Abstract
Management of pediatric spinal deformities requires an accurate prediction of growth spurts to allow for timely initiation of treatment and prevention of curve progression. Determining remaining growth potential is also important for avoiding prolonged unnecessary treatment, e.g. bracing for patients nearing skeletal maturity. Many clinical and radiological growth parameters have been developed to aid clinicians in growth prediction. Of these, several commonly used measures such as height and arm span growth trends, timing of menarche, and the Risser sign are mostly retrospective and lack strong predictive utility. Bone age assessments, such as digital skeletal age and the distal radius and ulna classification, are more accurate parameters, but further research is required to determine interethnic variations and develop their role in management decisions.
Keywords: Growth, Risser, Bone age, Distal radius and ulna, DRU
Introduction
Management of all types of scoliosis requires a thorough understanding of a child's remaining growth and timing of skeletal maturity. To optimize treatment outcomes, accurate prediction of growth rate is necessary to indicate when the risk for deformity progression is greatest. This allows interventions to be initiated without delay. Conversely, identifying when growth cessation occurs indicates when preventive measures can be stopped with minimal risk for further curve deterioration. Growth assessment is also necessary for the management of other pediatric deformities besides the spine. This includes limb length discrepancy, which can cause pelvic obliquity and indirectly induce lumbar scoliosis, and overall spine imbalance.
Both early onset scoliosis (EOS) and adolescent idiopathic scoliosis (AIS) require good understanding of patient growth potential to initiate timely appropriate treatment. For EOS, spinal deformities occur in young children and the curvature may deteriorate rapidly, causing cosmetic disfigurement and poor pulmonary development [1,2,3,4,5,6,7,8]. Lung development is particularly important for better life expectancy and is closely related to the size of the thoracic cage. As thoracic spine and thoracic cage growth are interrelated, the growth of the thoracic spine should receive special attention during decision-making. The length of the thoracic spine is typically used to indirectly represent thoracic cage volume as the thoracic cage is not as easily measured with conventional radiography. Hence, EOS patients are commonly managed with growing rod devices to prevent deformity progression while allowing for thoracic spine length gain. This growth translates into increased thoracic cage volume. Both traditional growing rods [9,10,11] and magnetically controlled growing rods [12,13,14,15,16] serve this purpose, with interval distractions to mimic normal spine growth. Successful utilization of growth modulation and tethers is also dependent on growth potential prediction [17,18].
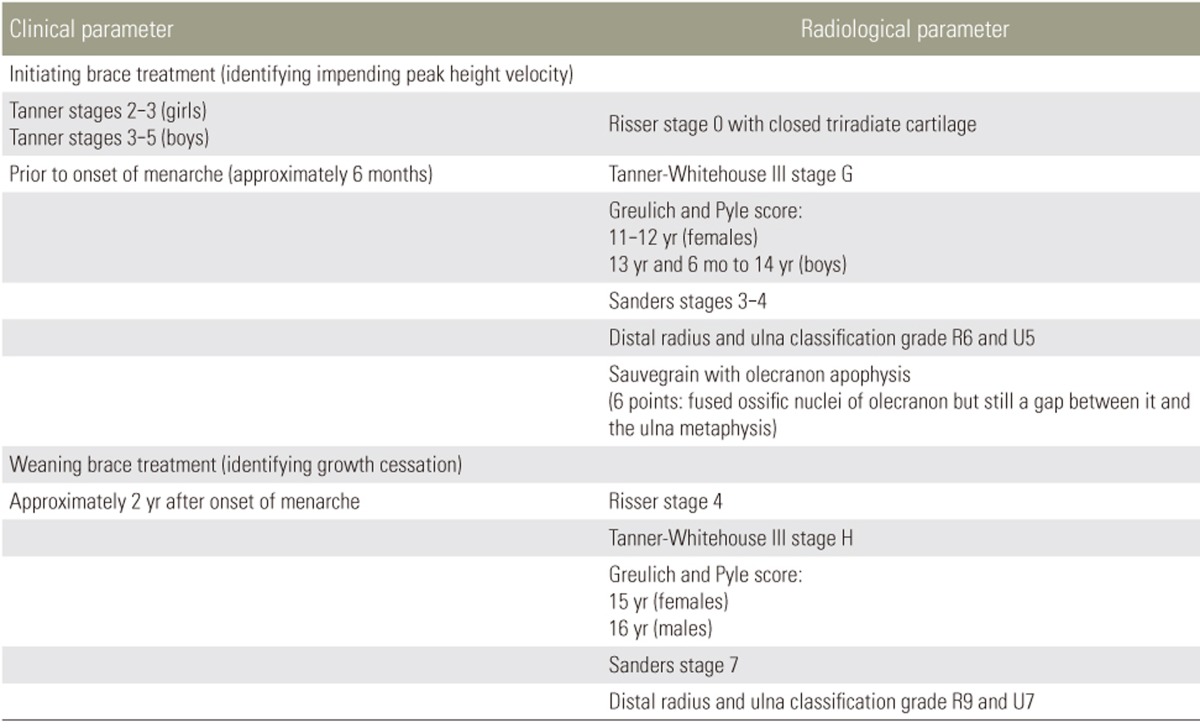
Proper management of AIS requires accurate identification of peak height velocity (PHV) and growth cessation. Predicting when PHV and growth cessation occur is essential for initiating and terminating brace treatment [19,20,21]. Bracing through PHV may help prevent curve progression [22], while stopping brace treatment at growth cessation prevents overuse [23,24,25]. Bracing should not be used indiscriminately as prolonged treatment in children has been shown to reduce spinal mobility, lead to poor body image and self-esteem, and worsen quality of life [26,27,28,29,30,31]. Regarding surgical correction, remaining growth potential is an important risk factor for predicting postoperative deterioration or Crankshaft phenomenon. For example, patients with open triradiate cartilage have higher risk of progression even after surgical correction and fusion in scoliosis [32,33].
The following review illustrates important concepts regarding growth phases in both early childhood and during adolescence. In addition, current evidence regarding each growth parameter and its utility for skeletal maturity assessment in scoliosis management is highlighted.
Growth Phases
Evaluating growth requires understanding of elapsed and remaining growth. Pediatric growth can be divided into three phases. The first two phases are more relevant to EOS management, while the third phase is more important for AIS. The first phase is from birth to age 5, the second from age 5 to 10, and the third is beyond 10 years and corresponds to the pubertal phase. The most rapid growth occurs from birth to 5 years and at puberty. However, these age ranges for these phases are only guidelines as there are likely large variations between ethnicities and in the timing of the growth spurt. Variation of up to 4 years in chronological age has been reported [34]. Nevertheless, a general approximation of a threefold increase in spine length can be expected between birth and skeletal maturity [32,35].
The growth spurt witnessed during puberty is a result of two events, known as adrenarche and gonadarche, which are characterized by a series of hormonal changes. Adrenarche precedes the pubertal growth spurt and indicates the period of 6–8 years where increased production of the adrenal androgens dehydroepiandrosterone (DHEA), androstenedione, and DHEA-sulfate (DHEA-S) occurs. These changes are a manifestation of pubarche with the development of pubic and axillary hair [36]. At this stage, the pubic hair is usually light and fine before it develops into a darker and coarser appearance later in puberty. Despite these changes, adrenarche has limited effects on actual growth gains [37,38]. However, the arrival of pubarche is an important event as it indicates the impending growth spurt seen in gonadarche. Here, a dramatic increase in gonadal steroids leads to thelarche (breast development) and menarche in girls and testicular enlargement and virilization in boys. A set of hormonal changes, namely increased secretion of growth hormone and gonadal steroids, occurs to trigger the adolescent pubertal growth spurt. Of the gonadal steroids, estrogen is the most important for stimulating the physis for linear growth, and this effect is dose dependent [39,40,41,42,43,44]. Lower doses of estrogen during early puberty stimulate growth, while higher doses in late puberty cause growth cessation. Growth hormones are also important as they act through insulin-like growth factor-1 (IGF-1) to also stimulate physeal growth. These changes manifest as secondary sexual characteristics; thus, their appearance suggests that the growth spurt has begun. Due to the timing of testosterone release, which is later than that of estrogen release, boys generally develop these secondary sexual characteristics and enter the pubertal growth spurt later than girls.
