Abstract
Purpose
Improvement of cure rates for patients treated with allogeneic hematopoietic stem-cell transplantation (HSCT) will require efforts to decrease treatment-related mortality from severe viral infections. Adoptively transferred virus-specific T cells (VSTs) generated from eligible, third-party donors could provide broad antiviral protection to recipients of HSCT as an immediately available off-the-shelf product.
Patient and Methods
We generated a bank of VSTs that recognized five common viral pathogens: Epstein-Barr virus (EBV), adenovirus (AdV), cytomegalovirus (CMV), BK virus (BKV), and human herpesvirus 6 (HHV-6). The VSTs were administered to 38 patients with 45 infections in a phase II clinical trial.
Results
A single infusion produced a cumulative complete or partial response rate of 92% (95% CI, 78.1% to 98.3%) overall and the following rates by virus: 100% for BKV (n = 16), 94% for CMV (n = 17), 71% for AdV (n = 7), 100% for EBV (n = 2), and 67% for HHV-6 (n = 3). Clinical benefit was achieved in 31 patients treated for one infection and in seven patients treated for multiple coincident infections. Thirteen of 14 patients treated for BKV-associated hemorrhagic cystitis experienced complete resolution of gross hematuria by week 6. Infusions were safe, and only two occurrences of de novo graft-versus host disease (grade 1) were observed. VST tracking by epitope profiling revealed persistence of functional VSTs of third-party origin for up to 12 weeks.
Conclusion
The use of banked VSTs is a feasible, safe, and effective approach to treat severe and drug-refractory infections after HSCT, including infections from two viruses (BKV and HHV-6) that had never been targeted previously with an off-the-shelf product. Furthermore, the multispecificity of the VSTs ensures extensive antiviral coverage, which facilitates the treatment of patients with multiple infections.
INTRODUCTION
Viral infections remain a major cause of post-transplantation morbidity and mortality in recipients of allogeneic hematopoietic stem-cell transplantation (HSCT), which adds substantially to the clinical and financial burden of transplantation.1-6 Though pharmacologic agents are available for some clinically problematic viruses, they are not always effective and can result in significant adverse effects. In contrast, the adoptive transfer of stem-cell donor-derived virus-specific T cells (VSTs) has shown efficacy for the treatment of viral pathogens.7-18 However, broader implementation of this therapeutic approach is limited by (1) the cost and complexity of individualized product manufacture, (2) the time needed for custom manufacturing, which may preclude the immediate availability of VSTs for urgent medical need, and (3) the requirement for seropositive donors—an issue of growing importance given the increasing use of younger, virus-naïve donors and cord blood as a source of stem cells.
One way to overcome these limitations and to supply antiviral protection to recipients of allogeneic HSCT would be to prepare and cryopreserve banks of VST lines from healthy seropositive donors, which would be available for immediate use as an off-the-shelf product. Promising results with this approach were first achieved with Epstein-Barr virus (EBV)–specific T cells for the treatment of EBV post-transplantation proliferative disorder19-21; our group and others extended the viral target range to include cytomegalovirus (CMV) and adenovirus (AdV).22,23 However, it was unknown whether banked VSTs would be effective against human herpesvirus 6 (HHV-6) and BK virus (BKV)—both frequent causes of morbidity and mortality that lack effective therapies.24 It was also unknown whether additional T-cell specificities for these two viruses could be incorporated into a multiple-virus–specific cell product. Therefore, we generated banks of pentavalent T-cell lines specific for 12 viral antigens from EBV, CMV, AdV, HHV-6, and BKV and administered them to 38 recipients of allogeneic HSCT with drug-refractory infections or diseases associated with all five viruses in a phase II clinical trial.
PATIENTS AND METHODS
Third-Party VST Bank
A total of 59 VST lines were manufactured and characterized by flow cytometry and virus specificity by interferon gamma (IFNγ) enzyme-linked immunospot (ELIspot) assay, as previously described.13 Lines were specific for the viral antigens hexon and penton (for AdV); IE1 and pp65 (for CMV); EBNA1, LMP2, and BZLF1 (for EBV); VP1 and large T (for BKV); and U11, U14 and U90 (for HHV-6). The selection of VST lines for infusion was based on the specificity of the line for the target virus through shared HLA alleles and the overall level of HLA match; the specificity through shared HLA alleles criterion took precedence.
Clinical Trial Design
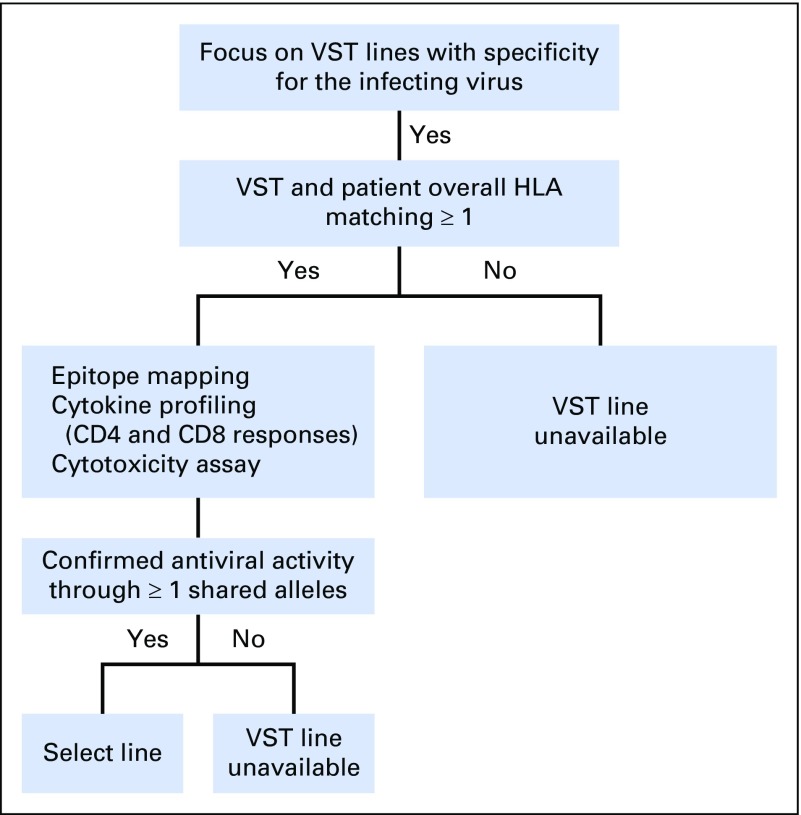
The phase II study was approved by the US Food and Drug Administration and the Baylor College of Medicine institutional review board. Patients initially gave their consent to search for a suitable VST line. If a line was available, on the basis of the selection criteria (Appendix Fig A1, online only), and if patients met eligibility criteria (Appendix Table A1, online only), they could consent to treatment and receive a single intravenous infusion of 2 × 107 partially HLA-matched VSTs/m2 with the option to receive a second infusion after 4 weeks and additional infusions at biweekly intervals thereafter. Therapy with standard antiviral medications could be continued at the discretion of the treating physician.
Safety End Points
Safety end points included acute grade 3 to 4 graft-versus-host disease (GVHD) within 42 days of the last dose of VSTs, infusion-related toxicities within 24 hours of infusion, and grade 3 to 5 nonhematologic adverse events related to the VSTs within 30 days of the last VST dose. Patients were also monitored for chronic GVHD for 12 months. Toxicities were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Clinical and Virologic End Points
Viral loads were monitored by quantitative polymerase chain reaction in laboratories approved by the Clinical Laboratory Improvement Amendments program. Clinical and virologic responses were assigned at week 6 (Appendix Table A2, online only). Clinical responses in individuals treated for BKV hemorrhagic cystitis (HC) were evaluated on the basis of clinical and laboratory documentation by three independent HSCT physicians (two were blinded) according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0, and also were graded on the grading system proposed by Bedi et al25 (Appendix Table A3, online only).
Immune Monitoring
IFNγ ELIspot analysis was used to determine the frequency of circulating T cells specific for viral antigens and peptides.13 When available, individual HLA-restricted epitope peptides (Genemed, San Antonio, TX) were used to track donor-derived VSTs postinfusion.26-36
Statistical Analysis
Descriptive statistics were calculated to summarize clinical characteristics. Comparisons were made between groups by using the nonparametric Wilcoxon rank sum test or Kruskal-Wallis exact test for continuous variables and the Fisher’s exact test for categoric variables.
