Abstract
Developing a novel vaccine that can be applied against multiple strains of influenza virus is of utmost importance to human health. Previously, we demonstrated that the intranasal introduction of Fc-fused IL-7 (IL-7-mFc), a long-acting cytokine fusion protein, confers long-lasting prophylaxis against multiple strains of influenza A virus (IAV) by inducing the development of lung-resident memory-like T cells, called TRM-like cells. Here, we further investigated the mechanisms of IL-7-mFc-mediated protective immunity to IAVs. First, we found that IL-7-mFc treatment augments the accumulation of pulmonary T cells in 2 ways: recruiting blood circulating T cells into the lung and expanding T cells at the lung parenchyma. Second, the blockade of T cell migration from the lymph nodes (LNs) with FTY720 treatment was not required for mounting the protective immunity to IAV with IL-7-mFc, suggesting a more important role of IL-7 in T cells in the lungs. Third, IL-7-mFc treatment also recruited various innate immune cells into the lungs. Among these cells, plasmacytoid dendritic cells (pDCs) play an important role in IL-7-mFc-mediated protective immunity through reducing the immunopathology and increasing IAV-specific cytotoxic T lymphocyte (CTL) responses. In summary, our results show that intranasal treatment with IL-7-mFc modulates pulmonary immune responses to IAV, affecting both innate and adaptive immune cells.
Keywords: Interleukin-7, Fc fusion protein, Orthomyxoviridae, Dendritic cells, T-Lymphocytes
INTRODUCTION
In the last few decades, it has been reported that avian influenza A viruses (IAVs), such as H5N1 and H7N9, can cross-infect humans with higher mortality than other strains of human-infectious influenza virus (1). IAV, which is the main influenza virus strain associated with pandemics, has a higher mutation rate and more frequent gene-reassortments than other strains of influenza virus (2). Due to these characteristics of IAV, current strategies using a trivalent vaccine and anti-virals against IAV infection, which have limited vaccine cross-reactivity and productivity and pose problems regarding resistance to anti-influenza drugs, respectively (3,4), are insufficient to protect against newly generated IAVs. In order to overcome these limitations, many studies have been conducted aiming to achieve effective universal protection against rapidly changing IAV strains using conserved IAV antigens (5,6). Based on these trials, it is suggested that the generation of antigen-specific, mucosal resident T cells in the airways seems to be the most promising approach for the protective immunity (7). Inducing both innate and adaptive immune responses is crucial for the control of viremia after IAV infection.
However, the current understanding of IAV infection suggests that an excessive host-immune response leads to immunopathology followed by respiratory dysfunction and mortality (8,9). In particular, neutrophils are critically associated with pathological inflammation and host mortality during IAV infection, although they are important in controlling the initial viral spread of IAVs (10,11). Although the role of CD4 and CD8 T cells in IAV-induced immunopathology has remained elusive in mice (8), several clinical observations suggest that severe immunopathology is frequently accompanied by defective adaptive immunity. For example, patients with a high pathogenic influenza infection exhibit poor CD8 cytotoxic T cell responses or even transient lymphopenia (12,13). Furthermore, pre-existing influenza-specific CD4 T cells in healthy humans confer a heterotypic immune response after IAV challenge, which was correlated with disease protection with less severe illness (14). Since the optimal disease protection against IAVs requires the orchestration of the immune response by T cells, we can speculate that the augmentation of pulmonary T cell-mediated immunity would provide a potential benefit to the host during IAV infection.
In a previous report, we demonstrated that a single intranasal pretreatment with Fc-fused IL-7 (IL-7-mFc) exerted protective effects against several IAV strains, which were dependent on the generation of lung-resident memory-like T cells, called TRM-like cells, by IL-7-mFc (15). Here, as a follow-up study, we investigated the cellular trafficking of IL-7-mFc-induced pulmonary TRM-like cells and potential roles of various innate immune cells in eliciting protective effects against IAVs by IL-7-mFc pretreatment.
MATERIALS AND METHODS
Animals
Female BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and housed under specific pathogen-free conditions in an approved animal facility at POSTECH Biotech Center and International Vaccine institute (Seoul, Korea). All mouse experiments were performed in accordance with the National Institutes of Health guidelines, and protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Pohang University of Science and Technology.
