determined by 1-way ANOVA and followed Tukey's multiple comparison test. p<0.05 was considered as significance. We analyzed all data with statistical Prism Software (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
Combination of MPL and CpG enhances DC activation and cytokine production
Adjuvants can improve vaccine efficacy by stimulating innate immune responses. To better understand adjuvant effects of MPL and CpG, we investigated in vitro activation of bone marrow derived DCs (Fig. 1). The immature DCs were harvested and then cultured with different dose combinations of MPL, CpG, or MPL+CpG. After 2-day culture, pro-inflammatory cytokines were measured in culture supernatants. MPL (0–5 µg/ml) or CpG (0–4 µg/ml) alone in a range tested was not effective in stimulating DCs to secrete IL-12p70 (IL-12) cytokine (Fig. 1A). Interestingly, combination of MPL (0.2–1 µg/ml) and CpG (1–4 µg/ml) showed synergistic effects on stimulating DCs to secrete IL-12. These synergistic effects were suppressed or not observed with a high concentration (5 µg/ml) of MPL (Fig. 1A). Both MPL and CpG either alone or combinations effectively stimulated DCs to secrete IL-6 (Fig. 1B). Either MPL or CpG alone stimulated DCs to secrete inflammatory cytokine TNF-α at a moderate level regardless of concentrations whereas combinations showed additive effects (Fig. 1C).
Figure 1.
In vitro activation of bone marrow-derived DCs by adjuvant stimulation. DCs were enriched from mouse bone marrow cells by treatment with mGM-CSF. (A-C) Cytokine levels secreted into the culture supernatants of DCs treated with different concentrations of MPL and CpG were measured by ELISA. For statistical analysis, Two-way ANOVA and Bonferroni post-multiple comparison tests were performed. (D-F) The immature DCs were cultured with MPL (0.2 μg/ml), CpG (1 μg/ml), or MPL (0.2 μg/ml)+CpG (1 μg/ml) for 2 days. Expression levels of DC activation markers were determined by flow cytometry. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
mGM-CSF, mouse granulocyte-macrophage colony stimulating factor.
*p<0.033; **p<0.002; ***p<0.001 between the indicated groups.
In vitro cultured DCs with MPL or CpG or combination of MPL+CpG were stained with DC activation markers and analyzed by flow cytometry. CD40 and CD86 activation markers on DCs were expressed at the highest level with combination of MPL+CpG (Fig. 1D and F). MPL more effectively stimulated the expression of CD40 and CD86 than CpG whereas levels of CD80 were similarly high in all stimulated DCs (Fig. 1D-F). Overall, compared to individual MPL or CpG, combination of MPL and CpG appears to be more effective in stimulating DCs particularly in IL-12 and TNF-α production as well as CD40 and CD86 marker expressions.
DCs with combination MPL and CpG treatment are effective in activating T cells
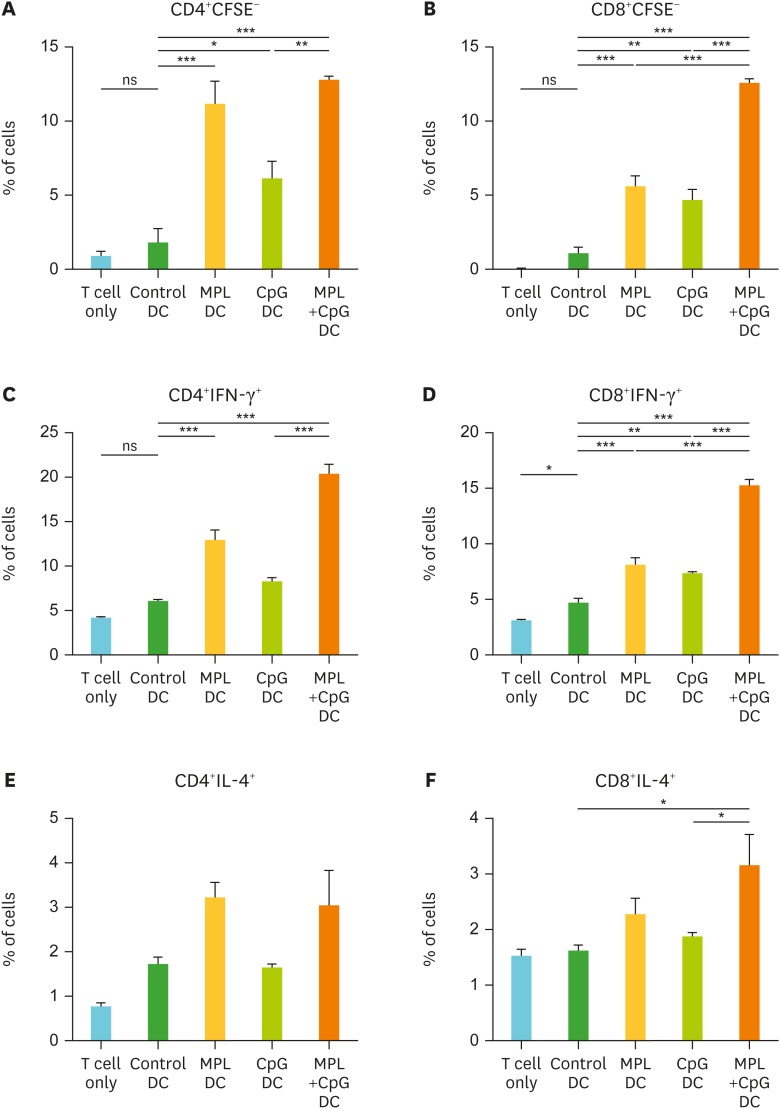
The stimulated DCs are known to activate allogeneic naïve T cell activation and proliferation (19). To determine the antigen presentation and activation ability of DCs to T cells, CFSE-labeled allogeneic lymphocytes (from C57BL/6 mice) were co-cultured with activated DCs (from BALB/c mice) that were pre-treated with MPL, CpG or MPL+CpG. After 5-day co-culture, the cell proliferation (CFSE negative) and cytokine productions of T cells were measured by flow cytometry (Fig. 2). MPL only and MPL+CpG pre-treated DC showed similarly higher levels of CD4 T cell proliferation than CpG alone treatment (Fig. 2A). Combination of MPL+CpG pre-treated DCs showed the highest levels of CD8 T cell proliferation, and IFN-γ producing CD4 and CD8 T cells meanwhile MPL or CpG single adjuvant treated DCs showed moderate effects (Fig. 2B-D). MPL alone or MPL+CpG combination treated DCs showed low to moderate effects on activating CD4 and CD8 T cells to secrete IL-4 but higher than those in DC controls (Fig. 2E and F). These results suggest that combination MPL+CpG stimulated DCs effectively proliferate T cells to secrete IFN-γ.