Understanding the timing of the above events is particularly important for AIS. Simply, the use of bracing depends on the timing of acceleration and deceleration phases of puberty [22]. The two phases are segregated by PHV or the maximum growth rate, which is highly prognostic for scoliosis progression. By accurately predicting when PHV occurs, clinicians can initiate brace treatment prior to PHV and until the deceleration phase to prevent curve deterioration [21]. The ability to accurately chart the slope of the deceleration phase is also important for brace weaning as the risk of curve progression is significantly reduced when limited growth remains. This will prevent brace-related complications and the overuse of bracing [26,27,28,29,30,31]. Currently, there is virtually no clinical utility of measuring hormonal changes in our scoliosis population; thus, prediction of growth velocity is reliant on clinical and radiographic parameters.
Growth Measurements
For the spine, several measurements (Table 1), including standing and sitting heights, arm span, T1–S1 length, and thoracic cage volume, are important for monitoring growth [45,46]. Standing height is a useful representation of overall growth, but since it incorporates the lower limbs, pelvis, and the skull, it may not be sensitive enough for monitoring spine growth in deformity management. Sitting height is thus a better marker for the spine as it eliminates the contribution of the lower limbs. Based on predominantly Caucasian data, the height of the spine accounts for ~60% of the total sitting height and the rest is accounted for by the head and pelvis [35]. During the first growth phase, typical gains in standing height are 25 cm in the first year and 12.5 cm in the second year [47]. This corresponds to a gain of 27 cm in total sitting height in the first growth period, with nearly 50% of it occurring in the first year of life. Until the fifth year, total height gain is approximately 8 cm per year. In the second growth phase, the sitting height increases at a slower rate of only 2.5 cm per year. For standing height, 5–5.5 cm of growth per year is expected. The third growth phase has an expected gain of 12–13 cm in sitting height and corresponds to the pubertal phase [46,48,49,50]. The remaining growth is approximately 18 cm for girls and 20 cm for boys at puberty. The expected sitting height represents trunk height and is 88 cm for girls and 92 cm for boys at the end of growth [35,45,46,49,50].
Table 1. Summary of common growth and maturity parameters used in managing pediatric spine deformities.
Arm span measurements are particularly important to record in addition to height gains in patients with scoliosis because curve deterioration may cause a reduction in overall height, and thus, mask the actual spine growth achieved [22]. Regardless, the correlation between arm span and standing height is nearly identical, so any change in arm span is quite representative of the height gains [51]. One study suggested that standing height is ~97% of arm span, with boys having a slightly greater proportion than girls [52]. This relationship is consistent into adulthood. A simple rule-of-thumb is that sitting height is arm span divided by two, while arm span divided by four is similar to T1–S1 spine length [46,48,49,50].
Actual clinical height measurements can be predicted by growth of individual spinal segments measured on imaging. Each thoracic vertebra and its disc have been reported to account for 2.5% of sitting height, while each lumbar segment accounts for 3.5% [50]. Measuring all thoracic and lumbar segments (T1–S1 length) is likely the most representative measurement of spine growth as it pertains only to the spinal column. For T1–S1, the measurement at birth is about 20 cm and reaches 45 cm at skeletal maturity. Hence, T1–S1 length accounts for ~50% of sitting height. Of this, two-thirds is contributed by the thoracic spine and one-third is contributed by the lumbar spine. During the first growth phase, T1–S1 increases by 2 cm per year; in the second phase, 1 cm per year; and during the third phase, 1.8 cm per year. By fraction, the thoracic spine (T1–T12) grows 1.3, 0.7, and 1.1 cm per year during the first, second, and third phases, respectively.
The ability to differentiate growth in the thoracic and lumbar vertebrae is useful for the assessment of thoracic cage growth. In particular, thoracic length (T1–T12) is important because of its relationship to the thoracic cavity and lung development. The largest potential for lung development is within 8 years of age. Premature cessation of alveolar proliferation is the main cause of reduced alveoli [53,54]. Development of the bronchial tree ends at 8 years of age; therefore, EOS significantly affects thoracic growth and lung development. T1–T12 measurements based on conventional radiographs are generally preferred to represent thoracic cage growth and avoid radiation exposure in young patients using computed tomography volumetry for measuring thoracic volume. A thoracic length of 18–22 cm is necessary to avoid respiratory insufficiency [55,56]. In general, thoracic circumference is 95% of sitting height during the first 5 years of life and at puberty [35,46,48].
Note that the above values for interval growth gains are only guidelines. They are the only available data regarding spine growth, but are based mostly on Caucasian populations. Although this is adequate for general treatment planning, certain therapies such as growing rod distractions or growth modulation surgeries require more accurate growth predictions and ethnic-specific values.
Clinical Parameters for Predicting Growth
The height and arm span parameters are important for determining growth, as discussed. However, they have practical limitations in spine management because they are unable to predict future growth. Change in these measurements only suggests trends, and actual growth rates can only be confirmed retrospectively. There is also an issue with the distal-to-proximal growth gradient, which suggests that more distal body parts, such as the foot, undergo the pubertal growth spurt earlier [57]. Thus, assessment of foot length may be an early indicator of peak growth velocity. Yet, it is not common for patients to have their foot length measured for curve progression prediction; hence, its utility is questionable. Shoe size difference may be an alternative and has been studied [58]. However, due to variations in branding, recall bias, and its mismatch with sitting height, which is more specific to the spine, its role in managing AIS is limited.
Secondary sexual characteristics are an important hall-mark of skeletal maturity. The Tanner staging for breast and pubic hair development in girls and scrotum and pubic hair in boys can be used for determining the rapid height gain phase [40,41,59]. They have been shown to be excellent maturity measurements but may not correspond well with PHV [60]. For girls, PHV occurs at Tanner stage 2 or 3, while for boys, it is between Tanner stages 3 and 5 [61]. Other characteristics such as voice change, sweat gland maturation, and axillary hair are also possible indicators, but are not well developed for clinical use and are difficult for spine surgeons to implement in their routine clinical assessments. Menarche is the exception and has been used frequently. However, it is a retrospective parameter for growth prediction and is markedly variable at onset. It usually occurs after PHV [60], and thus, its presence only indicates that PHV has occurred. The onset of menarche can only gauge the rate and timing of past skeletal growth, when the best opportunity for meaningful bracing has been missed [62]. The onset of menarche also does not consistently identify the termination of peak growth as delayed menarche is not uncommon [63].