RESULTS
Patients
We screened 56 recipients of allogeneic HSCT and identified a suitable VST line for 54 patients (96.6%). Of these, 16 did not receive VSTs, either because intervention was considered not required (n = 4) or because the patients were ineligible (n = 12; Appendix Table A4, online only). Of the 38 patients who were treated (Table 1), 31 received cells to treat a single virus, and seven patients were treated for two viral infections (Table 2). A total of 23 patients received a single infusion, 11 patients had two infusions, and four patients had three infusions of VSTs.
Table 1.
Patient Demographic and Clinical Characteristics
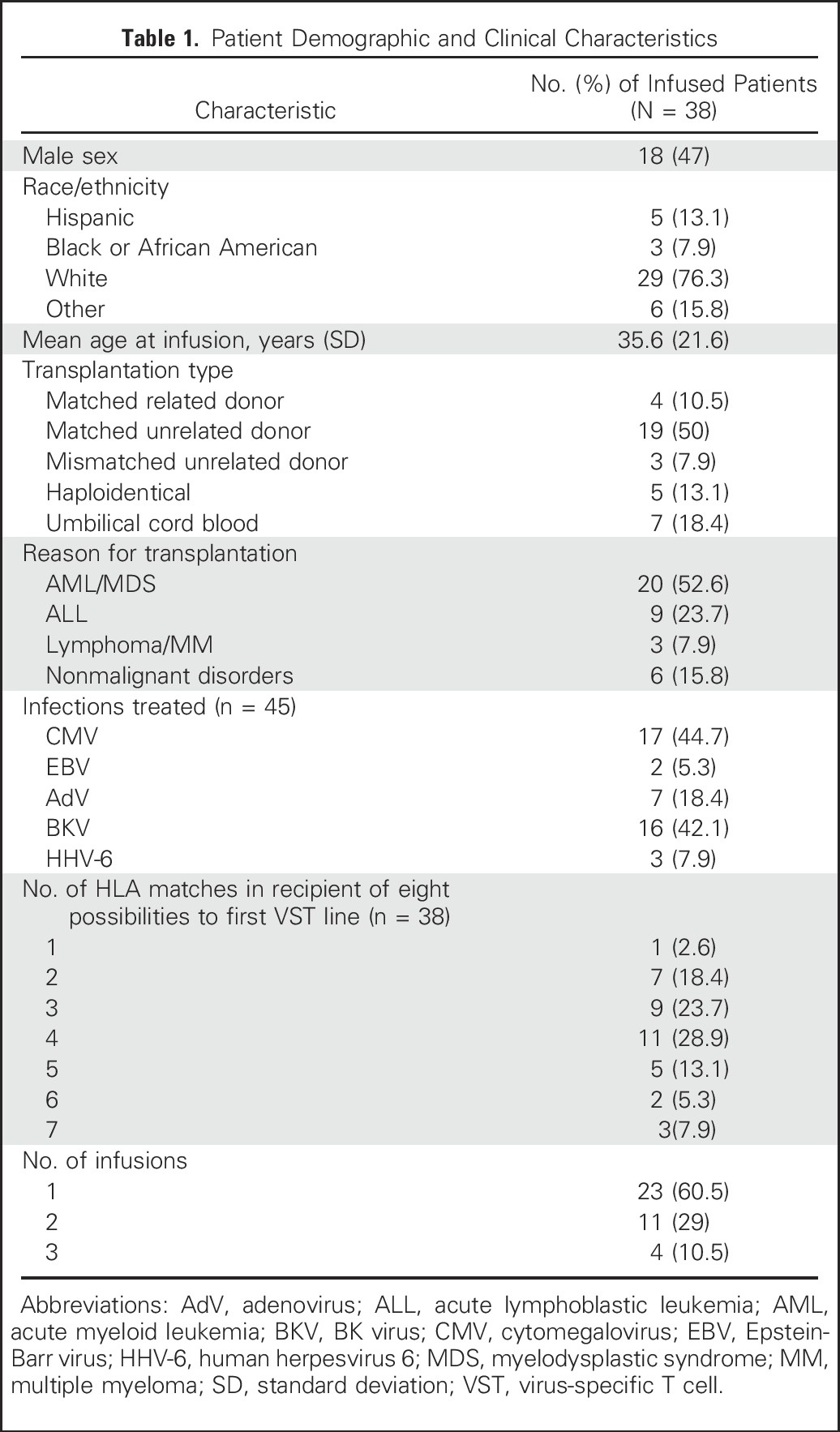
Table 2.
Outcomes After Treatment With Banked VSTs
VST Line Characteristics
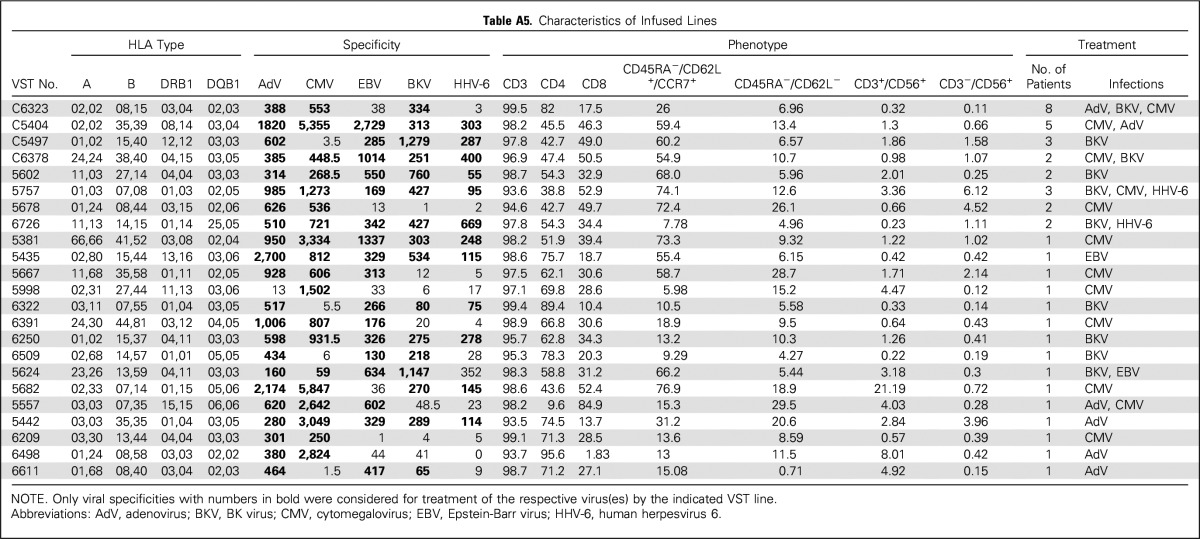
From the bank of 59 VST lines, 23 were administered to one to eight patients, with matching at one of eight to seven of eight HLA alleles (Appendix Table A5, online only). The infused cells were almost exclusively CD3+ T cells (mean ± SEM, 97.3% ± 0.4%). A mixture of CD4+ (60.4% ± 3.9%) and CD8+ (34.2% ± 3.6%) subsets expressed both central (CD45RA−/62L+/CCR7+: 39.1% ± 5.5%) and effector (CD45RA−/62L−/CCR7−: 11.8% ± 1.6%) memory markers and recognized the target viruses.