Preparation and treatment
The murine non-lytic Fc fusion of IL-7 was prepared as previously described (16). After being anesthetized with ketamine (100 mg/kg; Yuhan, Seoul, Korea) and xylazine hydrochloride (10 mg/kg; Bayer, Brussels, Belgium) in PBS intraperitoneally (i.p.), mice received 50 μl of the indicated dose of cytokines in PBS via the indicated routes with a micropipette or syringe. To analyze the mucosal resident T cell populations, we administered 60 μl of FTY720 (Cayman Chemical, Ann Arbor, MI, USA) in 0.1% BSA containing PBS i.p. twice at 3-day intervals starting the day of IL-7-mFc treatment. The depleting mAbs against mouse CD4 (GK1.5), mouse plasmacytoid dendritic cell antigen-1 (PDCA-1) (120G8), and polyclonal rat IgG were purchased from BioXcell (West Lebanon, NH, USA). Mice received 200 μg of each depleting mAb i.p. at −1, 0, 1, and 4 day post-IAV infection.
Virus infection
Influenza strain H5N2 (A/aquatic bird/ma81/2007) was kindly provided by Young Ki Choi at Chungbuk National University College of Medicine (Cheongju, Korea). Two weeks after the last immunization, mice were anesthetized and infected intranasally (i.n.) with 5 LD50 of H5N2. Body weight change and survival were monitored daily following infection, and groups with more than 50% of dead mice were excluded from the body weight graph. Mice that lost more than 30% of their initial body weight were euthanized.
Flow cytometry
Single-cell suspensions of lung homogenate were incubated with Fc-blocker (eBioscience, San Diego, CA, USA) in staining buffer (1% fetal bovine serum [FBS] in PBS) to prevent non-specific antibody staining. Cells were then stained with the following mAbs with staining buffer: antibodies against CD4, CD8, CD44, CD62L, PDCA-1, B220, CD3, CD11b, CD11c, Ly6c, MHC II (IA/IE), F4/80, SiglecF, and interferon (IFN)-γ (all from eBioscience), and antibodies against Ly6G, CD19, Gr-1, and CD45 (all from BD biosciences, San Jose, CA, USA). For the intracellular cytokine staining of IFNγ-producing CD8 T cells, lung homogenates were incubated for 6 h with HA peptide (residue 529–543, Peptron, Daejeon, Korea), brefeldin A (eBioscience), and DNAse I (Sigma-Aldrich, St. Louis, MO, USA) and then stained using Cytofix/Cytoperm following the manufacturer's protocol (BD Bioscience). All samples were evaluated with an LSR Fortessa cytometer (BD biosciences), and the data were analyzed with FlowJo software (Tree star, St. Ashland, OR, USA).
Bronchoalveolar lavage fluid (BALF) collection and lung homogenate preparation
The mice were anesthetized, and BALF was collected with 1 ml of PBS. After BALF collection, the lungs were collected and minced into small pieces and treated with type I collagenase (Gibco/Life Technology, Grand Island, NY, USA) and DNase I (Sigma-Aldrichs) at 37°C for 30–45 min. Tissue fragments were harvested and crushed through a 70-μm strainer (BD Biosciences) to generate single cell suspensions. The cells were then washed and resuspended in RPMI-1640 (Welgene, Daegu, Korea) containing 10% FBS (Hyclone, South Logan, UT, USA), 2-mercaptoethanol (Gibco/Life Technology), and antibiotics (Gibco/Life Technology).
Statistical analysis
A 2-tailed Student's t-test and 2-tailed Mann-Whitney U test were used to evaluate the differences between 2 groups. Differences in survival rates between groups were determined by a log-rank test.