Radiographic Parameters for Predicting Growth
1. Risser sign
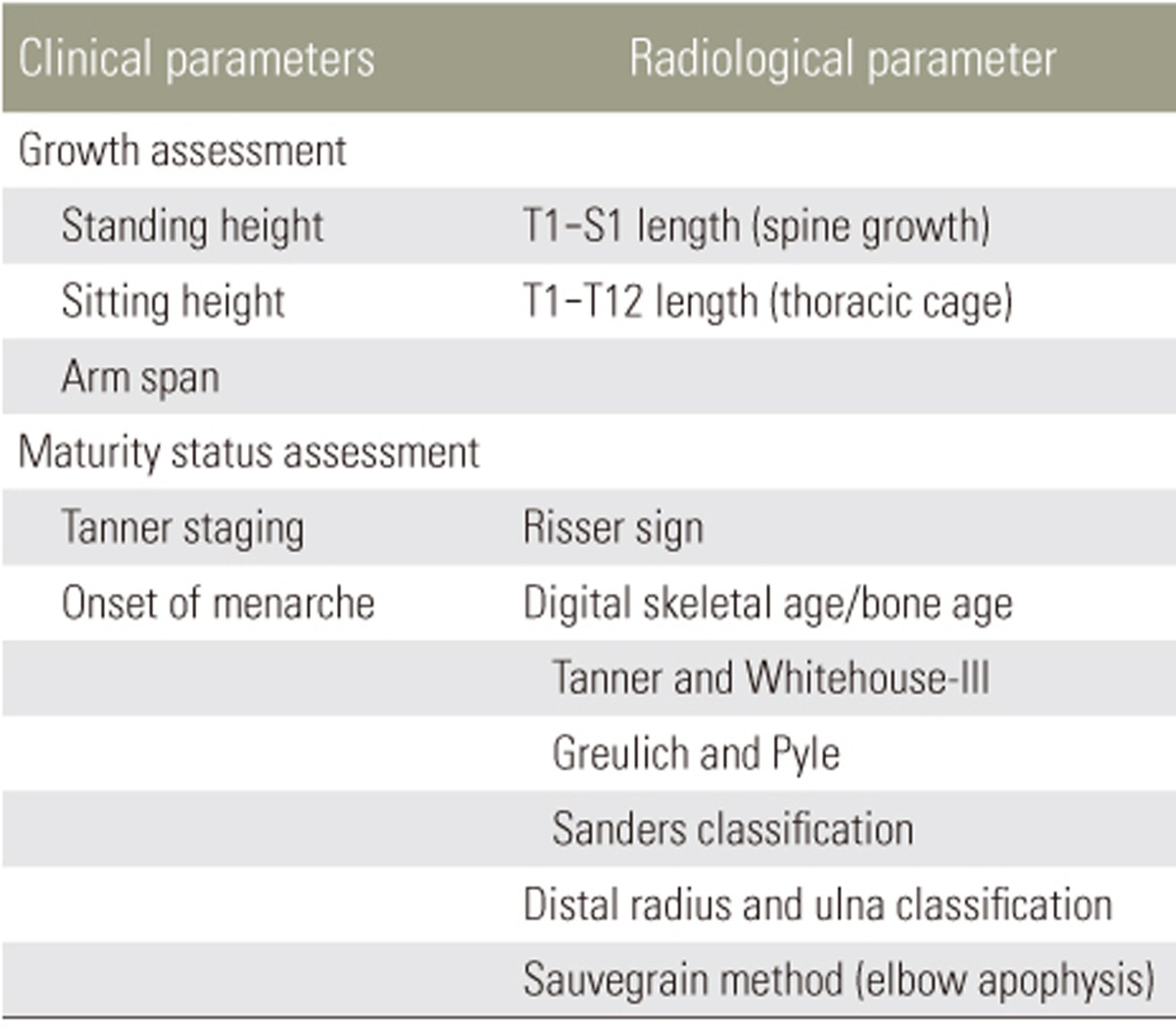
The Risser sign [64,65,66,67] is the most commonly used maturity indicator based on the stage of ossification of the iliac apophysis. Originally described by Risser in 1958 [62], the Risser sign has since been modified and two versions exist for use. In the US, the Risser sign incorporates five stages (Fig. 1), which are designated by dividing the iliac crest into four quarters on an anteroposterior radiograph. Risser stage 1 indicates ossification of the anterior lateral quarter, stage 2 indicates ossification of the anterolateral half, stage 3 indicates ossification of the anterior three-quarters, stage 4 indicates complete ossification, and stage 5 indicates fusion to the ilium. In the French version, the Risser sign has only four stages designated by dividing the apophysis into thirds with Risser stage 1 indicating the lateral third, stage 2 indicating the middle third, stage 3 indicating the medial third, and stage 4 indicating ossification/fusion of the apophysis [68]. Despite its advantages of simplicity and availability on the same spine radiograph used to assess scoliosis, there are several significant limitations to its utility. It is customary to use posteroanterior spine radiographs to reduce radiation of the breast tissue, and thus, visibility of the Risser sign is reduced with this view. Moreover, there are often difficulties interpreting the Risser sign. Although the iliac apophysis normally ossifies at the anterior superior iliac spine and extends posteriorly to the posterior superior iliac spine, there are exceptions (Fig. 2) where the iliac apophysis develops posteromedially to anterolaterally or in fragments [69].
Fig. 1. Example of Risser sign assessment (US version) with the right iliac crest. (A) Risser stage 0 with no ossification of the iliac apophysis. (B) Risser stage 1 with ossification of the anterior lateral quarter. (C) Risser stage 2 with ossification of the anterolateral half of the iliac apophysis. (D) Risser stage 3 with ossification of the anterior three-quarters. (E) Risser stage 4 indicates complete ossification of the iliac apophysis and Risser stage 4+ or 5− can be used to indicate capped apophysis, which suggests end of growth but not complete fusion as seen in Risser stage 5 (F).
Fig. 2. The Risser sign may not begin ossification at the anterolateral half of the iliac apophysis (A) and may not have complete ossification of the anterolateral aspect of the iliac apophysis even past Risser stage 2 (B).
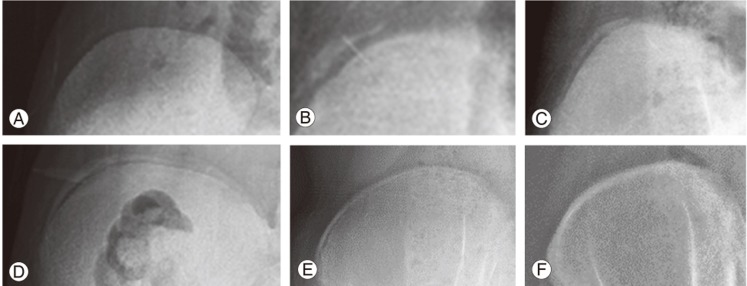
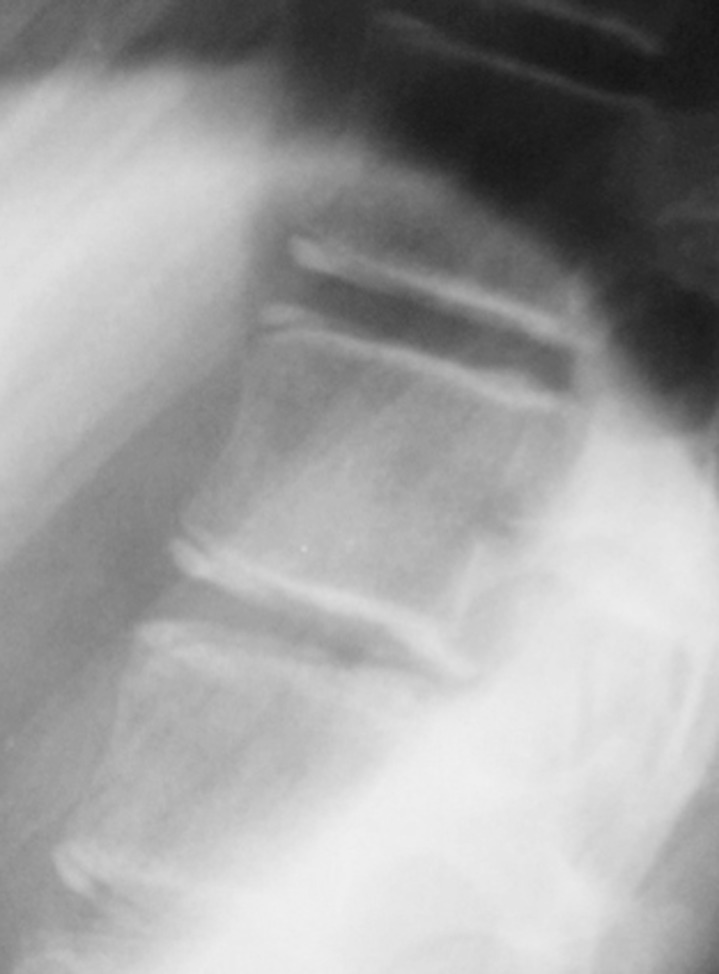
As a growth predictor, the Risser sign lacks the ability to predict PHV, which is most important for the timing of brace treatment [69,70,71,72,73]. As all children are Risser 0 prior to pubertal growth acceleration, little information can be provided prior to the growth spurt. Once the Risser sign proceeds to stage 1, it becomes a retrospective indicator that up to two-thirds of the pubertal growth spurt has occurred [74]. Dimeglio [35] suggested using the timing of triradiate cartilage (Fig. 3) closure to separate Risser 0 into two stages. Yet even with this modification, it has been shown to be suboptimal for predicting peak growth [75]. In addition, there are concerns of radiation exposure to the gonads. Despite using gonadal shields, common errors of inadequate positioning or even complete omission have been reported [76,77].
Fig. 3. Both patients with Risser stage 0 but with open triradiate cartilage (A) and closed triradiate cartilage (B).