Clinical Responses
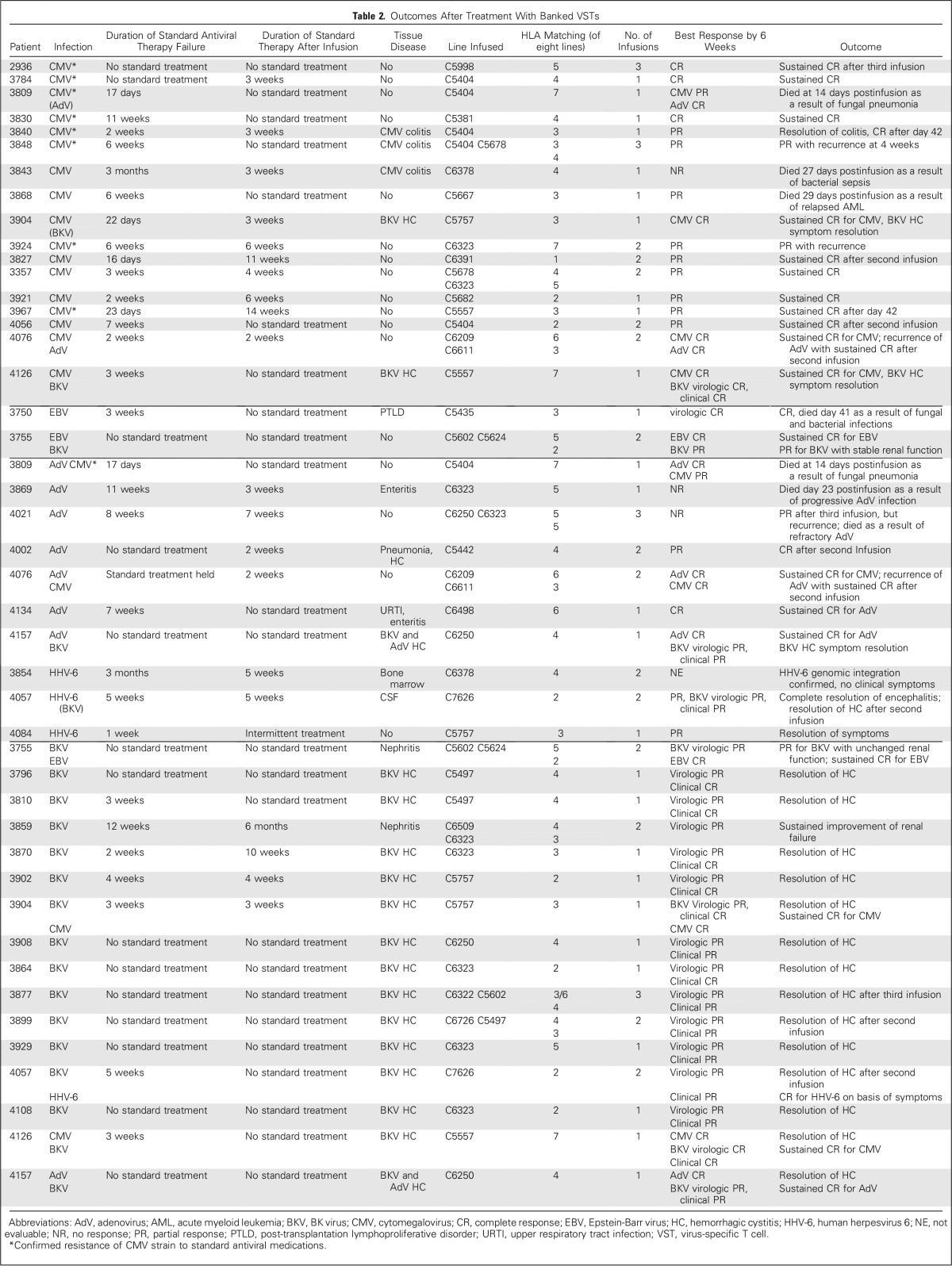
The cumulative clinical response rate in 37 evaluable patients was 91.9% (95% CI, 78.1% to 98.3%) after one VST infusion by week 6 (Fig 1A). Of 18 patients who were screened but did not receive VST therapy, 12 developed progressive disease (Appendix Table A4).
Fig 1.
Cumulative incidence response rate that is based on time to first complete response and/or partial response in (A) overall patients (n = 37); one patient with nonevaluable data was excluded; (B) patients with cytomegalovirus infections (n = 17); (C) patients with adenovirus infections (n = 7), and (D) patients with BK virus infections (n = 16).
CMV.
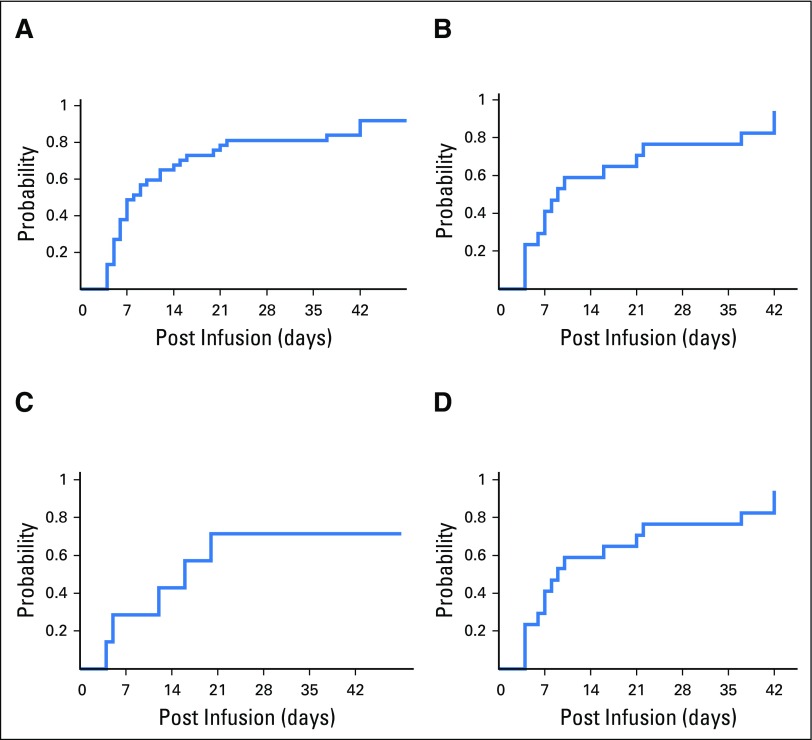
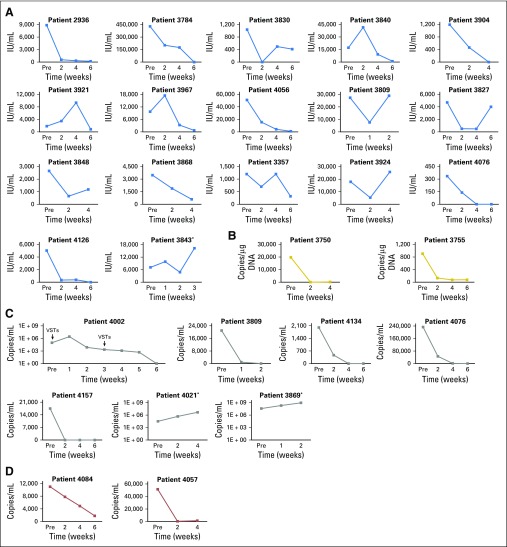
A total of 17 patients received VSTs for persistent CMV (Table 2), which in eight patients was confirmed by CMV gene sequencing to be resistant to conventional antiviral drugs. A total of 16 patients responded to VSTs with six complete responses (CRs) and 10 partial responses (PRs); the cumulative response rate was 94.1% (95% CI, 71.3% to 99.9%; Fig 1B) by week 6. Figure 2A summarizes the outcomes of all patients treated for CMV as assessed by sequential viral load measurement. Clinical benefit was achieved both in patients with refractory infections and in individuals with biopsy-proven CMV colitis (n = 3). Overall, of the 16 patients who responded to VST treatment, nine had a concomitant increase in circulating CMV-reactive T cells and a five-fold mean increase in spot-forming cells (SFCs) from 73 (± 59) SFCs/5 × 105 peripheral blood mononuclear cells (PBMCs) before infusion to 399 (± 141) SFCs/5 × 105 PBMCs after infusion (Fig 3A). For example, patient 2936 had refractory CMV that was resistant to ganciclovir, as confirmed by CMV antiviral resistance sequencing. Within 6 weeks of VST therapy, the virus was undetectable, which coincided with an increase in the frequency of circulating CMV-specific T cells (Appendix Fig A2A, online only). Patient 3840 had refractory CMV that was resistant to foscarnet and ganciclovir, as confirmed by CMV antiviral resistance sequencing, and had CMV inclusions evident on numerous ulcers detected in the ileum and colon (Appendix Fig A2B). Despite 3 weeks of treatment with cidofovir, the CMV titers of this patient continued to increase, which caused worsened abdominal cramping that required high-dose opioid treatment. After VST infusion, the viral titers decreased (Fig 3A) coincident with rapid symptomatic improvement and complete resolution of abdominal cramping without the need for additional narcotics by postinfusion week 4.