RESULTS AND DISCUSSION
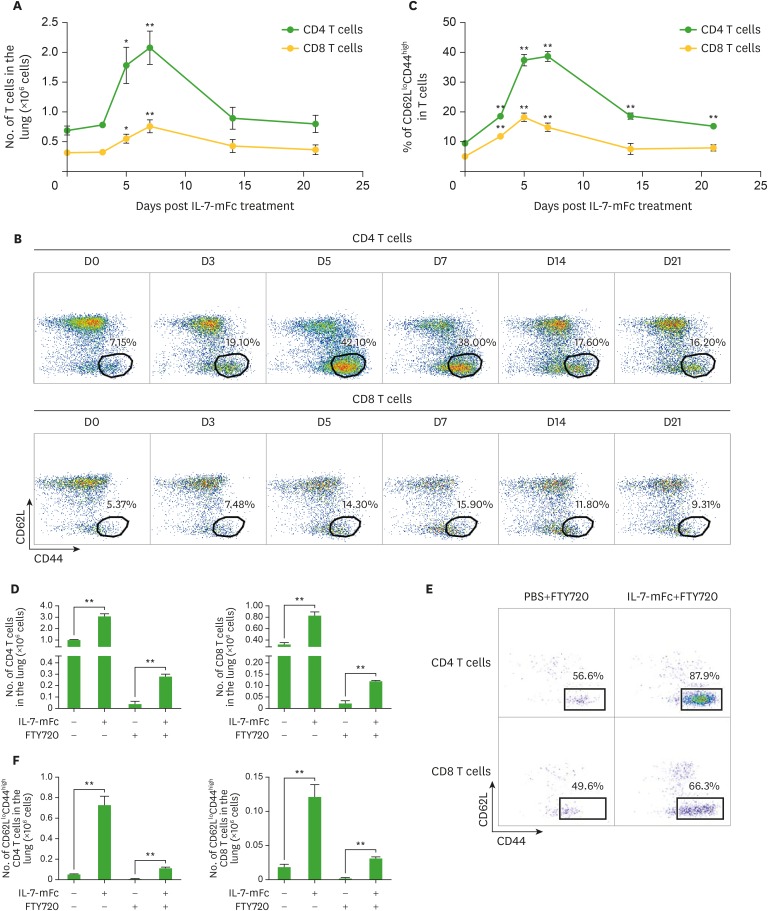
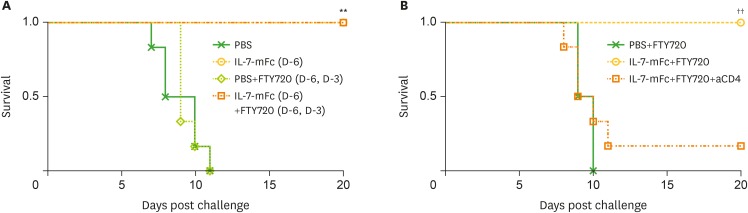
The receptor for IL-7, CD127, is primarily expressed by lymphocyte subsets, including T and B cells (17). Because IL-7-responsive pulmonary T cells are responsible for protection against IAV as shown in our previous report (15), we assessed the pulmonary T cell populations after IL-7-mFc treatment. Without IAV challenge, intranasal IL-7-mFc treatment alone transiently induced the expansion of both CD4 and CD8 cells in the lung 7 day after treatment, which gradually returned to normal levels after day 14 (Fig. 1A-C). As expected, among the T cell populations that increased, CD62LloCD44high effector/memory-phenotype populations of pulmonary CD4 and CD8 T cells were significantly increased by IL-7-mFc, and the increases were sustained until day 21. Based on our previous report, these T cells expressed CD11a and CD49d as tissue-retentive markers. Thus, these lung-resident CD62LloCD44high T cells induced by IL-7-mFc were designated TRM-like cells (15). Although we observed an increase in pulmonary T cells following IL-7-mFc treatment, it was not clear whether IL-7-mFc primarily expands T cells at the secondary lymphoid organs, such as the lymph nodes (LNs), from which T cells move to the lungs and become TRM-like cells. Otherwise, IL-7-mFc treatment may exert its main effect on the local pulmonary site by upregulating lung-resident T cells. To clarify this, we introduced IL-7-mFc via the i.n. route with FTY720, an inhibitor of sphingosine-1-phosphate receptor (S1PR), which blocks the migration of lymphocytes from the LNs (18). The number of CD8 and CD4 T cells in the lung was strongly decreased by FTY720, regardless of IL-7-mFc treatment, suggesting that the basal number of lung-resident T cells was maintained by the migration of T cells from the LNs (Fig. 1D). Notably, there was still a significant expansion of lung-resident CD4 and CD8 T cells induced by IL-7-mFc treatment in the presence of FTY720, and most of them showed CD62LloCD44high phenotypes (Fig. 1D-F). These results indicate that intranasal IL-7-mFc treatment can also recruit blood circulating T cells to the lung. Moreover, the protective effect of IL-7-mFc against lethal IAV infection was maintained in the mice that received FTY720 concomitantly (Fig. 2A), suggesting that at least the migration of LN T cells is not required for IL-7-mFc-induced protection. Since the protective immunity by IL-7-mFc pretreatment was largely mediated by pulmonary CD4 TRM-like cells, we also depleted CD4 T cells in this experimental setting. Similar to the previous results, the treatment with anti-CD4 mAb significantly abrogated the protective effect of IL-7-mFc (Fig. 2B). Together, these data imply that IL-7-mFc induces the trafficking of blood circulating T cells to the lung and may expand them at the local site, ultimately establishing TRM-like cells to protect against IAVs.