Similar problems exist for its utility as a predictor of growth cessation, and thus, brace weaning in AIS treatment [69,70,71,72,73]. Hoppenfeld et al. [72] suggested that up to 75.2% of children continue to grow despite apophyseal capping. This feature is even less useful in boys who typically have substantial growth remaining even at Risser stages 4 and 5 compared to girls [78]. In addition, reports suggest suggest that the iliac apophysis does not fuse until adulthood or not fuse at all; therefore, the presence of Risser stage 4 does not accurately represent the remaining growth potential [73,74,79]. Growth cessation has even been reported to occur as early as Risser stage 3 [22]. Hence, the Risser sign cannot be used to accurately predict whether further growth is expected or to gauge the risk of curve progression.
2. Digital skeletal age (bone age)
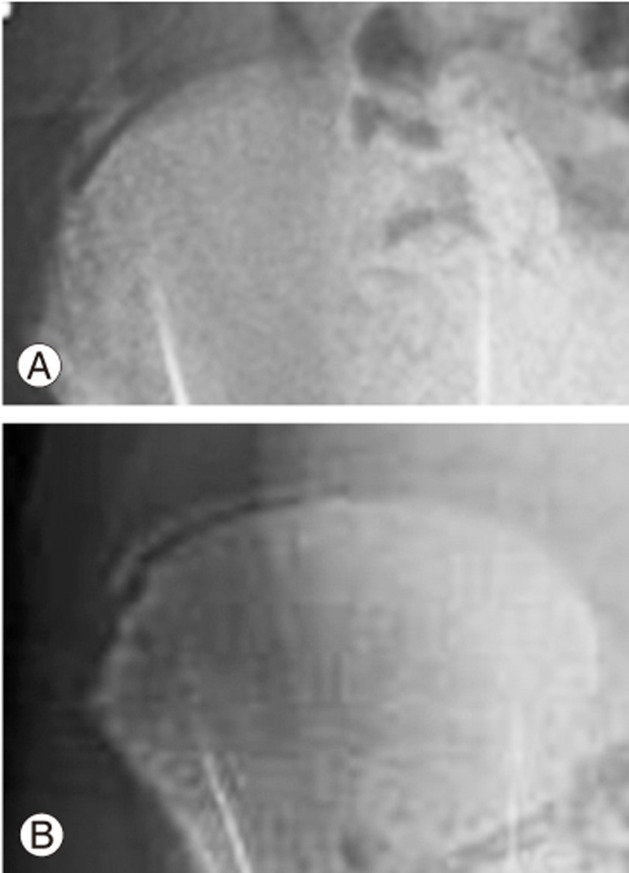
Bone age measurement is based on morphological changes in the epiphyseal–metaphyseal bone complexes of the non-dominant hand (usually left hand). In the hand, areas of the phalanges and the distal radius and ulna are more useful for growth determination, while carpal bones are only useful for growth prediction in those less than 9–12 years of age [80,81,82]. Skeletal maturity status is based on the stages of bone complex fusions. The epiphyseal region grows continuously until it caps and fuses with the metaphysis. Capping (Fig. 4) describes the shape of the ends of the epiphysis during the acceleration phase as a horn-like structure turning toward the metaphysis. The phalanges and metacarpals tend to fuse in a specific order, with the distal phalanges first, followed by the metacarpals, proximal phalanges, and finally the middle phalanges. Fusion begins at the center of physis and then spreads to the periphery. The relationship between the order of fusion and specific growth rate is currently unknown. However, collectively, this period is regarded as PHV. With hand bone aging, stage progression is nonlinear, suggesting that the skeletal age does not progress in parallel with chronological time. Essentially, the time elapsed between each stage is not the same; hence, bone age may only reflect maturity status in normal children rather than those with developmental problems [83,84].
Fig. 4. Appearance of epiphyseal–metaphyseal bone complex of the middle finger. (A) Epiphysis is not as wide as the metaphysis. (B) Epiphysis is as wide as the metaphysis. (C) Capping or horn-like structure of the ends of the epiphysis is formed. (D) Fusion of the epiphyseal–metaphyseal bone complex.
Two traditional techniques for measuring skeletal maturity, also known as digital skeletal age, were proposed by Tanner and Whitehouse (TW) [60,85,86] and Greulich and Pyle (GP) [87]. The TW1 method was developed in Britain in 1962 and was based on data collected from 3,000 British boys and girls. This method was subsequently modified into TW2 in 1975 and TW3 in 2002. The revisions included changes in scores for each stage and gender-specific standards for TW2 and moving the scores from ages 10–11 a year after the incorporation of North American and European data for TW3 [88]. Specific bone complexes (joint including two or more bones) were used to create scores for calculating the degree of maturation. A total of twenty bone complexes, including the radius, are included in the scoring system. The score is then matched to a table to estimate the bone age. The simpler but more subjective method is the GP method, which requires matching the patient's hand image to an atlas of morphological changes of the 28 bones in the hand to determine the maturity phase. The GP atlas is based on the premise that all bone complexes of the hand and wrist mature at the same rate, but this is, as suggested earlier, not true. The features in the atlas were created by Todd [89] using 1,000 children from the United States. Due to its simplicity and subjective nature, there are concerns of inaccuracy.
Due to the above-discussed drawbacks of the TW and GP scoring systems, their accuracy and clinical usefulness have been challenged. Intraobserver rater error can be up to 0.5 years for the TW method and 0.82 years for the GP method [90,91]. Sanders et al. [92] has since simplified bone age assessment into a more reasonable tool that has been shown to be predictive for peak growth in North American children. Eight stages were created to encompass the period from when girls and boys are 8 and 12 years old, respectively, until maturity. Of particular importance are stages 3 and 4, or the early and late adolescent rapid stages, respectively. Phalangeal capping is found at these stages, indicating PHV. Nevertheless, Sanders staging still requires physeal assessment of all digits, which is complex and cumbersome to utilize in a busy clinic setting.
3. The distal radius and ulna classification
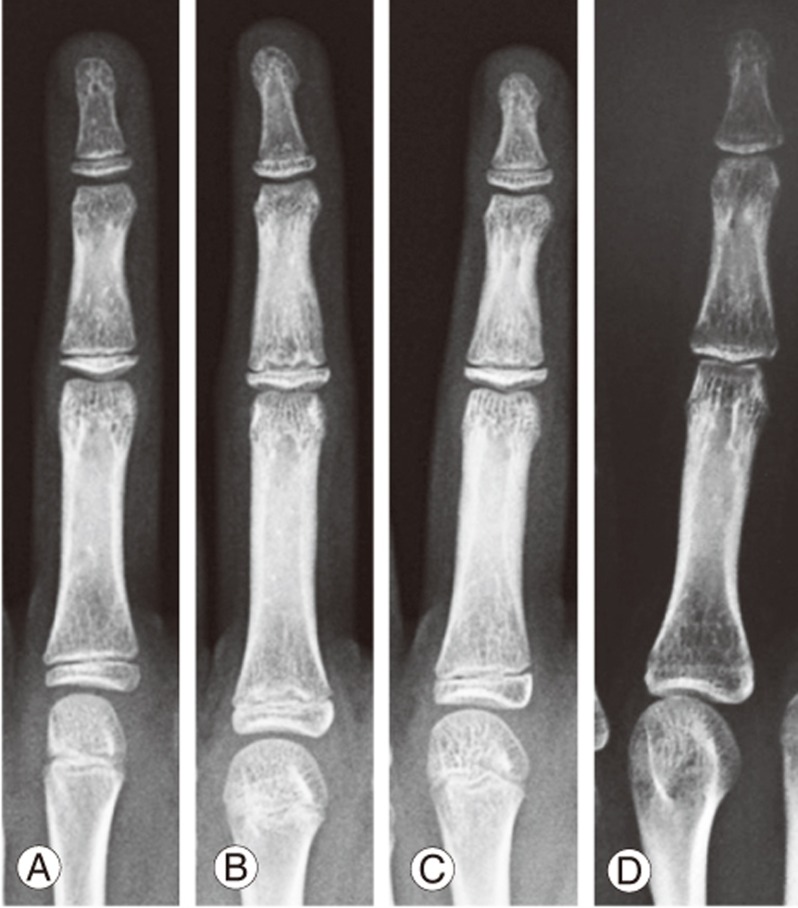
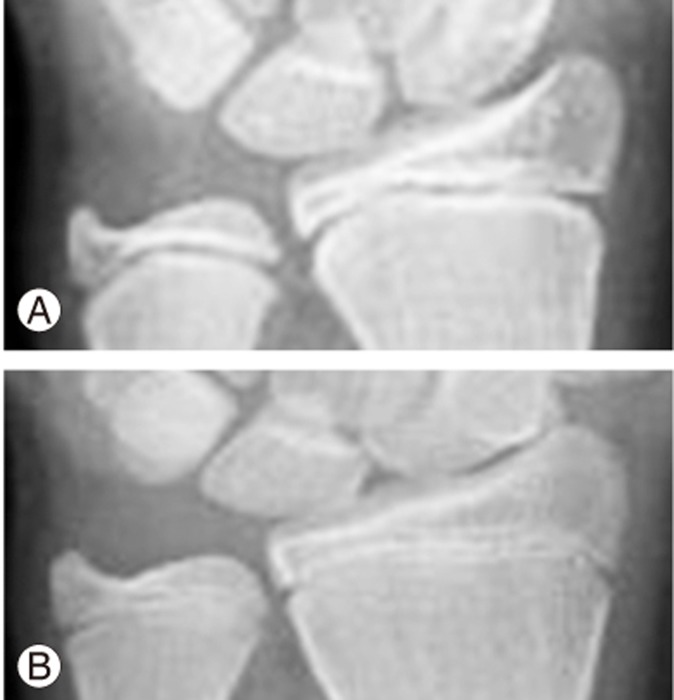