Figure 2.
In vitro proliferation and activation of T cells by adjuvant-activated DCs. DCs enriched from bone marrow cells were pre-activated by MPL (0.2 μg/ml), CpG (1 μg/ml), or MPL (0.2 μg/ml)+CpG (1 μg/ml) for 2 days. Allogeneic lymphocytes were harvested from spleens of C57BL/6 mice. CFSE-labeled lymphocytes and pre-activated DCs were co-cultured for 5 days. T cell proliferation and cytokine producing cells were determined by flow cytometry. (A) Proliferated CD4+ T cells. (B) Proliferated CD8+ T cells. (C) IFN-γ producing CD4+ T cells. (D) IFN-γ producing CD8+ T cells. (E) IL-4 producing CD4+ T cells. (F) IL-4 producing CD8+ T cells. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
ns, not significant between the indicated groups.
*p<0.033; **p<0.002; ***p<0.001.
Combination MPL and CpG adjuvants enhance IgG antibody responses to influenza vaccine
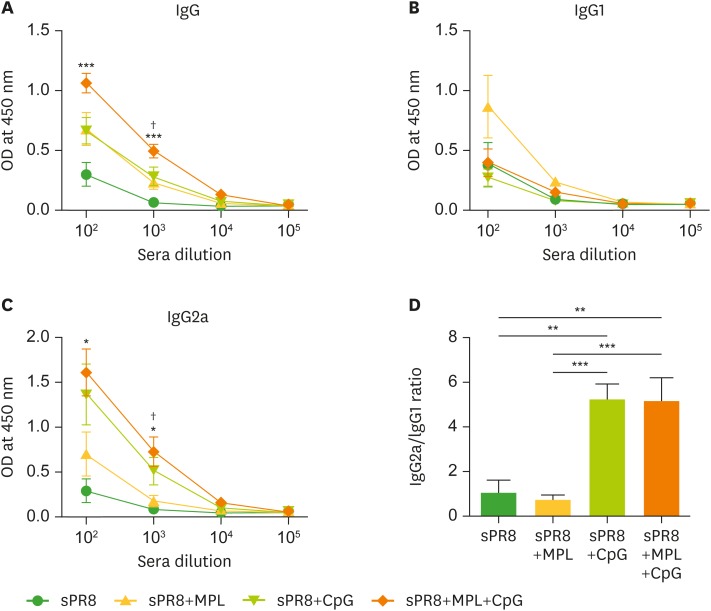
To determine the adjuvant effects of combination MPL+CpG in vivo, we immunized BALB/c mice with split A/PR8 virus vaccine (sPR8, 0.3 µg) only or sPR8 supplemented with MPL (1 µg), CpG (4 µg), or MPL+CpG (1 µg +4 µg, respectively) intramuscularly. After 3 weeks of prime vaccination, sera were collected and sPR8-specific antibody levels were measured by ELISA. The mice with combination MPL and CpG adjuvanted vaccine showed the highest levels of sPR8-specific IgG and IgG2a isotype antibodies (Fig. 3A and C). The MPL or CpG single adjuvanted groups showed similarly higher IgG levels than the sPR8 vaccine alone group (Fig. 3A). Interestingly, the sPR8+MPL group induced the highest levels of IgG1 isotype antibodies whereas CpG showed more effective adjuvant effects on enhancing IgG2a isotype antibodies than MPL (Fig. 3B and C). Higher IgG2a/IgG1 ratios were observed in the CpG and MPL+CpG combination groups compared to the MPL or sPR8 vaccine alone group (Fig. 3D).
Figure 3.
TLR agonist adjuvant effects on inducing IgG antibodies specific for influenza vaccine antigen. BALB/c mice (n=5) were immunized with sPR8 virus vaccine only or sPR8 virus vaccine in the presence of TLR agonist adjuvants (MPL, CpG, or MPL+CpG). Immune sera were taken 2 weeks after immunization and PR8 virus antigen-specific IgG antibody levels were measured by ELISA. IgG (A), IgG1 (B) and IgG2a (C) levels were shown in OD values at 450 nm. (D) IgG2a/IgG1 ratio was calculated at 102 times sera dilution. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
*p<0.033; **p<0.002; ***p<0.001 between the indicated groups or compared to sPR8 group; †p<0.033 compared to sPR8+MPL.