Figure 1.
Effect of FTY720 treatment on the expansion of pulmonary TRM-like cells following the intranasal introduction of IL-7-mFc. Mice (BALB/c, n=6) in each group were treated with PBS or IL-7-mFc i.n. (A-C) Pulmonary CD4 and CD8 T cells were analyzed for the level of CD62L and CD44 at each indicated time point after IL-7-mFc treatment. The number of CD4 and CD8 T cells in the lung (A) and CD62LloCD44high population in each T cell population (B, C) are shown as representative plots and graphs, respectively. (D-F) Mice were treated with IL-7-mFc i.n., and at the same time, they were also treated twice with 60 μg of FTY720 i.p. at 3-day intervals. The absolute number of total pulmonary CD4 and CD8 T cells (D) and the CD62LloCD44high population (F) in each T cell population were analyzed 6 day post-IL-7-mFc treatment. (E) Representative plots for TRM-like cells in the lung are shown. The data are representative of 2 independent experiments and expressed as the mean±standard error of mean.
*p<0.05, **p<0.01 by Student's t-test.
Figure 2.
Effect of FTY720 treatment on IL-7-mFc-mediated protection against IAV. (A) Mice (BALB/c, n=6) in each group were treated with PBS or IL-7-mFc i.n. At the same time, some mice were also treated twice with 60 μg of FTY720 at 3-day intervals. Mice were challenged with a lethal dose of H5N2 6 day post-IL-7-mFc treatment. Survival rates are shown. The data are representative of 2 independent experiments. (B) Mice were treated as described in (A) and treated with 200 μg of anti-CD4 mAb at −1, 0, 1, and 3 day post-challenge. The data are representative of 2 independent experiments.
**p<0.01 by log-rank test compared with PBS controls, ††p<0.01 by log-rank test comparing the IL-7-mFc and FTY720 groups.
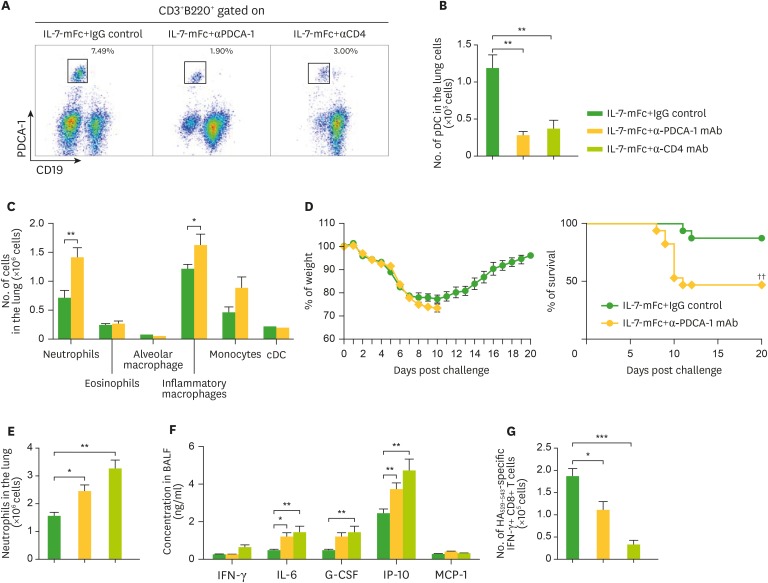
Although pulmonary T cells played an indispensable role in IL-7-mFc-mediated IAV protection, we also found that various innate-type immune cells also accumulated in the lung following IL-7-mFc pretreatment (Table 1). Those increases in innate cells peaked around day 7 but remained several weeks after IL-7-mFc treatment for certain populations, including monocytes, eosinophils, and alveolar macrophages. Since the protective role of plasmacytoid dendritic cells (pDCs) against IAV infection was reported previously (19,20), we aimed to address the role of pDCs in IL-7-mFc-mediated IAV protection. Because some pDCs do express CD4 surface antigen and because anti-CD4 mAb almost abolished the protective effects of IL-7-mFc previously, we first tested how efficiently the anti-PDCA-1 and anti-CD4 mAb treatment depleted pDCs. As shown in Fig. 3A, the accumulation of pDCs induced by IL-7-mFc treatment in the lung was dramatically reduced by both mAbs compared to the induced by the control mAb, which is summarized in Fig. 3B. In addition, anti-PDCA-1 mAb treatment