Fig 2.
Treatment outcomes in patients infected with cytomegalovirus (CMV), Epstein-Barr virus (EBV), adenovirus, and human herpesvirus 6 (HHV-6). Depiction of plasma viral load before (pre) and after (post) infusion of virus-specific T cells in all patients treated for (A) CMV infection, (B) EBV infection, (C) adenovirus infection, and (D) HHV-6 infection. (*) Nonresponders.
Fig 3.
Frequency of viral-specific T cells (VSTs) in vivo in the peripheral blood before (pre) and after (post) infusion, as measured by interferon gamma enzyme-linked immunospot (ELIspot) assay after stimulation with viral pepmixes. Results are expressed as spot-forming cells (SFCs) per 5 × 105 input cells with specificity for patients with (A) cytomegalovirus, (B) Epstein-Barr virus, (C) adenovirus, (D) human herpesvirus 6, and (E) BK virus. (F-K) Frequency of T cells in peripheral blood as measured by interferon gamma ELIspot assay after stimulation with epitope-specific peptides with restriction to HLA antigens exclusive to the VST line or the recipient, or shared between the two.
EBV.
Both patients treated for EBV achieved a virologic CR by week 6 (Table 2; Fig 2B). The CR in one patient was associated with a concomitant increase in circulating EBV-specific T cells (Fig 3B).
AdV.
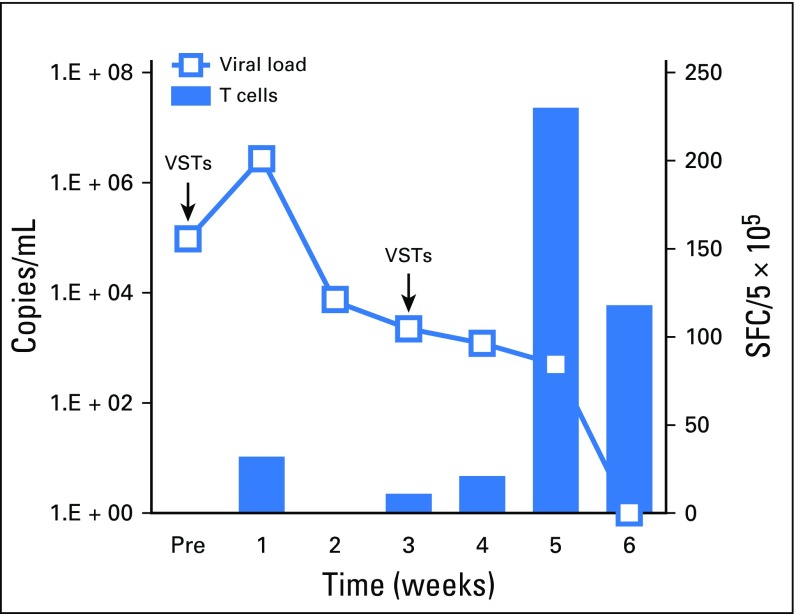
Seven patients received VSTs for persistent AdV (Table 2), which resulted in four CRs, one PR, two nonresponses, and a cumulative response rate of 71.4% (Fig 1C and Fig 2C). Clinical benefit was associated with an increase in the frequency of circulating AdV-specific T cells in four patients (Fig 3C). Responders included one patient (patient 4002) with AdV pneumonitis and HC who experienced a PR—a 93% reduction in viral load after an initial infusion of VSTs followed by complete virologic and clinical remission after a second infusion of the same VST line, with a corresponding increase in AdV-specific T cells (Appendix Fig A3, online only).
HHV-6.
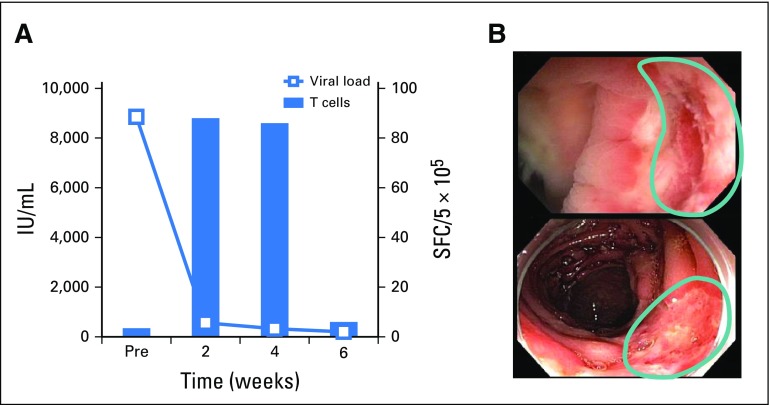
VSTs were infused to three patients to treat HHV-6 reactivations (Table 2), and the response rate was 67% (PRs [n = 2] and not evaluable [n = 1]). Patient 4084 had elevated viral titers along with fevers and symptoms of bone marrow suppression, including neutropenia. After treatment, symptoms resolved and the viral load decreased, which was associated with the detection of HHV-6–specific T cells in the peripheral blood (Fig 2D and Fig 3D). Patient 4057, who presented with decreased alertness and responsiveness, was infused with VSTs to treat refractory HHV-6 encephalitis and BKV HC. Within 24 hours, the patient exhibited improved alertness and sustained normalization of mental status within 1 week, which correlated with a decrease in HHV-6 viral titers from 51,500 to 1,200 copies/mL (Fig 2D). Finally, Patient 3854 received VSTs to treat persistently elevated HHV-6 levels in the absence of clinical symptoms. Though the viral load decreased postinfusion, it was not eliminated, and subsequent investigation confirmed the presence of chromosomally integrated HHV-6, which is associated with indefinitely elevated HHV-6 titers.37 Therefore, given a lack of a clinical or virologic end point, this patient was excluded from additional analysis.
BKV.
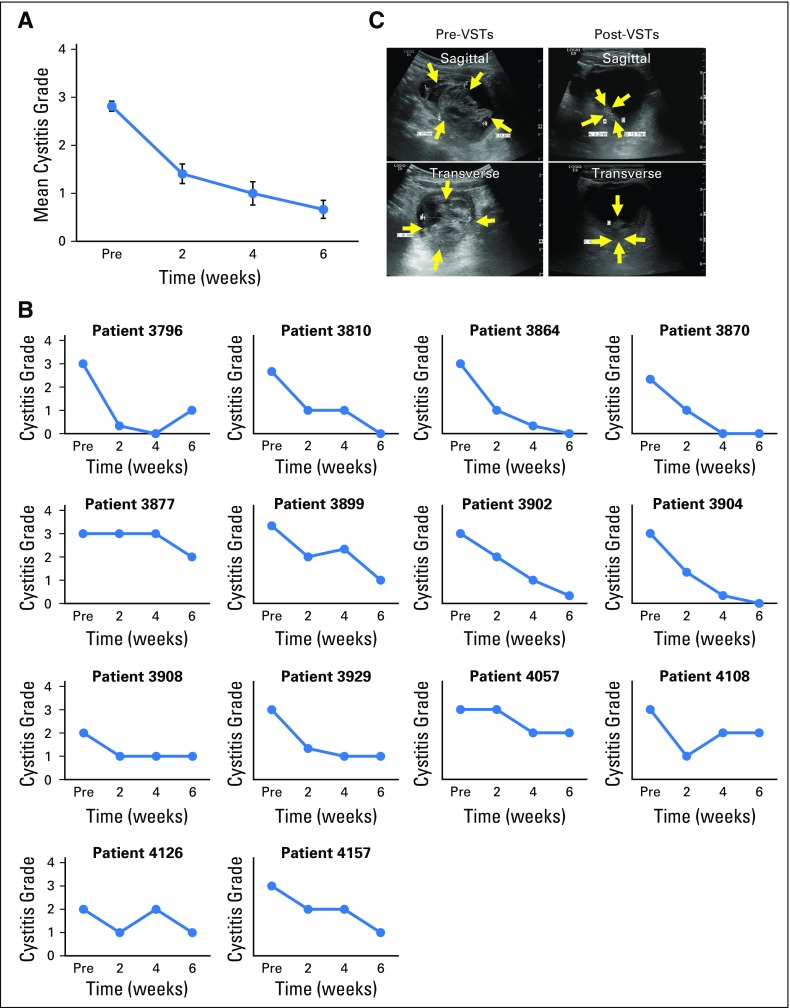
A total of 16 patients with tissue disease (BKV-associated HC [n = 14] and BKV-associated nephritis [n = 2]) were treated with VSTs; all 16 patients (100%; 95% CI, 79.4% to 100%; Fig 1D) achieved clinical benefit (Table 2) associated with a median decrease of 85.5% in urinary viral load by week 6 and of 96% by week 12 postinfusion. Both patients treated for biopsy-proven nephritis responded virologically to VSTs; in one patient, response was associated with a decrease in creatinine from 1.7 mg/dL before infusion to 1.25 mg/dL at week 6 postinfusion and sustained improved renal function. In addition, 13 of the 14 patients with HC had complete resolution of gross hematuria by week 6 postinfusion, but symptomatic improvement was notably more rapid as captured with the National Cancer Institute cystitis grading scale. Before VSTs, these patients with HC exhibited moderate (grade 2, n = 3) to severe (grade 3, n = 11) symptoms and an average cystitis grade of 2.8 ± 0.1. However, within 2 weeks, the symptomatic severity had decreased by at least one grade in 12 of these 14 patients and continued to decline thereafter (Figs 4A and 4B). Seven of the responding patients had a concomitant increase in the frequency of circulating BKV-specific T cells (mean increase, from 13 ± 10 to 245 ± 114 SFCs/5 × 105 PBMCs; Fig 3E). One example is patient 3899, who was in secondary graft failure after HSCT and BKV HC, which precluded him from advancement to a second HSCT because of profound hematuria with significant transfusion requirements. The patient had developed a large bladder clot and was unable to tolerate standard antiviral treatment (Fig 4C, left panel). After VST infusion, his hematuria resolved, the bladder clot decreased by 98% within 3 weeks (reduction, from 66.1 to 1.4 cm3; Fig 4C, right panel), and he successfully proceeded to transplantation.