Both MPL and CpG adjuvants enhance protective efficacy of influenza vaccination
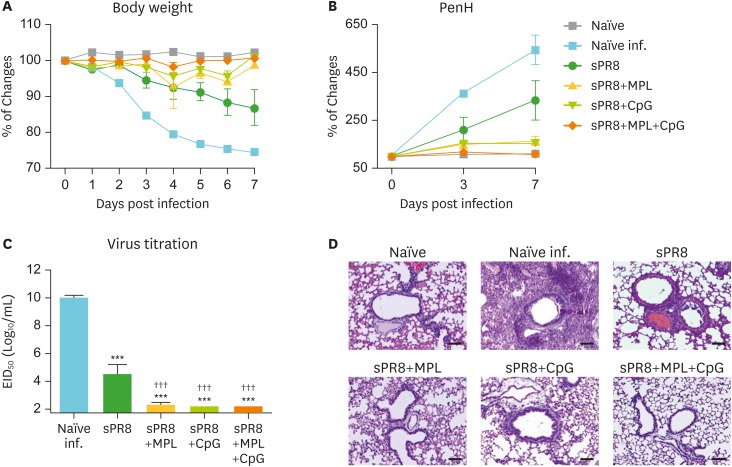
At 6 weeks after single dose vaccination, mice were challenged with a lethal dose of A/RP8 virus to determine the vaccine efficacy. Naïve mice upon infection showed most severe BW loss and highest levels of PenH values as a measure of resistance to air inhalation (naïve infection, Fig. 4A and B). The sPR8 vaccine alone group showed moderate but significant BW loss and increases in PenH. The combination MPL+CpG group was well protected against A/PR8 challenge as evidenced by no changes in BW and in PenH, similar to naïve uninfected mice (Fig. 4A and B). The MPL and CpG alone adjuvant groups displayed a transiently slight loss (3%–7%) in BW but recovered back to normal weight and there were no significant differences among the adjuvanted groups.
Figure 4.
MPL and CpG adjuvant effects on improving protective efficacy of influenza vaccination after lethal virus infection. The immunized mice (n=5) were infected with A/PR8 virus (2×LD50) after 6 weeks of immunization. BW (A) and PenH (B) were measured for 7 days after infection and % changes were calculated based on the day 0. (C) Lung samples were harvested day 7 post infection. Lung virus titers of each immunized mice were measured by using embryonated eggs. EID50 were shown. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed. (D) Lung histopathology. Intact lungs were harvested at day 7 post infection, fixed, processed, and stained with hematoxylin and eosin.
EID50, 50% egg infection dose; inf., infection.
***p<0.001 compared to naïve infection group; †††p<0.001 compared to sPR8 group.
At day 7 post infection, lung samples of the infected mice were harvested to measure virus titers and lung histopathology (Fig. 4C and D). The naïve infection group showed the highest levels of lung viral titers (10 Log10) and severe histopathology with infiltrates. Also, the sPR8 vaccine group exhibited significant lung viral loads and thickening of airway epithelial layers in the histology despite lower than those in naïve infection mice. The adjuvanted vaccine groups showed significantly lower levels of virus titers near to the detection limit. Little thickening of airway epithelial layers was observed in the histology of the CpG group (Fig. 4C and D). These data support that MPL and CpG adjuvants in single dose vaccination enhance protective efficacy of sPR8 vaccine.
Combination MPL and CpG adjuvanted vaccination prevents inflammatory innate immune responses due to virus infection
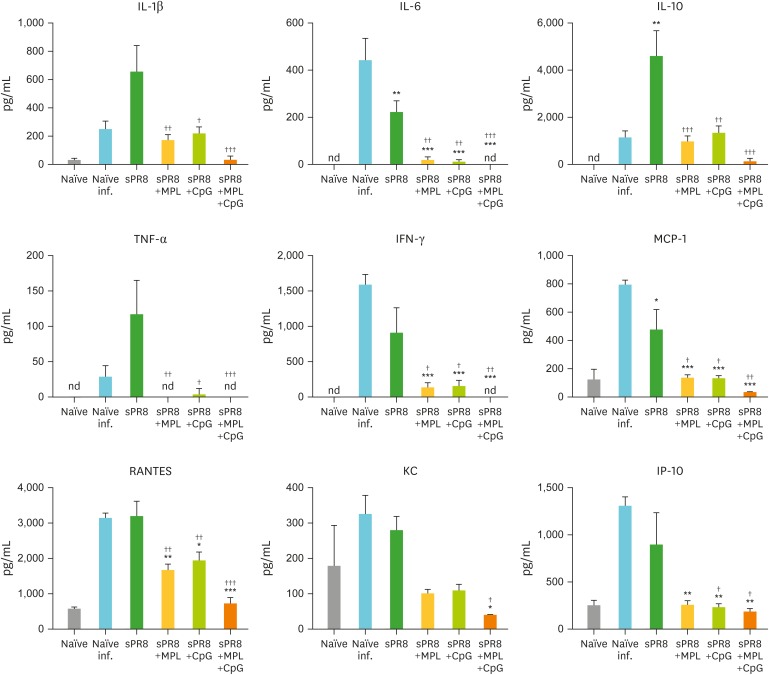
For further details of protective efficacy, we collected lungs at day 7 post infection and measured cytokine levels in lung extract (Fig. 5) and analyzed lung cell populations (Fig. 6). The naïve infection group showed the highest levels of inflammatory cytokines (IL-6 and IFN-γ) and chemokines (MCP-1, RANTES, KC, and IP-10). The sPR8 vaccine alone group exhibited significantly higher levels of IL-1β, IL-6, IL-10, TNF-α, IFN-γ, MCP-1, RANTES, KC, and IP-10 compared to those in the adjuvanted vaccine groups (Fig. 5). The single adjuvant MPL and CpG groups displayed substantial levels of cytokines (IL-1β, IL-10, and IFN-γ) and chemokines (MCP-1 and RANTES) compared to the combination MPL+CpG group which is almost free of inflammation similar to naïve uninfected mice (Fig. 5).