did not deplete any other myeloid cells in the lung, including conventional DCs (cDCs) (Fig. 3C). This result suggests that it is possible that the use of anti-CD4 mAb in a previous study not only nullified CD4 T cells but also dampened pDCs. Therefore, it is necessary to separate the potential protective role of pDCs by using anti-PDCA-1 mAb, which specifically depletes pDCs, when challenging with IAV. Interestingly, reducing pDCs in the lung alone decreased the protective immunity mediated by IL-7-mFc treatment (Fig. 3D). Since IL-7-mFc pretreatment confers protection against IAV by alleviating the immunopathology in the lung and augmenting IAV-specific cytotoxic T lymphocytes (CTLs), we also determined whether the depletion of pDCs modulates these parameters during IAV infection, as compared with those seen following the depletion of CD4-positive cells, including CD4 T cells and pDCs. The typical signs of immunopathology in IAV infection, such as infiltration of neutrophils (Fig. 3E) and levels of inflammation-associated molecules in the BALF (Fig. 3F), were enhanced by anti-PDCA-1 treatment, which were further aggravated by anti-CD4 treatment. Similarly, IAV-specific CTLs were partially diminished by anti-PDCA-1, while anti-CD4 almost completely abolished CTL generation (Fig. 3G). Taken together, these results indicate that although CD4 TRM-like cells induced by IL-7-mFc treatment play a quintessential role in protecting against IAV infection, some innate pulmonary cells, such as pDCs, also contribute to establishing protective immunity.
Table 1. Intranasal administration of IL-7-mFc induces expansion of innate immune cells at the lung.
| Type of Cells | Day 0 | Day 7 | Day 14 | Day 21 |
|---|---|---|---|---|
| Monocytes (CD11b+CD11c−IAIE−F4/80-Ly6c+) | 0.557±0.039 | 0.928±0.093** | 0.445±0.033 | 0.369±0.047* |
| Neutrophils (CD11b+Ly6g+Ly6c−F4/80−) | 0.377±0.081 | 1.452±0.133*** | 0.233±0.035 | 0.231±0.022 |
| Eosinophil (CD11b+CD11cintSiglecF+IAIE−) | 0.127±0.027 | 1.629±0.280*** | 0.631±0.074*** | 0.238±0.142 |
| Inflammatory Macrophages (CD11c+CD11b+IAIE+F4/80+Ly6c+) | 0.189±0.021 | 0.411±0.072* | 0.138±0.012 | 0.152±0.012 |
| Alveolar macrophage (CD11b+CD11c+SiglecF+IAIE+F4/80+) | 0.627±0.057 | 0.235±0.044*** | 0.357±0.026*** | 0.520±0.037 |
| Plasmacytoid DC (B220+CD19−PDCA-1+CD11c+) | 0.034±0.004 | 0.144±0.010*** | 0.044±0.009 | 0.047±0.009 |
Mice (BALB/c, n=5 per group) were received 1 μg of IL-7-mFc i.n. at 0, 7, 14, 21 days prior to the sacrifice. Absolute number of immune cells in the total lung homogenate at each indicated time point were calculated by population ratio and total cell number with flow cytometry. Results are representative of two independent experiments and expressed as mean±standard error of mean.
*p<0.05, **p<0.01, ***p<0.001 by student's t-test compared with day 0.
Figure 3.