An alternative to the maturity parameters discussed is the distal radius and ulna (DRU) classification, as reported by Luk et al. [93]. The DRU classification incorporates the whole range of growth phases with 11 radius grades (R1–R11) and 9 ulna grades (U1–U9). As it is solely based on the morphology of two physeal plates, it is more user-friendly than digital skeletal age. The classification has been shown to be a reproducible classification with excellent intra- and interrator reliability and is further simplified for clinical use [94,95]. As an example, for AIS patients, each grade from R6–R11 and U5–U9 (Fig. 5) can be distinguished by one feature, thereby reducing the time needed for grading. A large-scale, thorough comparison of the DRU, Risser sign, age of menarche, and digit physeal capping and fusion was performed for a Chinese population [22]. The DRU was superior to the Risser sign, with its larger spread of grades prior to PHV so that both acceleration and deceleration phases of growth can be determined during puberty [22]. For peak growth, DRU grades of R6 (77.5% sensitivity and 78.9% specificity) and U5 (77.3% sensitivity and 81.6% specificity) were most predictive. For growth cessation, DRU grades of R9 (71.5% sensitivity, 86.6% specificity) and U7 (85.1% sensitivity, 75.9% specificity) were most predictive. Hence, it is useful for determining the timing of brace initiation and weaning. Despite evidence showing that the DRU matches well with spine growth and has strong utility for scoliosis management, its predictability of curve progression is yet to be determined. Similarly, its role in the management of EOS patients may be expanded as the full breadth of its grading scheme incorporates the younger age range as well. It may have a potential role in management guidance during the first two growth phases and for monitoring of thoracic cage development. Further study is necessary to define its role in brace management, growth guidance, and definitive fusion surgery.
Fig. 5. The distal radius and ulna classification showing two important time points in the pubertal growth period. (A) Radius grade 6 and ulna grade 5 indicate the peak growth spurt and are identified with similar epiphyseal and metaphyseal widths at the radius without capping and ulna epiphysis and metaphysis with similar widths without rounding of the medial physis. (B) Radius grade 9 and ulna grade 7 indicate growth cessation, which is identified as capped radial and ulnar borders of the radius epiphysis with central physis narrowing and initial fusion and ulna medial side narrowing or fused physis.
Other Markers
Other skeletal parameters reported in the literature include the fusion of rib heads and ring apophysis (Fig. 6) of vertebrae [72]. Although these parameters are routinely visible on the same spine radiograph, there are limitations in predicting growth due to inadequate evidence for growth prediction, and the ring apophysis may be open until the middle or late twenties [96]. The upper cervical growth centers may also be used as potential evaluators of growth [97,98,99,100]. Most of the work related to these growth centers is done by dentistry due to feasibility of acquiring images of the oral cavity. However, its usefulness for the spine is yet to be revealed.
Fig. 6. Appearance of rib apophysis at the anterosuperior and anteroinferior aspects of the vertebral endplates.
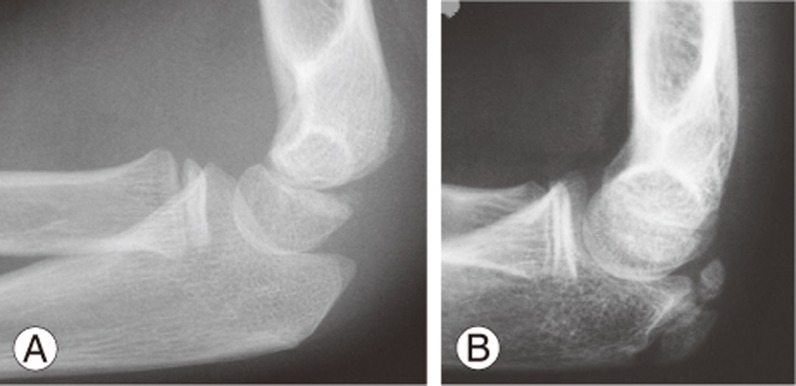
Skeletal age can also be measured from the elbow (Fig. 7) using the Sauvegrain method [101], which has also been shown to have excellent interobserver correlation and reproducibility in determining the growth spurt [102]. However, its comparison with other methods is unknown, and the olecranon apophysis fuses before growth cessation (usually around the time of Risser stage 1); hence, it is likely limited for complete management of scoliosis. For full scoring (27-point scoring system), the method requires assessment of four growth centers (lateral condyle and epicondyle, trochlea, olecranon apophysis, and proximal radial epiphysis), which results in two elbow radiographs (anteroposterior and lateral radiographs of the left elbow). Hence, increased radiation exposure is required as compared to other growth parameters.
Fig. 7. Elbow olecranon assessment demonstration with only a lateral radiograph. Olecranon prior to ossification (A) and olecranon morphology at the initiation of the growth acceleration phase of puberty (B).
Metabolic and hormonal biomarkers have also been investigated in the past, including measurement of DHEA, DHEA-S, IGF-1, estradiol, alkaline phosphatase, osteocalcin, and testosterone. IGF-1 has the best correlation to curve progression, but it is not as strong as the correlation to digital skeletal age [61]. Its role is limited in clinical practice due to its availability, cost, and overall lack of evidence supporting its prediction capabilities.
Conclusions
Most of the current values for natural growth are based on Caucasian data; thus, there is a need for data regarding other ethnicities. Growth information must be gender- and ethnicity-specific to avoid misinterpretation of growth patterns. Accurate prediction of growth phases and remaining growth potential is important for successful treatment (Table 2) of pediatric scoliosis. The ability to initiate timely interventions, such as growth guidance surgery in EOS or bracing in AIS, depends on our understanding of thoracic cage and lung development and the growth acceleration phase during puberty. There are a multitude of clinical and radiological parameters and their respective classification schemes that aid us in defining key growth phases. Despite the accuracy and vast utility of some classification tools, they are only guidelines and should never replace the insight of the clinician in the decision-making process.
Table 2. Suggested parameters for initiating and weaning brace treatment in adolescent idiopathic scoliosis.