Fig 4.
Treatment outcomes in BK virus–infected patients (n = 14). BK virus–associated hemorrhagic cystitis symptom score before infusion and at 2, 4, and 6 weeks after infusion of virus-specific T cells (VSTs); the grade is based on the National Cancer Institute hemorrhagic cystitis grading scale. Results are presented as (A) mean (± SEM) grade of all patients and (B) individually for each patient. (C) Example of a patient with BK virus–associated hemorrhagic cystitis. Ultrasound imaging depicts the blood clot in the bladder, shown (left) preinfusion and (right) 3 weeks after infusion.
Dual Infections.
Seven patients received VSTs for two viral infections, and all treatments were able to control both viruses with a single infusion. CMV, AdV, and EBV were cleared in all cases, and all patients with BKV HC and the patient with HHV-6 encephalitis had clinical improvement or disease resolution.
Durability.
Of the 34 patients who achieved a PR or CR after a single infusion of VSTs, seven had a subsequent recurrence (median, 10 weeks), which was successfully treated in six patients. One (n = 5) or two (n = 1) additional infusions provided durable benefit.
Repeat Infusions.
Overall, 15 patients with no response (n = 1), PR (n = 7), or recurrence (n = 7) received a second infusion of the same (n = 8) or a different (n = 7) VST line at a median interval of 39 days (range, 21 to 214 days) after the initial infusion, which produced clinical benefit in 10 patients (77%; CR [n = 1] and PRs [n = 9]). Four patients received a third VST infusion, and three of the patients (75%) responded to therapy (CRs [n = 2], PR [n = 1], and no response [n = 1]).
In Vivo T-Cell Persistence
To evaluate the in vivo longevity of these partially HLA-matched VSTs, we interrogated the specificity of circulating T-cells postinfusion, and we discriminated between infused versus endogenous cells on the basis of peptide-epitope specificity in 16 clinical responders with adequate PBMC numbers and available reagents (Appendix Table A6, online only). In 11 patients (69%), we confirmed the persistence of VSTs from the infused line for up to 12 weeks (Figs 3F to 3K). For example, Figure 3F shows the longitudinal analysis of T-cell responses in patient 3929, who received VST line C6323 to treat persistent BKV HC. At 2 weeks post-infusion the dominant detectable specificities represented epitopes presented in the context of alleles that were either unique to the infused VST line or shared between the line and patient. Over time, the frequency of third-party–derived specificities declined, coincident with endogenous immune reconstitution. Figures 3G through 3K show similar patterns of activity in other VST responders.
In patient 3864, short tandem repeats analysis was performed in addition to epitope profiling (Fig 3H), which showed that third-party VSTs comprised 4% of all circulating T cells after peak expansion and persisted for a total of 12 weeks until BKV clearance.
Clinical Safety
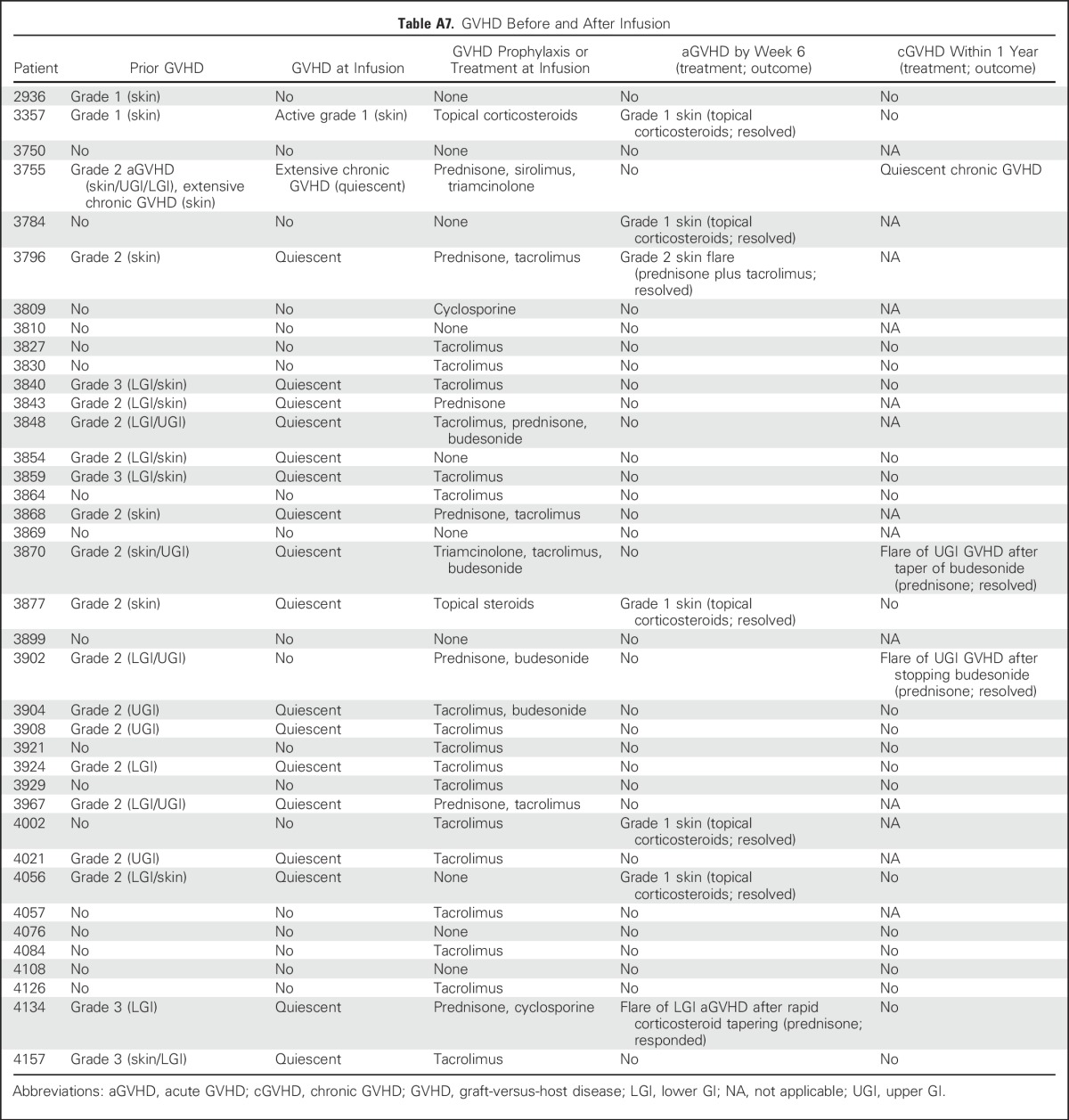
All infusions were well tolerated. Except for one patient who developed an isolated fever within 24 hours of infusion, no immediate toxicities were observed. None of the patients developed cytokine release syndrome. Nineteen patients (50%) had prior grade 2 to 4 GVHD (grade 2, n = 15; grade 3, n = 4), which was quiescent at the time of VST infusion (Appendix Table A7, online only). After infusion, one patient developed recurrent grade 3 GI GVHD after rapid corticosteroid taper, and five patients developed recurrent (n = 3) or de novo (n = 2) grade 1 to 2 skin GVHD, which resolved with the administration of topical treatments (n = 4) and reinitiation of corticosteroid treatment after a taper (n = 1). In long-term follow-up, two patients had a flare of upper-GI GVHD, which resolved after a brief corticosteroid course. Finally, one patient who received VSTs as treatment of BKV HC experienced transient hydronephrosis and a decrease in renal function associated with a concomitant bacterial urinary tract infection that resolved within 2 weeks.