Role of pDCs in IL-7-mFc-mediated protection against IAV. Mice (BALB/c, n=6) were treated with 200 μg of anti-PDCA-1, anti-CD4 antibody, or IgG control (D-1, D0, D4, D7) after IL-7-mFc treatment and challenge. (A-C) Representative plots and absolute number of pDCs and other myeloid cells in the lung of IL-7-mFc-treated mice measured 9 dpi. (D) Mice were challenged with a lethal dose of H5N2. Weight and survival of the mice were monitored daily. Absolute number of neutrophils (E) in the lung of IL-7-mFc treated mice and inflammatory cytokines and chemokines (F) at 9 dpi were also measured in the BALF. (G) Antigen-specific CD8+ T cell response was assessed by intracellular cytokine staining of IFNγ after HA529–543 stimulation. Results are representative of 2 independent experiment and expressed as the mean±standard error of mean.
*p<0.05, **p<0.01, ***p<0.001; ††p<0.01 by log-rank test.
Aside from the mucosal T cells, we observed increases in some pulmonary innate immune cells, including pDCs, which showed a clear difference compared to neutrophils. Mucosal innate immune cells play crucial roles in protecting against respiratory virus infection. Although there are reports suggesting a pathological role of pDCs in IAV infection (19), the previous study by Soloff et al. (20) showed that pDC ablation resulted in increased inflammatory cytokine production from cDCs and exudate macrophages, indicating a suppressive effect of pDCs on the inflammatory response to influenza infection in the lung (19). To reduce the immunopathology, pDCs can present viral antigens to induce antigen-specific CD8 T cells for an anti-viral immune response, as previously described (19,20). Furthermore, it is possible that pDCs directly reduce the immunopathology by producing type I IFN (21), since type I IFNs limit IAV-induced pulmonary inflammation by direct resolution of the viral load and production of IL-10 (22). In addition, virus-activated pDCs were reported to induce the differentiation of IL-10- and transforming growth factor (TGF)-β1-producing regulatory T (Treg) cells (23); therefore, pDCs might play a similar regulatory role in IAV infection. Taken together, treatment with IL-7-mFc has multiple effects to balance the innate immunity against IAV infection, as well as modulate the pulmonary T cell response.
ACKNOWLEDGEMENTS
We thank Young Ki Choi (Chungbuk National University, College of Medicine, Cheongju, Korea) for providing influenza virus and Man Ki Song for organizing the challenge experiment at the International Vaccine institute.
This work was supported in part by the Ministry of Science, ICT and Future Planning (2017M3A9C8033570), the Korea Health Technology R&D Project through the Korea Health funded by the Ministry of Health & Welfare, Republic of Korea (HI14C2640), the grant from Cooperative Research Program for Agriculture Science and Technology Development under project number PJ011316 (Rural Development Administration, Republic of Korea), Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (116011-03-2-SB010), andBK21 Plus funded by the Ministry of Education, Korea (10Z20130012243).
Abbreviations
- BALF
bronchoalveolar lavage fluid
- CTL
cytotoxic T lymphocyte
- i.n.
intranasally
- i.p.
intraperitoneally
- IAV
Influenza A virus
- IFN
interferon
- IL-7-mFc
Fc-fused interleukin-7
- LN
lymph node
- pDC
plasmacytoid dendritic cell
- PDCA-1
plasmacytoid dendritic cell antigen-1
Footnotes
Conflict of Interest: The authors declare no competing conflicts of interest.
- Conceptualization: Kang MC, Choi DH, Lee SW, Sung YC.
- Data curation: Kang MC, Park HW.
- Formal analysis: Kang MC, Choi DH, Choi YW, Park Y.
- Funding acquisition: Lee SW, Sung YC.
- Supervision: Lee SW, Sung YC.
- Writing - original draft: Kang MC, Park HW, Lee SW.
- Writing - review & editing: Kang MC, Park HW, Lee SW.