These are only suggested guidelines. In general, for bone age assessment, the peak height velocity occurs with capping of the digits and growth cessation occurs with closed digit physes and narrowing/near closure of the radius and ulna physes. One or multiple parameters may be used in conjunction but cannot replace the decision-making ability of the clinician. Decisions also depend on the patient's Cobb angle status and the patient's willingness to undergo brace treatment. Those with a very minor curve ≤20° generally should not be braced until deterioration is witnessed, even at peak height velocity. Those who are near maturity but with a significant curve approaching 40° may benefit from a longer bracing period to reduce the risk of deterioration into adulthood.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Akbarnia BA, Emans JB. Complications of growth-sparing surgery in early onset scoliosis. Spine (Phila Pa 1976) 2010;35:2193–2204. doi: 10.1097/BRS.0b013e3181f070b5. [DOI] [PubMed] [Google Scholar]
- 2.Bess S, Akbarnia BA, Thompson GH, et al. Complications of growing-rod treatment for early-onset scoliosis: analysis of one hundred and forty patients. J Bone Joint Surg Am. 2010;92:2533–2543. doi: 10.2106/JBJS.I.01471. [DOI] [PubMed] [Google Scholar]
- 3.Campbell RM, Jr, Smith MD, Mayes TC, et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85-A:399–408. doi: 10.2106/00004623-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Cheung JP, Smith MD, Mayes TC. Management of early-onset scoliosis [Internet] London: British Editorial Society of Bone and Joint Surgery; 2013. [cited 2017 Jun 2]. Available from: http://www.boneandjoint.org.uk/content/focus/management-early-onset-scoliosis. [Google Scholar]
- 5.Goldberg CJ, Gillic I, Connaughton O, et al. Respiratory function and cosmesis at maturity in infantile-onset scoliosis. Spine (Phila Pa 1976) 2003;28:2397–2406. doi: 10.1097/01.BRS.0000085367.24266.CA. [DOI] [PubMed] [Google Scholar]
- 6.James JI. Idiopathic scoliosis; the prognosis, diagnosis, and operative indications related to curve patterns and the age at onset. J Bone Joint Surg Br. 1954;36-B:36–49. doi: 10.1302/0301-620X.36B1.36. [DOI] [PubMed] [Google Scholar]
- 7.James JI, Lloyd-Roberts GC, Pilcher MF. Infantile structural scoliosis. J Bone Joint Surg Br. 1959;41-B:719–735. doi: 10.1302/0301-620X.41B4.719. [DOI] [PubMed] [Google Scholar]
- 8.Redding GJ, Mayer OH. Structure-respiration function relationships before and after surgical treatment of early-onset scoliosis. Clin Orthop Relat Res. 2011;469:1330–1334. doi: 10.1007/s11999-010-1621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akbarnia BA, Breakwell LM, Marks DS, et al. Dual growing rod technique followed for three to eleven years until final fusion: the effect of frequency of lengthening. Spine (Phila Pa 1976) 2008;33:984–990. doi: 10.1097/BRS.0b013e31816c8b4e. [DOI] [PubMed] [Google Scholar]
- 10.Akbarnia BA, Marks DS, Boachie-Adjei O, Thompson AG, Asher MA. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine (Phila Pa 1976) 2005;30(17 Suppl):S46–S57. doi: 10.1097/01.brs.0000175190.08134.73. [DOI] [PubMed] [Google Scholar]
- 11.Winter RB, Moe JH, Lonstein JE. Posterior spinal arthrodesis for congenital scoliosis: an analysis of the cases of two hundred and ninety patients, five to nineteen years old. J Bone Joint Surg Am. 1984;66:1188–1197. [PubMed] [Google Scholar]
- 12.Cheung JP, Bow C, Samartzis D, Ganal-Antonio AK, Cheung KM. Clinical utility of ultrasound to prospectively monitor distraction of magnetically controlled growing rods. Spine J. 2016;16:204–209. doi: 10.1016/j.spinee.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 13.Cheung JP, Bow C, Samartzis D, Kwan K, Cheung KM. Frequent small distractions with a magnetically controlled growing rod for early-onset scoliosis and avoidance of the law of diminishing returns. J Orthop Surg (Hong Kong) 2016;24:332–337. doi: 10.1177/1602400312. [DOI] [PubMed] [Google Scholar]
- 14.Cheung JP, Cahill P, Yaszay B, Akbarnia BA, Cheung KM. Special article: Update on the magnetically controlled growing rod: tips and pitfalls. J Orthop Surg (Hong Kong) 2015;23:383–390. doi: 10.1177/230949901502300327. [DOI] [PubMed] [Google Scholar]
- 15.Cheung JP, Samartzis D, Cheung KM. A novel approach to gradual correction of severe spinal deformity in a pediatric patient using the magnetically-controlled growing rod. Spine J. 2014;14:e7–e13. doi: 10.1016/j.spinee.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 16.Cheung KM, Cheung JP, Samartzis D, et al. Magnetically controlled growing rods for severe spinal curvature in young children: a prospective case series. Lancet. 2012;379:1967–1974. doi: 10.1016/S0140-6736(12)60112-3. [DOI] [PubMed] [Google Scholar]
- 17.Betz RR, D'Andrea LP, Mulcahey MJ, Chafetz RS. Vertebral body stapling procedure for the treatment of scoliosis in the growing child. Clin Orthop Relat Res. 2005;(434):55–60. doi: 10.1097/01.blo.0000163472.46511.a8. [DOI] [PubMed] [Google Scholar]
- 18.Betz RR, Kim J, D'Andrea LP, Mulcahey MJ, Balsara RK, Clements DH. An innovative technique of vertebral body stapling for the treatment of patients with adolescent idiopathic scoliosis: a feasibility, safety, and utility study. Spine (Phila Pa 1976) 2003;28:S255–S265. doi: 10.1097/01.BRS.0000092484.31316.32. [DOI] [PubMed] [Google Scholar]
- 19.Danielsson AJ, Hasserius R, Ohlin A, Nachemson AL. A prospective study of brace treatment versus observation alone in adolescent idiopathic scoliosis: a follow-up mean of 16 years after maturity. Spine (Phila Pa 1976) 2007;32:2198–2207. doi: 10.1097/BRS.0b013e31814b851f. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama T. Bracing adolescent idiopathic scoliosis: a systematic review of the literature of effective conservative treatment looking for end results 5 years after weaning. Disabil Rehabil. 2008;30:786–791. doi: 10.1080/09638280801889782. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein SL, Dolan LA, Wright JG, Dobbs MB. Effects of bracing in adolescents with idiopathic scoliosis. N Engl J Med. 2013;369:1512–1521. doi: 10.1056/NEJMoa1307337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung JP, Cheung PW, Samartzis D, Cheung KM, Luk KD. The use of the distal radius and ulna classification for the prediction of growth: peak growth spurt and growth cessation. Bone Joint J. 2016;98-B:1689–1696. doi: 10.1302/0301-620X.98B12.BJJ-2016-0158.R1. [DOI] [PubMed] [Google Scholar]
- 23.Katz DE, Durrani AA. Factors that influence outcome in bracing large curves in patients with adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2001;26:2354–2361. doi: 10.1097/00007632-200111010-00012. [DOI] [PubMed] [Google Scholar]
- 24.Landauer F, Wimmer C, Behensky H. Estimating the final outcome of brace treatment for idiopathic thoracic scoliosis at 6-month follow-up. Pediatr Rehabil. 2003;6:201–207. doi: 10.1080/13638490310001636817. [DOI] [PubMed] [Google Scholar]
- 25.Rahman T, Bowen JR, Takemitsu M, Scott C. The association between brace compliance and outcome for patients with idiopathic scoliosis. J Pediatr Orthop. 2005;25:420–422. doi: 10.1097/01.bpo.0000161097.61586.bb. [DOI] [PubMed] [Google Scholar]
- 26.Cheung KM, Cheng EY, Chan SC, Yeung KW, Luk KD. Outcome assessment of bracing in adolescent idiopathic scoliosis by the use of the SRS-22 questionnaire. Int Orthop. 2007;31:507–511. doi: 10.1007/s00264-006-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Climent JM, Sanchez J. Impact of the type of brace on the quality of life of adolescents with spine deformities. Spine (Phila Pa 1976) 1999;24:1903–1908. doi: 10.1097/00007632-199909150-00007. [DOI] [PubMed] [Google Scholar]