DISCUSSION
In this phase II study, we administered third-party VSTs to recipients of allogeneic HSCT with drug-refractory infections associated with five of the most frequent infectious causes of post-transplantation morbidity and mortality. We could identify a suitable VST line for 54 of the 56 patients screened for study participation, of whom 38 were infused. Before study entry, the majority of these patients had experienced failure of at least two lines of conventional agents or were infected with drug-resistant viral variants; nevertheless, VSTs produced a 92% overall CR or PR rate (CMV, 94%; BKV, 100%; EBV, 100%; AdV, 71%; and HHV-6, 67%), which included responses in all seven patients who had multiple coexisting infections. To our knowledge, this trial is the first to extend the use of banked VSTs to five viruses, including BKV and HHV-6.
In recipients of HSCT, viral reactivations of BKV are frequent and are associated with severe pain, hematuria, and renal disease.4,38,39 No antiviral drugs for BKV have been approved or tested in randomized clinical trials. Cidofovir, which is most often administered to treat BK-associated HC, is associated with major drug-related adverse effects that include myelotoxicity and nephrotoxicity; cidofovir is frequently ineffective and offers a survival rate of just 14% in the third of patients whose disease fails to respond.4,40 In this study, we treated 16 patients with BKV disease without significant treatment-related toxicities, and benefit—including complete resolution of gross hematuria in 13 of 14 patients with BKV-associated HC by week 6 postinfusion—was achieved in all patients. In contrast, four of five patients with HC who were screened for study participation but not treated with VSTs experienced disease progression. In some patients, symptomatic improvement of HC without reduction in BK viruria was observed, which is consistent with previous reports that virus titers in urine in patients with significant gross hematuria show no clear association with symptoms38 and that asymptomatic viremia or viruria is not necessarily clinically relevant.41-43
Both evaluable patients treated for HHV-6, one of whom had neurologic impairment caused by HHV-6 encephalitis, responded to VSTs. There was a decrease in HHV-6 levels and resolution of clinical symptoms in each case.
Incorporation of these additional specificities into multiple-virus VSTs did not compromise activity against CMV, AdV, or EBV. When antiviral activities of the new pentavalent and previous triple-virus lines were compared, the magnitude of the in vitro response for these viruses was similar. Not surprisingly, therefore, clinical benefit was maintained. Indeed, in our previous phase II study, response rates for CMV, AdV, and EBV were 74%, 78%, and 67%, respectively, in patients with similar disease characteristics; response rates were 94%, 71%, and 100%, respectively, in this study. We found no correlation between viral load reduction and HLA matching in patients who received low (one to three alleles matched) versus high (four to eight matching alleles) HLA-matched VST lines (P = .961). Indeed, a CR was achieved in a patient treated with a VST line matched at just a single HLA allele, with strong antiviral activity through this shared allele, which highlights the importance of careful VST line selection. In this study, the majority of responders had a detectable expansion in the frequency of circulating VSTs postinfusion. The epitope profiling method enabled us to track the presence of functional VSTs of third-party origin over time, which persisted for up to 12 weeks, in 11 patients. Third-party T cells predominated immediately postinfusion, but this early expansion did not come at the expense of increased alloreactivity; we observed only two occurrences of (mild) de novo GVHD. Thereafter, the numbers declined coincident both with resolution of viral infection and with endogenous T-cell recovery.
Although a randomized trial will be required to definitively assess the value of banked VSTs, this study strongly suggests that off-the-shelf multiple-virus–directed VSTs are a safe and effective broad-spectrum approach to treat severe viral infections after HSCT. These VSTs can be rapidly and cost-effectively produced in scalable quantities with excellent long-term stability, which facilitates the broad implementation of this therapy. More widespread and earlier use of this modality could minimize both drug-related and virus-associated complications and thereby decrease treatment-related mortality in recipients of allogeneic HSCT.
ACKNOWLEDGMENT
We thank all those who participated in this trial; the clinicians, their staff, and their patients: Amy Reyna for study coordination; Sara Richman and Deborah Lyon for quality assurance and quality control; Huimin Zhang and her team for cell processing; and Bridget Medina for regulatory assistance.
Appendix
Fig A1.
Decision algorithm for virus-specific T-cell (VST) selection.
Fig A2.
Examples of patients infected with cytomegalovirus (CMV): (A) Patient 2936 with CMV infection. Viral load (left y-axis) and frequency of CMV-directed T cells in peripheral blood (right y-axis) before infusion and at 2, 4, and 6 weeks after infusion as measured by interferon gamma enzyme-linked immunospot assay. (B) Endoscopic picture of patient 3840 with CMV colitis that depicts ulcers (in circled areas) in the ileum and colon before infusion. SFC, spot-forming cell.
Fig A3.
Example of a patient infected with adenovirus; patient 4002 with adenovirus respiratory tract infection; plasma viral load (left y-axis) and frequency of virus-specific T cells (VSTs; right y-axis) in peripheral blood over time. SFC, spot-forming cell.
Table A1.
Eligibility Criteria
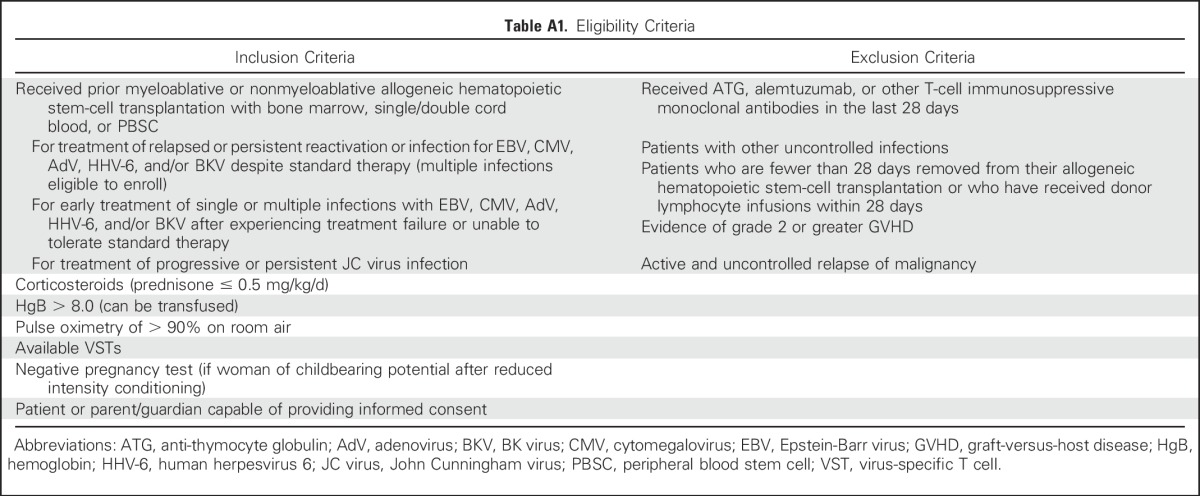
Table A2.