Figure 5.
Cytokines and chemokines in lung samples after lethal virus infection of mice. Lung samples were harvested from the immunized mice (n=5) day 7 post A/PR8 virus infection. Cytokine and chemokine levels of each lung samples were measured by ELISA. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison test were performed.
*p<0.033; **p<0.002; ***p<0.001 compared to Naïve infection group; †p<0.033; ††p<0.002; †††p<0.001 compared to sPR8 group.
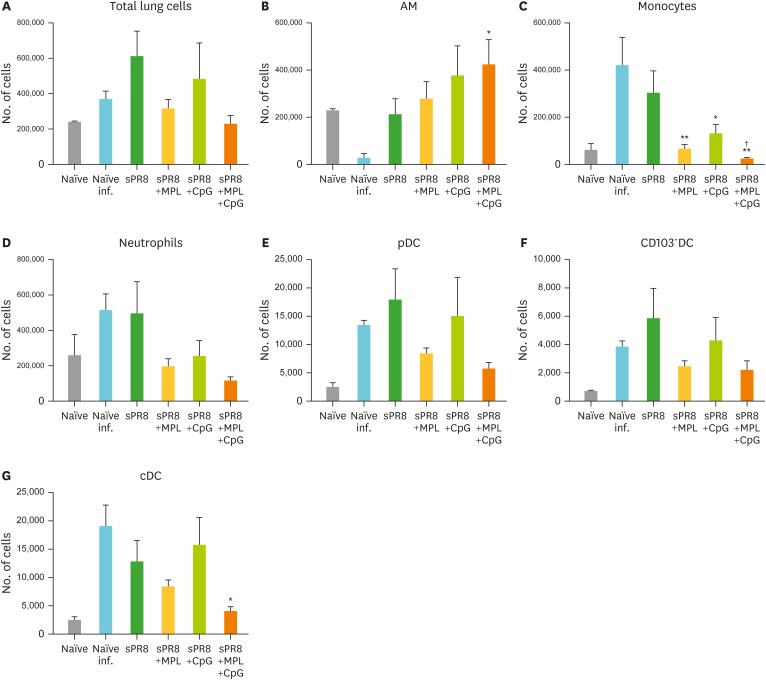
Figure 6.
Cellular infiltration in lungs after lethal virus infection. The immunized mice (n=5) were infected with A/PR8 virus (2×LD50) after 6 weeks of immunization. Lung samples were harvested day 7 post infection, and cell phenotypes were determined by flow cytometry and calculated by multiplying cell percentages with total cell numbers. (A) Total lung cells. (B) AMs; CD11b−CD11c+F4/80+. (C) Monocytes; CD11b+F4/80+Ly6Chigh. (D) Neutrophils; CD11b+F4/80−Ly6c+. (E) pDCs; CD45+F4/80−CD11c+MHCIIhighB220+. (F) CD103 + DC; CD45+F4/80−CD11c+MHCIIhighCD11b−CD103+. (G) cDC; CD45+F4/80−CD11c+MHCIIhighCD11b+. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
pDC, plasmacytoid dendritic cell; cDC, conventional DC.
*p<0.033; **p<0.002 compared to naïve infection group; †p<0.033 compared to sPR8 group.
At 7 days after challenge, the lung cells were harvested and stained with the cell phenotype specific marker antibodies. Relatively high cell numbers were observed in the lungs from naïve infection, sPR8, and CpG adjuvanted mice whereas total lung cell numbers in mice with combination MPL+CpG adjuvanted vaccination were similar to those in naïve uninfected mice (Fig. 6A). The alveolar macrophage (AM) is the first defense cell in the airways. AM populations were significantly reduced in the naïve infection group whereas the vaccinated groups showed similar or higher numbers of AM than the naïve infection group (Fig. 6B). Higher numbers of monocytes and neutrophils were recruited by influenza virus infection in the naïve infection and sPR8 only vaccinated groups than the adjuvanted groups (Fig. 6C and D). Especially, the MPL+CpG combination group showed lower numbers of monocytes, neutrophils, plasmacytoid DCs, CD103+ resident DCs, and conventional DCs compared to the CpG group (Fig. 6C-G). These results suggest that combination MPL+CpG adjuvanted vaccination more effectively prevents the induction of inflammatory cytokines and cellular infiltrates into the lungs due to influenza virus infection.