- 28.Noonan KJ, Dolan LA, Jacobson WC, Weinstein SL. Long-term psychosocial characteristics of patients treated for idiopathic scoliosis. J Pediatr Orthop. 1997;17:712–717. [PubMed] [Google Scholar]
- 29.Odermatt D, Mathieu PA, Beausejour M, Labelle H, Aubin CE. Electromyography of scoliotic patients treated with a brace. J Orthop Res. 2003;21:931–936. doi: 10.1016/S0736-0266(03)00038-X. [DOI] [PubMed] [Google Scholar]
- 30.Ugwonali OF, Lomas G, Choe JC, et al. Effect of bracing on the quality of life of adolescents with idiopathic scoliosis. Spine J. 2004;4:254–260. doi: 10.1016/j.spinee.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Vasiliadis E, Grivas TB, Savvidou O, Triantafyllopoulos G. The influence of brace on quality of life of adolescents with idiopathic scoliosis. Stud Health Technol Inform. 2006;123:352–356. [PubMed] [Google Scholar]
- 32.Dubousset J, Herring JA, Shufflebarger H. The crankshaft phenomenon. J Pediatr Orthop. 1989;9:541–550. doi: 10.1097/01241398-198909010-00008. [DOI] [PubMed] [Google Scholar]
- 33.Sanders JO, Herring JA, Browne RH. Posterior arthrodesis and instrumentation in the immature (Risser-grade-0) spine in idiopathic scoliosis. J Bone Joint Surg Am. 1995;77:39–45. doi: 10.2106/00004623-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 35.Dimeglio A. Growth in pediatric orthopaedics. J Pediatr Orthop. 2001;21:549–555. [PubMed] [Google Scholar]
- 36.Auchus RJ. The physiology and biochemistry of adrenarche. Endocr Dev. 2011;20:20–27. doi: 10.1159/000321209. [DOI] [PubMed] [Google Scholar]
- 37.Plant TM, Barker-Gibb ML. Neurobiological mechanisms of puberty in higher primates. Hum Reprod Update. 2004;10:67–77. doi: 10.1093/humupd/dmh001. [DOI] [PubMed] [Google Scholar]
- 38.Saenger P, Dimartino-Nardi J. Premature adrenarche. J Endocrinol Invest. 2001;24:724–733. doi: 10.1007/BF03343917. [DOI] [PubMed] [Google Scholar]
- 39.Cutler GB., Jr The role of estrogen in bone growth and maturation during childhood and adolescence. J Steroid Biochem Mol Biol. 1997;61:141–144. [PubMed] [Google Scholar]
- 40.Grumbach MM. Estrogen, bone, growth and sex: a sea change in conventional wisdom. J Pediatr Endocrinol Metab. 2000;13(Suppl 6):1439–1455. doi: 10.1515/jpem-2000-s619. [DOI] [PubMed] [Google Scholar]
- 41.Grumbach MM. The role of estrogen in the male and female: evidence from mutations in synthesis and action. Horm Res. 2000;53(Suppl 3):23–24. doi: 10.1159/000023527. [DOI] [PubMed] [Google Scholar]
- 42.Lee PA, Witchel SF. The influence of estrogen on growth. Curr Opin Pediatr. 1997;9:431–436. doi: 10.1097/00008480-199708000-00020. [DOI] [PubMed] [Google Scholar]
- 43.Nilsson O, Chrysis D, Pajulo O, et al. Localization of estrogen receptors-alpha and -beta and androgen receptor in the human growth plate at different pubertal stages. J Endocrinol. 2003;177:319–326. doi: 10.1677/joe.0.1770319. [DOI] [PubMed] [Google Scholar]
- 44.Styne DM. The regulation of pubertal growth. Horm Res. 2003;60:22–26. doi: 10.1159/000071222. [DOI] [PubMed] [Google Scholar]
- 45.Akbarnia BA, Campbell RM, Dimeglio A, et al. Fusionless procedures for the management of early-onset spine deformities in 2011: what do we know? J Child Orthop. 2011;5:159–172. doi: 10.1007/s11832-011-0342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dimeglio A, Bonnel F, Canavese F. Normal growth of the spine and thorax. In: Akbarnia BA, Yazici M, Thompson GH, editors. The growing spine. New York: Springer; 2009. pp. 11–41. [Google Scholar]
- 47.Dimeglio A, Canavese F. The growing spine: how spinal deformities influence normal spine and thoracic cage growth. Eur Spine J. 2012;21:64–70. doi: 10.1007/s00586-011-1983-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charles YP, Dimeglio A, Marcoul M, Bourgin JF, Marcoul A, Bozonnat MC. Influence of idiopathic scoliosis on three-dimensional thoracic growth. Spine (Phila Pa 1976) 2008;33:1209–1218. doi: 10.1097/BRS.0b013e3181715272. [DOI] [PubMed] [Google Scholar]
- 49.Dimeglio A. Growth of the spine before age 5 years. J Pediatr Orthop B. 1993;1:102–107. [Google Scholar]
- 50.Dimeglio A. Growth in pediatric orthopaedics. In: Morrissy RT, Weinstein SL, editors. Lovell and Winter's pediatric orthopaedics. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 33–62. [Google Scholar]
- 51.Cheng JC, Leung SS, Chiu BS, et al. Can we predict body height from segmental bone length measurements? A study of 3,647 children. J Pediatr Orthop. 1998;18:387–393. [PubMed] [Google Scholar]
- 52.Jarzem PF, Gledhill RB. Predicting height from arm measurements. J Pediatr Orthop. 1993;13:761–765. doi: 10.1097/01241398-199311000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Berend N, Rynell AC, Ward HE. Structure of a human pulmonary acinus. Thorax. 1991;46:117–121. doi: 10.1136/thx.46.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeGroodt EG, van Pelt W, Borsboom GJ, Quanjer PH, van Zomeren BC. Growth of lung and thorax dimensions during the pubertal growth spurt. Eur Respir J. 1988;1:102–108. [PubMed] [Google Scholar]
- 55.Karol LA, Johnston C, Mladenov K, Schochet P, Walters P, Browne RH. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am. 2008;90:1272–1281. doi: 10.2106/JBJS.G.00184. [DOI] [PubMed] [Google Scholar]
- 56.Pehrsson K, Larsson S, Oden A, Nachemson A. Long-term follow-up of patients with untreated scoliosis: a study of mortality, causes of death, and symptoms. Spine (Phila Pa 1976) 1992;17:1091–1096. doi: 10.1097/00007632-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 57.Tanner JM. Growth at adolescence. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- 58.Busscher I, Kingma I, Wapstra FH, Bulstra SK, Verkerke GJ, Veldhuizen AG. The value of shoe size for prediction of the timing of the pubertal growth spurt. Scoliosis. 2011;6:1. doi: 10.1186/1748-7161-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanner JM. Normal growth and techniques of growth assessment. Clin Endocrinol Metab. 1986;15:411–451. doi: 10.1016/s0300-595x(86)80005-6. [DOI] [PubMed] [Google Scholar]
- 60.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanders JO, Browne RH, Cooney TE, Finegold DN, McConnell SJ, Margraf SA. Correlates of the peak height velocity in girls with idiopathic scoliosis. Spine (Phila Pa 1976) 2006;31:2289–2295. doi: 10.1097/01.brs.0000236844.41595.26. [DOI] [PubMed] [Google Scholar]
- 62.Risser JC. The Iliac apophysis; an invaluable sign in the management of scoliosis. Clin Orthop. 1958;11:111–119. [PubMed] [Google Scholar]
- 63.Chang SH, Tzeng SJ, Cheng JY, Chie WC. Height and weight change across menarche of schoolgirls with early menarche. Arch Pediatr Adolesc Med. 2000;154:880–884. doi: 10.1001/archpedi.154.9.880. [DOI] [PubMed] [Google Scholar]
- 64.Risser JC. Iliac apophysis. Clin Orthop Relat Res. 1977;(122):366. [PubMed] [Google Scholar]
- 65.Risser JC. The classic: The iliac apophysis: an invaluable sign in the management of scoliosis. 1958. Clin Orthop Relat Res. 2010;468:643–653. doi: 10.1007/s11999-009-1095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urbaniak JR, Schaefer WW, Stelling FH., 3rd Iliac apophyses: prognostic value in idiopathic schliosis. Clin Orthop Relat Res. 1976;(116):80–85. [PubMed] [Google Scholar]
- 67.Zaoussis AL, James JI. The iliac apophysis and the evolution of curves in scoliosis. J Bone Joint Surg Br. 1958;40-B:442–453. doi: 10.1302/0301-620X.40B3.442. [DOI] [PubMed] [Google Scholar]