References
- 1.Cowling BJ, Jin L, Lau EH, Liao Q, Wu P, Jiang H, Tsang TK, Zheng J, Fang VJ, Chang Z, et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet. 2013;382:129–137. doi: 10.1016/S0140-6736(13)61171-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. 2008;26(Suppl 4):D49–D53. doi: 10.1016/j.vaccine.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurt AC, Ernest J, Deng YM, Iannello P, Besselaar TG, Birch C, Buchy P, Chittaganpitch M, Chiu SC, Dwyer D, et al. Emergence and spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antiviral Res. 2009;83:90–93. doi: 10.1016/j.antiviral.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson I, Nicholson KG. Influenza: vaccination and treatment. Eur Respir J. 2001;17:1282–1293. doi: 10.1183/09031936.01.00084301. [DOI] [PubMed] [Google Scholar]
- 5.Jegaskanda S, Reading PC, Kent SJ. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J Immunol. 2014;193:469–475. doi: 10.4049/jimmunol.1400432. [DOI] [PubMed] [Google Scholar]
- 6.Pica N, Palese P. Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med. 2013;64:189–202. doi: 10.1146/annurev-med-120611-145115. [DOI] [PubMed] [Google Scholar]
- 7.Shane HL, Klonowski KD. Every breath you take: the impact of environment on resident memory CD8 T cells in the lung. Front Immunol. 2014;5:320. doi: 10.3389/fimmu.2014.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damjanovic D, Small CL, Jeyanathan M, McCormick S, Xing Z. Immunopathology in influenza virus infection: uncoupling the friend from foe. Clin Immunol. 2012;144:57–69. doi: 10.1016/j.clim.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Kuiken T, Riteau B, Fouchier RA, Rimmelzwaan GF. Pathogenesis of influenza virus infections: the good, the bad and the ugly. Curr Opin Virol. 2012;2:276–286. doi: 10.1016/j.coviro.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Brandes M, Klauschen F, Kuchen S, Germain RN. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell. 2013;154:197–212. doi: 10.1016/j.cell.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tate MD, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One. 2011;6:e17618. doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji H, Gu Q, Chen LL, Xu K, Ling X, Bao CJ, Tang FY, Qi X, Wu YQ, Ai J, et al. Epidemiological and clinical characteristics and risk factors for death of patients with avian influenza A H7N9 virus infection from Jiangsu Province, Eastern China. PLoS One. 2014;9:e89581. doi: 10.1371/journal.pone.0089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maines TR, Szretter KJ, Perrone L, Belser JA, Bright RA, Zeng H, Tumpey TM, Katz JM. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol Rev. 2008;225:68–84. doi: 10.1111/j.1600-065X.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 15.Kang MC, Choi DH, Choi YW, Park SJ, Namkoong H, Park KS, Ahn SS, Surh CD, Yoon SW, Kim DJ, et al. Intranasal introduction of Fc-fused interleukin-7 provides long-lasting prophylaxis against lethal influenza virus infection. J Virol. 2015;90:2273–2284. doi: 10.1128/JVI.02768-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam HJ, Song MY, Choi DH, Yang SH, Jin HT, Sung YC. Marked enhancement of antigen-specific T-cell responses by IL-7-fused nonlytic, but not lytic, Fc as a genetic adjuvant. Eur J Immunol. 2010;40:351–358. doi: 10.1002/eji.200939271. [DOI] [PubMed] [Google Scholar]
- 17.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langlois RA, Legge KL. Plasmacytoid dendritic cells enhance mortality during lethal influenza infections by eliminating virus-specific CD8 T cells. J Immunol. 2010;184:4440–4446. doi: 10.4049/jimmunol.0902984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soloff AC, Weirback HK, Ross TM, Barratt-Boyes SM. Plasmacytoid dendritic cell depletion leads to an enhanced mononuclear phagocyte response in lungs of mice with lethal influenza virus infection. Comp Immunol Microbiol Infect Dis. 2012;35:309–317. doi: 10.1016/j.cimid.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jewell NA, Vaghefi N, Mertz SE, Akter P, Peebles RS, Jr, Bakaletz LO, Durbin RK, Flaño E, Durbin JE. Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo. J Virol. 2007;81:9790–9800. doi: 10.1128/JVI.00530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arimori Y, Nakamura R, Yamada H, Shibata K, Maeda N, Kase T, Yoshikai Y. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral Res. 2013;99:230–237. doi: 10.1016/j.antiviral.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Hong J, Gong ZJ. Human plasmacytoid dendritic cells from patients with chronic hepatitis B virus infection induce the generation of a higher proportion of CD4(+) and CD25(+) regulatory T cells compared with healthy patients. Hepatol Res. 2008;38:362–373. doi: 10.1111/j.1872-034X.2007.00279.x. [DOI] [PubMed] [Google Scholar]