MPL and CpG combination adjuvanted vaccination does not induce inflammatory T cells due to influenza virus infection in the airways and lungs
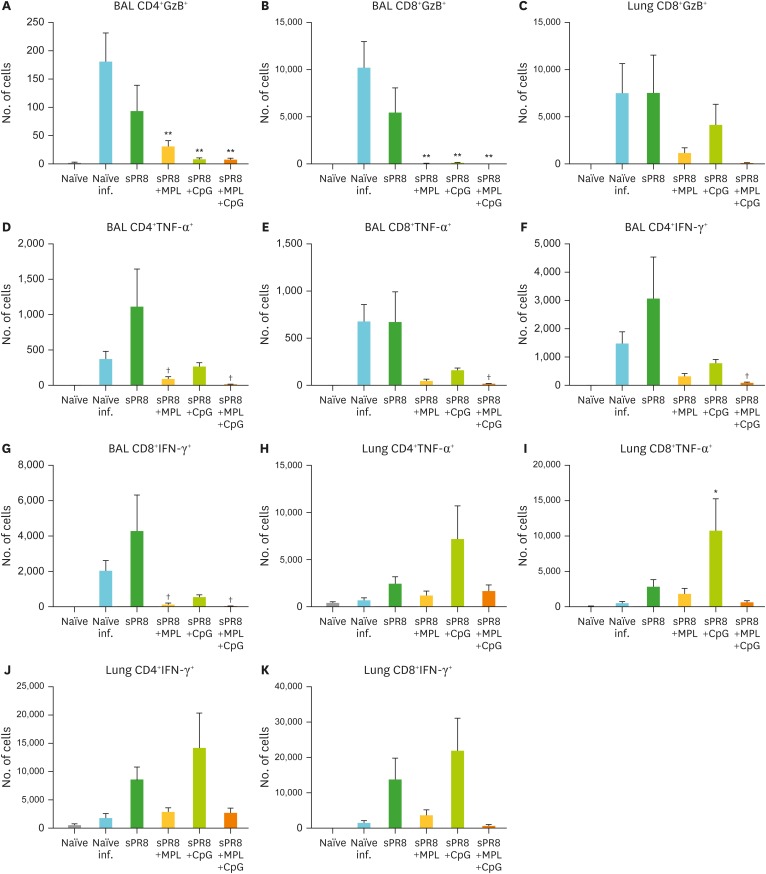
We investigated T cell responses in the BAL and lung samples after influenza virus infection. Naïve and sPR8 immune mice induced high levels of CD4 and CD8 T cells producing granzyme B (GzB+) in the BAL (Fig. 7A and B) and GzB+ CD8 T cells in lungs (Fig. 7C) at day 7 post infection compared to the adjuvanted vaccine groups. The naïve and sPR8 vaccine groups induced CD4 and CD8 T cells secreting TNF-α and IFN-γ in the BAL at higher levels than adjuvanted vaccination after infection (Fig. 7D-G). TNF-α secreting lung CD4 and CD8 T cells were induced at the highest level in the CpG group among the groups (Fig. 7H and I). IFN-γ producing CD4 and CD8 T cells were detected in the lungs from the CpG adjuvant and sPR8 vaccine alone groups compared to those in the naïve, MPL adjuvant, and combination MPL+CpG groups after challenge (Fig. 7J and K). Interestingly, GzB+, TNF-α, and IFN-γ expressing T cells were at the lowest levels in the BAL and lung samples from the combination MPL+CpG and MPL adjuvant groups (Fig. 7).
Figure 7.
Cytokine producing T cells after immunization and lethal virus infection. The immunized mice were infected with a lethal dose (2×LD50) of A/PR8 virus after 6 weeks of immunization. Lung and BAL samples were harvested day 7 post infection. Intracellular cytokine staining was performed after incubation with MHCI and II-restricted peptides for CD8 and CD4 T cell stimulation as described in the Materials and Methods section. The cytokine producing cell numbers were calculated by multiplying cell percentages with total cell numbers. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
*p<0.033; **p<0.002 compared to naïve infection group; †p<0.033 compared to sPR8 group.
MPL and CpG adjuvanted vaccination enhances antigen-specific cytokine producing splenocytes
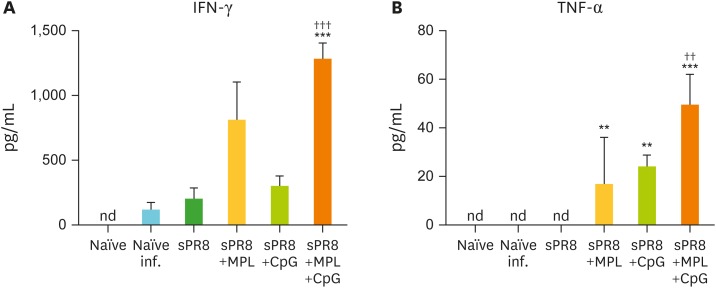
To elucidate the cellular immune responses in the systemic sites of secondary lymphoid organs of the vaccinated mice, we harvested the spleen cells from the immunized mice at day 7 post infection and cultured the cells with inactivated A/PR8 virus. After 3-day culture, cytokine levels in culture supernatants were measured by ELISA (Fig. 8). In contrast to the local sites of virus infected lungs, spleen cells from naïve, naïve mice with infection, and sPR8 vaccinated mice did not produce meaningful levels of IFN-γ and TNF-α. Splenocytes from MPL or CpG single adjuvanted mice secreted moderate levels of IFN-γ and TNF-α cytokines. Notably, the MPL+CpG combination group induced significant levels of both IFN-γ and TNF-α cytokines in spleen cell cultures after antigen-specific stimulation (Fig. 8A and B).
Figure 8.
Cytokine production of spleen cells from the immunized mice after in vitro antigen stimulation. Spleen cells were harvested from the immunized mice day 7 post infection and then cultured with inactivated A/PR8 virus stimulation. After 3 days culture, cytokine levels in supernatants were determined by ELISA. All results were shown in mean±SEM. For statistical analysis, One-way ANOVA and Tukey's post-multiple comparison tests were performed.
nd, not detected or values below detection limit.