- 68.Bitan FD, Veliskakis KP, Campbell BC. Differences in the Risser grading systems in the United States and France. Clin Orthop Relat Res. 2005;(436):190–195. doi: 10.1097/01.blo.0000160819.10767.88. [DOI] [PubMed] [Google Scholar]
- 69.Shuren N, Kasser JR, Emans JB, Rand F. Reevaluation of the use of the Risser sign in idiopathic scoliosis. Spine (Phila Pa 1976) 1992;17:359–361. doi: 10.1097/00007632-199203000-00020. [DOI] [PubMed] [Google Scholar]
- 70.Biondi J, Weiner DS, Bethem D, Reed JF., 3rd Correlation of Risser sign and bone age determination in adolescent idiopathic scoliosis. J Pediatr Orthop. 1985;5:697–701. doi: 10.1097/01241398-198511000-00013. [DOI] [PubMed] [Google Scholar]
- 71.Dhar S, Dangerfield PH, Dorgan JC, Klenerman L. Correlation between bone age and Risser's sign in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 1993;18:14–19. doi: 10.1097/00007632-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Hoppenfeld S, Lonner B, Murthy V, Gu Y. The rib epiphysis and other growth centers as indicators of the end of spinal growth. Spine (Phila Pa 1976) 2004;29:47–50. doi: 10.1097/01.BRS.0000103941.50129.66. [DOI] [PubMed] [Google Scholar]
- 73.Little DG, Sussman MD. The Risser sign: a critical analysis. J Pediatr Orthop. 1994;14:569–575. doi: 10.1097/01241398-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 74.Little DG, Song KM, Katz D, Herring JA. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am. 2000;82:685–693. doi: 10.2106/00004623-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 75.Nault ML, Parent S, Phan P, Roy-Beaudry M, Labelle H, Rivard M. A modified Risser grading system predicts the curve acceleration phase of female adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2010;92:1073–1081. doi: 10.2106/JBJS.H.01759. [DOI] [PubMed] [Google Scholar]
- 76.Fawcett SL, Barter SJ. The use of gonad shielding in paediatric hip and pelvis radiographs. Br J Radiol. 2009;82:363–370. doi: 10.1259/bjr/86609718. [DOI] [PubMed] [Google Scholar]
- 77.Gul A, Zafar M, Maffulli N. Gonadal shields in pelvic radiographs in pediatric patients. Bull Hosp Jt Dis. 2005;63:13–14. [PubMed] [Google Scholar]
- 78.Karol LA, Johnston CE, 2nd, Browne RH, Madison M. Progression of the curve in boys who have idiopathic scoliosis. J Bone Joint Surg Am. 1993;75:1804–1810. doi: 10.2106/00004623-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 79.Wang WW, Xia CW, Zhu F, et al. Correlation of Risser sign, radiographs of hand and wrist with the histological grade of iliac crest apophysis in girls with adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2009;34:1849–1854. doi: 10.1097/BRS.0b013e3181ab358c. [DOI] [PubMed] [Google Scholar]
- 80.Pietka E, Gertych A, Pospiech S, Cao F, Huang HK, Gilsanz V. Computer-assisted bone age assessment: image preprocessing and epiphyseal/metaphyseal ROI extraction. IEEE Trans Med Imaging. 2001;20:715–729. doi: 10.1109/42.938240. [DOI] [PubMed] [Google Scholar]
- 81.Tanner JM, Whitehouse RH, Marshall WA, Healy MJ, Goldstein H. Skeletal maturity and prediction of adult height (TW2 Method) New York: Academic; 1965. [Google Scholar]
- 82.Kirks DR. Practical pediatric imaging: diagnostic radiology of infants and children. Boston: Little, Brown and Co.; 1984. [Google Scholar]
- 83.Buckler JM. Skeletal age changes in puberty. Arch Dis Child. 1984;59:115–119. doi: 10.1136/adc.59.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parfitt AM. Misconceptions (1): epiphyseal fusion causes cessation of growth. Bone. 2002;30:337–339. doi: 10.1016/s8756-3282(01)00668-8. [DOI] [PubMed] [Google Scholar]
- 85.Tanner JM, Whitehouse RH, Hughes PC, Carter BS. Relative importance of growth hormone and sex steroids for the growth at puberty of trunk length, limb length, and muscle width in growth hormone-deficient children. J Pediatr. 1976;89:1000–1008. doi: 10.1016/s0022-3476(76)80620-8. [DOI] [PubMed] [Google Scholar]
- 86.Tanner JM, Whitehouse RH, Marubini E, Resele LF. The adolescent growth spurt of boys and girls of the Harpenden growth study. Ann Hum Biol. 1976;3:109–126. doi: 10.1080/03014467600001231. [DOI] [PubMed] [Google Scholar]
- 87.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Stanford: Stanford University Press; 1959. [Google Scholar]
- 88.Tanner JM, Whitehouse RH, Marshall WA, Healy MJ, Goldstein H. Assessment of skeleta l maturity and prediction of adult height (TW3 Method) New York: Academic Press; 1975. [Google Scholar]
- 89.Todd TW. Atlas of skeletal maturation (hand) St. Louis: C. V. Mosby Co.; 1937. [Google Scholar]
- 90.Bull RK, Edwards PD, Kemp PM, Fry S, Hughes IA. Bone age assessment: a large scale comparison of the Greulich and Pyle, and Tanner and Whitehouse (TW2) methods. Arch Dis Child. 1999;81:172–173. doi: 10.1136/adc.81.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thodberg HH, Kreiborg S, Juul A, Pedersen KD. The BoneXpert method for automated determination of skeletal maturity. IEEE Trans Med Imaging. 2009;28:52–66. doi: 10.1109/TMI.2008.926067. [DOI] [PubMed] [Google Scholar]
- 92.Sanders JO, Khoury JG, Kishan S, et al. Predicting scoliosis progression from skeletal maturity: a simplified classification during adolescence. J Bone Joint Surg Am. 2008;90:540–553. doi: 10.2106/JBJS.G.00004. [DOI] [PubMed] [Google Scholar]
- 93.Luk KD, Saw LB, Grozman S, Cheung KM, Samartzis D. Assessment of skeletal maturity in scoliosis patients to determine clinical management: a new classification scheme using distal radius and ulna radiographs. Spine J. 2014;14:315–325. doi: 10.1016/j.spinee.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 94.Cheung JP, Samartzis D, Cheung PW, Cheung KM, Luk KD. Reliability analysis of the distal radius and ulna classification for assessing skeletal maturity for patients with adolescent idiopathic scoliosis. Global Spine J. 2016;6:164–168. doi: 10.1055/s-0035-1557142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheung JP, Samartzis D, Cheung PW, Leung KH, Cheung KM, Luk KD. The distal radius and ulna classification in assessing skeletal maturity: a simplified scheme and reliability analysis. J Pediatr Orthop B. 2015;24:546–551. doi: 10.1097/BPB.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 96.Albert AM, Maples WR. Stages of epiphyseal union for thoracic and lumbar vertebral centra as a method of age determination for teenage and young adult skeletons. J Forensic Sci. 1995;40:623–633. [PubMed] [Google Scholar]
- 97.Grave K, Townsend G. Hand-wrist and cervical vertebral maturation indicators: how can these events be used to time Class II treatments? Aust Orthod J. 2003;19:33–45. [PubMed] [Google Scholar]
- 98.Grave K, Townsend G. Cervical vertebral maturation as a predictor of the adolescent growth spurt. Aust Orthod J. 2003;19:25–32. [PubMed] [Google Scholar]
- 99.Hassel B, Farman AG. Skeletal maturation evaluation using cervical vertebrae. Am J Orthod Dentofacial Orthop. 1995;107:58–66. doi: 10.1016/s0889-5406(95)70157-5. [DOI] [PubMed] [Google Scholar]
- 100.Minars M, Burch J, Masella R, Meister M. Predicting skeletal maturation using cervical vertebrae. Todays FDA. 2003;15:17–19. [PubMed] [Google Scholar]
- 101.Sauvegrain J, Nahum H, Bronstein H. Study of bone maturation of the elbow. Ann Radiol (Paris) 1962;5:542–550. [PubMed] [Google Scholar]
- 102.Dimeglio A, Charles YP, Daures JP, de Rosa V, Kabore B. Accuracy of the Sauvegrain method in determining skeletal age during puberty. J Bone Joint Surg Am. 2005;87:1689–1696. doi: 10.2106/JBJS.D.02418. [DOI] [PubMed] [Google Scholar]