Definitions of Response
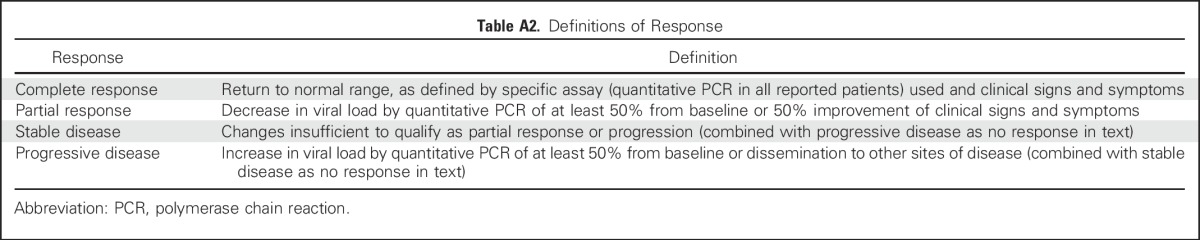
Table A3.
Cystitis Grading Scale
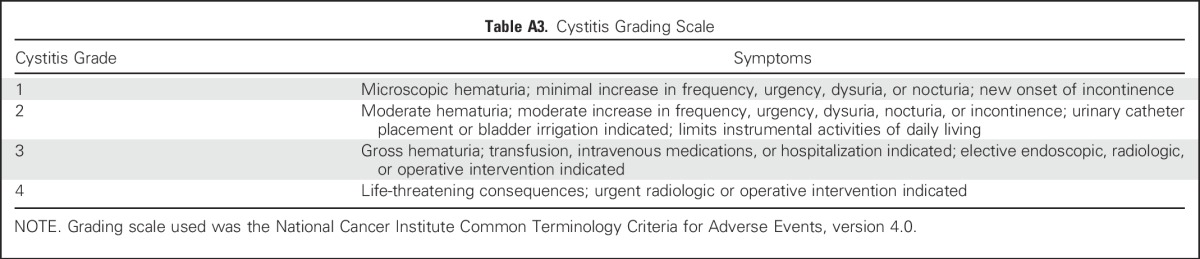
Table A4.
Outcome of Patients Who Did Not Receive VSTs
Table A5.
Characteristics of Infused Lines
Table A6.
VST Tracking Studies in Patients With and Without Detectable Third-Party VSTs
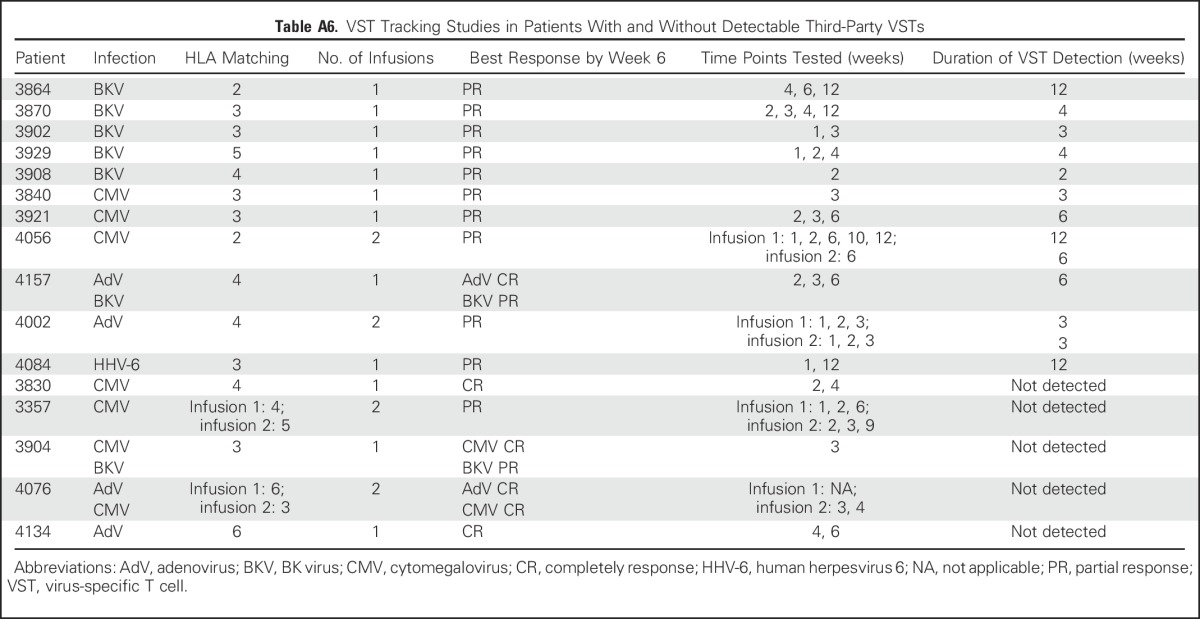
Table A7.
GVHD Before and After Infusion
Footnotes
Supported by a National Heart, Lung, and Blood Institute Production Assistance for Cellular Therapy grant (to B.O. for the clinical virus-specific T-cell manufacture of this investigator-initiated study); by a Conquer Cancer Foundation/American Society for Clinical Oncology grant (to B.O. for correlative studies); and by Dan L. Duncan Cancer Center Support Grant No. P30CA125123 (for shared resources). B.O. was supported by an educational National Institutes of Health K12 faculty fellowship grant at Texas Children’s Hospital.
The funding sources had no role in trial design, data collection, interpretation of data, or decision to publish.
Clinical trial information: NCT02108522.
Listen to the podcast by Dr Nikiforow at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Helen E. Heslop, Ann M. Leen, Bilal Omer
Administrative support: Bambi J. Grilley
Collection and assembly of data: Ifigeneia Tzannou, Anastasia Papadopoulou, Swati Naik, Kathryn Leung, Caridad A. Martinez, Carlos A. Ramos, George Carrum, Ghadir Sasa, Ayumi Watanabe, Manik Kuvalekar, Adrian P. Gee, Bambi J. Grilley, Robert A. Krance, Ann M. Leen, Bilal Omer
Data analysis and interpretation: Ifigeneia Tzannou, Swati Naik, Kathryn Leung, Caridad A. Martinez, George Carrum, Premal Lulla, Meng-Fen Wu, Hao Liu, Stephen Gottschalk, Malcolm K. Brenner, Cliona M. Rooney, Helen E. Heslop, Ann M. Leen, Bilal Omer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Off-the-Shelf Virus-Specific T Cells to Treat BK virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Ifigeneia Tzannou
Consulting or Advisory Role: Viracyte
Anastasia Papadopoulou
No relationship to disclose
Swati Naik
Travel, Accommodations, Expenses: Bellicum Pharmaceuticals
Kathryn Leung
No relationship to disclose
Caridad A. Martinez
No relationship to disclose
Carlos A. Ramos
Consulting or Advisory Role: Novartis
Research Funding: Tessa Therapeutics
Patents, Royalties, Other Intellectual Property: Tessa Therapeutics
George Carrum
No relationship to disclose
Ghadir Sasa
No relationship to disclose
Premal Lulla
No relationship to disclose
Ayumi Watanabe
No relationship to disclose
Manik Kuvalekar
No relationship to disclose
Adrian P. Gee
No relationship to disclose
Meng-Fen Wu
No relationship to disclose
Hao Liu
No relationship to disclose
Bambi J. Grilley
Consulting or Advisory Role: Lokon, Viracyte (through QB Regulatory)
Other Relationship: QB Regulatory
Robert A. Krance
No relationship to disclose
Stephen Gottschalk
Consulting or Advisory Role: AbbVie, Celgene, Merrimack, GlaxoSmithKline, Servier
Patents, Royalties, Other Intellectual Property: Filed patent applications focused on cell therapy for cancer (8; Inst), on oncolytic viruses (1; Inst), and on cancer gene therapy (1; Inst)
Malcolm K. Brenner
Stock or Other Ownership: Bluebird Bio, Tessa Therapeutics, Viracyte, Viracyte (I), Marker Therapeutics, Marker Therapeutics (I)
Consulting or Advisory Role: Cell Medica, Formula Pharmaceuticals, Nankwest, Unum Therapeutics, Poseida, Bluebird Bio
Research Funding: Celgene, Tessa Therapeutics
Patents, Royalties, Other Intellectual Property: Patents in the field of cellular immunotherapy, Patents in the field of cellular immunotherapy (I)
Cliona M. Rooney
Stock or Other Ownership: Tessa Therapeutics, Bluebird Bio
Consulting or Advisory Role: Cell Medica, Tessa Therapeutics
Research Funding: Tessa Therapeutics
Patents, Royalties, Other Intellectual Property: Patents in the field of cellular immunotherapy
Helen E. Heslop
Stock or Other Ownership: Viracyte, Marker Therapeutics
Consulting or Advisory Role: Chimerix
Research Funding: Celgene (Inst), Cell Medica (Inst)
Patents, Royalties, Other Intellectual Property: Cell Medica
Ann M. Leen
Stock or Other Ownership: Viracyte, Marker Therapeutics
Honoraria: Cell Medica
Patents, Royalties, Other Intellectual Property: Patents in the field of cell therapy (received royalty payments)
Travel, Accommodations, Expenses: Cell Medical, Viracyte
Bilal Omer
Patents, Royalties, Other Intellectual Property: One patent pending in the field of cell therapy
REFERENCES
- 1.Locatelli F, Crotta A, Ruggeri A, et al. : Analysis of risk factors influencing outcomes after cord blood transplantation in children with juvenile myelomonocytic leukemia: A EUROCORD, EBMT, EWOG-MDS, CIBMTR study. Blood 122:2135-2141, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gratwohl A, Brand R, Frassoni F, et al. : Cause of death after allogeneic hematopoietic stem cell transplantation (HSCT) in early leukaemias: An EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant 36:757-769, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Styczynski J, Einsele H, Gil L, et al. : Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: A comprehensive review of reported cases. Transpl Infect Dis 11:383-392, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Gilis L, Morisset S, Billaud G, et al. : High burden of BK virus-associated hemorrhagic cystitis in patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 49:664-670, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Jain NA, Lu K, Ito S, et al. : The clinical and financial burden of pre-emptive management of cytomegalovirus disease after allogeneic stem cell transplantation: Implications for preventative treatment approaches. Cytotherapy 16:927-933, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broers AE, van Der Holt R, van Esser JW, et al. : Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell–depleted stem cell transplantation. Blood 95:2240-2245, 2000 [PubMed] [Google Scholar]