**p<0.002; ***p<0.001 compared to naïve infection group; ††p<0.002; †††p<0.001 compared to sPR8 group.
DISCUSSION
Most subunit vaccines require adjuvants to enhance the efficacy of vaccination. A strategy of combination adjuvants is expected to help develop safe and effective vaccines since replicating live attenuated vaccines stimulate multiple immune receptors. We found distinctive properties of MPL, CpG, and combination MPL+CpG TLR agonists as vaccine adjuvants in stimulating DCs in vitro in the aspects of secreting cytokines and activating T cells. Influenza single dose vaccination of mice in the presence of combination MPL+CpG resulted in additive adjuvant effects on enhancing IgG and IgG2a isotype antibodies, preventing inflammatory cytokines and infiltrates of innate and adaptive immune cells with potential inflammatory and cytotoxic T cell responses due to virus infection in the airways. This study provides new insight into correlating in vitro activities and in vivo efficacy of MPL+CpG adjuvant.
We determined in vitro activities of MPL and CpG adjuvants in stimulating DCs at a range of low doses (0–5 µg). The levels of cytokines secreted by DCs were not proportionally correlated with the concentrations of MPL and CpG. Either TLR4 agonist MPL or TLR9 agonist CpG alone stimulated DCs to secrete IL-6 and TNF-α. CpG but not MPL stimulated DCs to produce IL-12p70. Combination MPL+CpG showed synergistic and additive effects on producing IL-12p70 and TNF-α respectively during DC stimulation but a high dose (5 µg) of MPL significantly inhibited the IL-12p70 production. MPL (0.2 µg) was more effective in upregulating CD40 and CD86 activation markers on DCs and in activating CD4 T cells via DCs than CpG (1 µg). Combination of MPL+CpG showed additive effects on upregulating CD40 and CD86 markers on DCs and in activating CD4 and CD8 T cells via DCs. Consistent with the results in this study, the synergistic effects of combination TLR4 and 9 agonists on IL-1β production but not on IL-6 were demonstrated with stimulation of peripheral blood mononuclear cells isolated from healthy adults (20). Differential outcomes of in vitro DC stimulation and combination MPL+CpG effects might be due to a difference in the signaling pathway of MPL and CpG. Toll-interleukin 1 receptor domain-containing adapter inducing IFN-β (TRIF) and TRIF-related adaptor molecule (TRAM) are known to be partially involved in the TLR4 ligand signaling in addition to MyD88 (21,22). The TLR9 CpG signaling pathway is mainly dependent on the MyD88 adaptor molecule (23).
The safety of adjuvants is a critical issue of consideration in vaccination. Lower doses of adjuvants but still exhibiting adjuvanticity would be expected to reduce undesirable side effects. Based on in vitro DC stimulation results, we applied low doses of MPL (1 µg), CpG (4 µg), and MPL+CpG (1 µg+4 µg, respectively) to single-dose vaccination with inactivated split influenza vaccine. A wide range of CpG from 10–100 µg was used as vaccine adjuvant in mice (15,24,25). Also, high doses (5–100 µg) of MPL were applied to enhance the efficacy of vaccines (24,26,27,28). Thus, the dose of 1 µg MPL and 4 µg CpG is in a low range compared to those used in previous studies. In vivo adjuvant effects appeared to be dose dependent since 4 µg dose of MPL resulted in higher IgG antibodies after vaccination of C57BL/6 mice than those in 1 µg MPL, and combination MPL+CpG adjuvant effects were prominent (data not shown). We have not observed any abnormal reactions on the site of vaccination. The assessment of safety and side effects of vaccines and adjuvants is limited in preclinical animal studies, and should be carried out in the phase I clinical studies. Although MPL increased total IgG antibodies to sPR8 vaccine, IgG2a/IgG1 ratios were similar to those in the sPR8 vaccine only group whereas CpG and MPL+CpG adjuvanted groups induced higher IgG2a/IgG1 ratios, suggesting that CpG is a main adjuvant biasing a Th1 pattern of IgG2a immune responses to inactivated split influenza vaccine in BALB/c mice. CpG in combination with conventional oil-based adjuvants was shown to enhance IgG2a isotype antibody and IFN-γ in splenocytes (15,29). Also, combination of TLR3+TLR9 agonist adjuvants was shown to enhance CD8 T cell responses to DNA vaccines (30).
AMs are the major cell population and the first defense line in lung. In case of respiratory virus infection, the AMs fight against the pathogen, and are depleted in the lung during the infection, and return to normal levels after recovery. Upon influenza virus infection, AMs were reported to be reduced to lower levels correlating to disease and mortality (31). AMs were also known to release inflammatory cytokines to control viral replication (32,33). In response to inflammatory cytokines and chemokines, innate inflammatory cells such as neutrophils, monocytes and DCs are recruited to the sites of viral infection. Thus, the percentages of the AMs are decreased as well during the infection. Therefore, naïve mice after influenza virus infection showed the lowest numbers of AMs. In contrast, the mice with adjuvanted vaccination maintained or increased the levels of AMs in lungs after influenza virus infection.
Pathogenic influenza virus infection led to severe lung inflammation characterized as proinflammatory cytokine storm and cellular infiltrates (34,35). Naïve infection or sPR8 vaccine alone immune mice exhibited high levels of proinflammatory cytokines and infiltrates of innate cells and lymphocytes into the lungs. These data indicate severe inflammatory disease due to virus infection and suggest insufficient protection by a single dose sPR8 vaccination. For effective protection, 2-time (prime and boost) influenza vaccination is recommended in naïve individuals. The strong adaptive immune development (IgG antibodies) was developed even by single vaccination with MPL+CpG adjuvant, the replication of influenza virus was well controlled in the MPL+CpG adjuvanted mice so that there was no BW loss, lung inflammation and cytokine production as well as inflammatory innate cellular infiltration. This complete protection might be resulted from high levels of antigen-specific antibodies by MPL+CpG adjuvant combination. In contrast, the sPR8 vaccine only group or unvaccinated mice showed high levels of inflammatory cytokines and cellular infiltration, which is because of ineffective control of influenza virus replication.
Consistent with previous studies, influenza virus infection induces T cells with cytotoxic activity in granule exocytosis and the engagement of TNF family members (36). Effector T cells produce excess cytokines and chemokines contributing to cytotoxic activity of damaging the tissue, although the primary role of which is to eliminate infected epithelial cells (37,38). In the intracellular staining and flow cytometry analysis of BAL and lung cells post infection, we observed that high levels of GzB+ CD4 and CD8 T cells in BAL and lung samples were induced at high levels in naïve mice with infection and in sPR8 alone immune mice. Also, relatively high levels of TNF-α+ and IFN-γ+ BAL CD4 and CD8 T cells were detected in sPR8 vaccine and naïve infection mice. The high levels of GzB+ effector T cells in the airway are correlated with lung viral loads. Previous studies demonstrated that effector T cells with cytotoxic function are located in the airways (BAL) from naïve infection or sPR8 vaccine alone mice. Both GzB+ and TNF-α+ CD4 and CD8 T cells can initiate apoptotic effects on target cells (37,38,39,40). The CpG adjuvanted mice induced a substantial level of GzB+ CD8 T cells and the highest levels of TNF-α+ and IFN-γ+ CD4 and CD8 T cells in lungs post infection. These results suggest that CpG adjuvant effectively primes cytotoxic T cells being recruited into the lungs upon infection. Excess cytotoxic T cells may also contribute to tissue damage as a low to moderate level of weight loss was observed in the CpG adjuvanted vaccine group. Inactivated respiratory syncytial virus vaccine with CpG adjuvant was reported to enhance pulmonary pathology of alveolitis and interstitial pneumonitis after virus challenge of CpG adjuvant vaccinated mice (25). Mice with combination MPL+CpG adjuvant was found to prevent inflammatory cytokines and innate immune cell infiltrates due to influenza virus infection in lungs, similar to naïve uninfected mice.
Vaccination with MPL+CpG is expected to induce strong DC activation and adaptive immune responses. The spleen is one of the secondary lymphoid tissues and most spleen cells are composed of lymphocytes. The purpose of using splenocytes was to determine the antigen-specific cellular responses in the systemic sites such as spleens (rather than mucosal sites) from the vaccinated groups. In contrast to effector T cells in lungs, we observed high levels of IFN-γ and TNF-α secreting spleen cells in the adjuvanted vaccine groups.
The humoral responses (high levels of antigen-specific antibodies) in the MPL+CpG combination group appeared to play a main role in the protection against virus infection. High levels of antibodies can bind the pathogen and protect the host by preventing virus attachment and controlling viral replication. Effective control of lung viral replication may contribute to preventing the induction of effector T cells in the BAL and lungs from the mice with MPL+CpG combination adjuvant vaccination.
ACKNOWLEDGEMENTS
This work was supported by National Institute of Allergy and Infectious Diseases at the National Institutes of Health grants AI105170 (S.M.K.), AI119366 (S.M.K.), and AI093772 (S.M.K.).
Abbreviations
- A/PR8
strain A/Puerto Rico/8/1934 H1N1
- AM
alveolar macrophage
- BAL
bronchoalveolar lavage
- BW
body weight
- DC
dendritic cell
- GzB+
granzyme B
- IFN
interferon
- IP-10
Interferon gamma-induced protein 10
- KC
keratinocyte-derived chemokine
- MCP-1
monocyte chemoattractant protein 1
- MHC
major histocompatibility complex
- MPL
monophosphoryl lipid A
- PenH
enhanced pause
- RANTES
regulated on activation, normal T cell expressed and secreted
- SEM
standard error of mean
- sPR8
split A/PR8 vaccine
- Th1
T helper type 1
- TLR
toll-like receptor
- TNF
tumor necrosis factor
Footnotes
Conflict of Interest: The authors declared no potential conflicts of interest.
Author Contributions: Conceptualization: Ko EJ, Kang SM. Data curation: Ko EJ, Kang SM. Formal analysis: Ko EJ. Funding acquisition: Kang SM. Investigation: Ko EJ, Lee YT, Lee Y, Kim KH. Writing - original draft: Ko EJ, Kang SM. Writing - review & editing: Ko EJ, Kang SM.