- 7.Tzannou I, Leen AM: Preventing stem cell transplantation-associated viral infections using T-cell therapy. Immunotherapy 7:793-810, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heslop HE, Slobod KS, Pule MA, et al. : Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 115:925-935, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feucht J, Opherk K, Lang P, et al. : Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood 125:1986-1994, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Einsele H, Roosnek E, Rufer N, et al. : Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99:3916-3922, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Leen AM, Myers GD, Sili U, et al. : Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med 12:1160-1166, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Gerdemann U, Katari UL, Papadopoulou A, et al. : Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther 21:2113-2121, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papadopoulou A, Gerdemann U, Katari UL, et al. : Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV-6 infections after HSCT. Sci Transl Med 6:242ra83, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peggs KS, Thomson K, Samuel E, et al. : Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis 52:49-57, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Icheva V, Kayser S, Wolff D, et al. : Adoptive transfer of Epstein-Barr virus (EBV) nuclear antigen 1-specific T cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol 31:39-48, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Tzannou I, Leen AM: Accelerating immune reconstitution after hematopoietic stem cell transplantation. Clin Transl Immunology 3:e11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peggs KS, Verfuerth S, Pizzey A, et al. : Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 362:1375-1377, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Blyth E, Clancy L, Simms R, et al. : Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood 121:3745-3758, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Feuchtinger T, Richard C, Joachim S, et al. : Clinical grade generation of hexon-specific T cells for adoptive T-cell transfer as a treatment of adenovirus infection after allogeneic stem cell transplantation. J Immunother 31:199-206, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. : Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood 119:2644-2656, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vickers MA, Wilkie GM, Robinson N, et al. : Establishment and operation of a Good Manufacturing Practice–compliant allogeneic Epstein-Barr virus (EBV)-specific cytotoxic cell bank for the treatment of EBV-associated lymphoproliferative disease. Br J Haematol 167:402-410, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leen AM, Bollard CM, Mendizabal AM, et al. : Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121:5113-5123, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qasim W, Derniame S, Gilmour K, et al. : Third-party virus-specific T cells eradicate adenoviraemia but trigger bystander graft-versus-host disease. Br J Haematol 154:150-153, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Ljungman P, de la Camara R, Cordonnier C, et al. : Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant 42:227-240, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Bedi A, Miller CB, Hanson JL, et al. : Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J Clin Oncol 13:1103-1109, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Gerdemann U, Keukens L, Keirnan JM, et al. : Immunotherapeutic strategies to prevent and treat human herpesvirus 6 reactivation after allogeneic stem cell transplantation. Blood 121:207-218, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halawi M, Khan N, Blake N: Identification of novel CD8+ T cell epitopes in human herpesvirus 6B U11 and U90. Immun Inflamm Dis 3:118-131, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iampietro M, Morissette G, Gravel A, et al. : Human herpesvirus 6B immediate-early I protein contains functional HLA-A*02, HLA-A*03, and HLA-B*07 class I restricted CD8(+) T-cell epitopes. Eur J Immunol 44:3573-3584, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Leen AM, Christin A, Khalil M, et al. : Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol 82:546-554, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leen AM, Sili U, Vanin EF, et al. : Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood 104:2432-2440, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Sukdolak C, Tischer S, Dieks D, et al. : CMV-, EBV- and ADV-specific T cell immunity: Screening and monitoring of potential third-party donors to improve post-transplantation outcome. Biol Blood Marrow Transplant 19:1480-1492, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Hislop AD, Taylor GS, Sauce D, et al. : Cellular responses to viral infection in humans: Lessons from Epstein-Barr virus. Annu Rev Immunol 25:587-617, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Kondo E, Akatsuka Y, Kuzushima K, et al. : Identification of novel CTL epitopes of CMV-pp65 presented by a variety of HLA alleles. Blood 103:630-638, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Li Pira G, Bottone L, Ivaldi F, et al. : Generation of cytomegalovirus (CMV)-specific CD4 T-cell lines devoid of alloreactivity, by use of a mixture of CMV-phosphoprotein 65 peptides for reconstitution of the T helper repertoire. J Infect Dis 191:215-226, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Paston SJ, Dodi IA, Madrigal JA: Progress made towards the development of a CMV peptide vaccine. Hum Immunol 65:544-549, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Wiesner M, Zentz C, Hammer MH, et al. : Selection of CMV-specific CD8+ and CD4+ T cells by mini-EBV-transformed B cell lines. Eur J Immunol 35:2110-2121, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Clark DA, Nacheva EP, Leong HN, et al. : Transmission of integrated human herpesvirus 6 through stem cell transplantation: Implications for laboratory diagnosis. J Infect Dis 193:912-916, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Silva Lde P, Patah PA, Saliba RM, et al. : Hemorrhagic cystitis after allogeneic hematopoietic stem-cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica 95:1183-1190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azzi A, Cesaro S, Laszlo D, et al. : Human polyomavirus BK (BKV) load and haemorrhagic cystitis in bone marrow transplantation patients. J Clin Virol 14:79-86, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Cesaro S, Hirsch HH, Faraci M, et al. : Cidofovir for BK virus-associated hemorrhagic cystitis: A retrospective study. Clin Infect Dis 49:233-240, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Giraud G, Priftakis P, Bogdanovic G, et al. : BK-viruria and haemorrhagic cystitis are more frequent in allogeneic haematopoietic stem cell transplant patients receiving full conditioning and unrelated-HLA-mismatched grafts. Bone Marrow Transplant 41:737-742, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Lee YJ, Zheng J, Kolitsopoulos Y, et al. : Relationship of BK polyoma virus (BKV) in the urine with hemorrhagic cystitis and renal function in recipients of T cell-depleted peripheral blood and cord blood stem cell transplantations. Biol Blood Marrow Transplant 20:1204-1210, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung AY, Suen CK, Lie AK, et al. : Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood 98:1971-1978, 2001 [DOI] [PubMed] [Google Scholar]