References
- 1.Centers for Disease Control and Prevention (CDC) Estimates of deaths associated with seasonal influenza --- United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057–1062. [PubMed] [Google Scholar]
- 2.Domínguez A, Godoy P, Torner N. The effectiveness of influenza vaccination in different groups. Expert Rev Vaccines. 2016;15:751–764. doi: 10.1586/14760584.2016.1142878. [DOI] [PubMed] [Google Scholar]
- 3.Heikkinen T, Heinonen S. Effectiveness and safety of influenza vaccination in children: European perspective. Vaccine. 2011;29:7529–7534. doi: 10.1016/j.vaccine.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 5.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 7.Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2011;29:3341–3355. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godaly G, Young DB. Mycobacterium bovis bacille Calmette Guerin infection of human neutrophils induces CXCL8 secretion by MyD88-dependent TLR2 and TLR4 activation. Cell Microbiol. 2005;7:591–601. doi: 10.1111/j.1462-5822.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 10.Kundi M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev Vaccines. 2007;6:133–140. doi: 10.1586/14760584.6.2.133. [DOI] [PubMed] [Google Scholar]
- 11.Szarewski A. Cervarix®: a bivalent vaccine against HPV types 16 and 18, with cross-protection against other high-risk HPV types. Expert Rev Vaccines. 2012;11:645–657. doi: 10.1586/erv.12.42. [DOI] [PubMed] [Google Scholar]
- 12.McAleer JP, Vella AT. Educating CD4 T cells with vaccine adjuvants: lessons from lipopolysaccharide. Trends Immunol. 2010;31:429–435. doi: 10.1016/j.it.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heeg K, Zimmermann S. CpG DNA as a Th1 trigger. Int Arch Allergy Immunol. 2000;121:87–97. doi: 10.1159/000024303. [DOI] [PubMed] [Google Scholar]
- 15.Ioannou XP, Gomis SM, Karvonen B, Hecker R, Babiuk LA, van Drunen Littel-van den Hurk S. CpG-containing oligodeoxynucleotides, in combination with conventional adjuvants, enhance the magnitude and change the bias of the immune responses to a herpesvirus glycoprotein. Vaccine. 2002;21:127–137. doi: 10.1016/s0264-410x(02)00378-x. [DOI] [PubMed] [Google Scholar]
- 16.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- 18.Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, Kang SM. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J Virol. 2009;83:4489–4497. doi: 10.1128/JVI.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko EJ, Byon YY, Jee Y, Shin T, Park SC, Hahn TW, Joo HG. Maturation of bone marrow-derived dendritic cells by a novel β-glucan purified from Paenibacillus polymyxa JB115. J Vet Sci. 2011;12:187–189. doi: 10.4142/jvs.2011.12.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Timmermans K, Plantinga TS, Kox M, Vaneker M, Scheffer GJ, Adema GJ, Joosten LA, Netea MG. Blueprints of signaling interactions between pattern recognition receptors: implications for the design of vaccine adjuvants. Clin Vaccine Immunol. 2013;20:427–432. doi: 10.1128/CVI.00703-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 23.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Hancock GE, Heers KM, Pryharski KS, Smith JD, Tiberio L. Adjuvants recognized by toll-like receptors inhibit the induction of polarized type 2 T cell responses by natural attachment (G) protein of respiratory syncytial virus. Vaccine. 2003;21:4348–4358. doi: 10.1016/s0264-410x(03)00482-1. [DOI] [PubMed] [Google Scholar]
- 25.Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J Virol. 2003;77:13156–13160. doi: 10.1128/JVI.77.24.13156-13160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 27.Prince GA, Denamur F, Deschamps M, Garçon N, Prieels JP, Slaoui M, Thiriart C, Porter DD. Monophosphoryl lipid A adjuvant reverses a principal histologic parameter of formalin-inactivated respiratory syncytial virus vaccine-induced disease. Vaccine. 2001;19:2048–2054. doi: 10.1016/s0264-410x(00)00417-5. [DOI] [PubMed] [Google Scholar]
- 28.Blanco JC, Boukhvalova MS, Pletneva LM, Shirey KA, Vogel SN. A recombinant anchorless respiratory syncytial virus (RSV) fusion (F) protein/monophosphoryl lipid A (MPL) vaccine protects against RSV-induced replication and lung pathology. Vaccine. 2014;32:1495–1500. doi: 10.1016/j.vaccine.2013.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wack A, Baudner BC, Hilbert AK, Manini I, Nuti S, Tavarini S, Scheffczik H, Ugozzoli M, Singh M, Kazzaz J, et al. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 2008;26:552–561. doi: 10.1016/j.vaccine.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 30.Grossmann C, Tenbusch M, Nchinda G, Temchura V, Nabi G, Stone GW, Kornbluth RS, Uberla K. Enhancement of the priming efficacy of DNA vaccines encoding dendritic cell-targeted antigens by synergistic toll-like receptor ligands. BMC Immunol. 2009;10:43. doi: 10.1186/1471-2172-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith AM, Smith AP. A critical, nonlinear threshold dictates bacterial invasion and initial kinetics during influenza. Sci Rep. 2016;6:38703. doi: 10.1038/srep38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tate MD, Pickett DL, van Rooijen N, Brooks AG, Reading PC. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. J Virol. 2010;84:7569–7580. doi: 10.1128/JVI.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tate MD, Schilter HC, Brooks AG, Reading PC. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral Immunol. 2011;24:77–88. doi: 10.1089/vim.2010.0118. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Zhou YH, Yang ZQ. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2016;13:3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokota S, Imagawa T, Miyamae T, Ito S, Nakajima S, Nezu A, Mori M. Hypothetical pathophysiology of acute encephalopathy and encephalitis related to influenza virus infection and hypothermia therapy. Pediatr Int. 2000;42:197–203. doi: 10.1046/j.1442-200x.2000.01204.x. [DOI] [PubMed] [Google Scholar]
- 36.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 37.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 38.Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J Exp Med. 2011;208:167–180. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hua L, Yao S, Pham D, Jiang L, Wright J, Sawant D, Dent AL, Braciale TJ, Kaplan MH, Sun J. Cytokine-dependent induction of CD4+ T cells with cytotoxic potential during influenza virus infection. J Virol. 2013;87:11884–11893. doi: 10.1128/JVI.01461-13. [DOI] [PMC free article] [PubMed] [Google Scholar]