Abstract
Nucleic acid molecules are important therapeutic agents in the field of antisense oligonucleotide, RNA interference, and gene therapies. Since nucleic acids are not able to cross cell membranes and enter efficiently into cells on their own, the development of efficient, safe, and precise delivery systems is the crucial challenge for development of nucleic acid therapeutics. For the delivery of nucleic acids to their intracellular site of action, either the cytosol or the nucleus, several extracellular and intracellular barriers have to be overcome. Multifunctional carriers may handle the different special requirements of each barrier. The complexity of such macromolecules however poses a new hurdle in medical translation, which is the chemical production in reproducible and well-defined form. Solid-phase assisted synthesis (SPS) presents a solution for this challenge. The current review provides an overview on the design and SPS of precise sequence-defined synthetic carriers for nucleic acid cargos.
Keywords: Artificial carriers, Nucleic acid delivery, Peptide-based carriers, Solid-phase assisted synthesis, Sequence-defined synthetic carriers
Introduction
Administration of nucleic acids with therapeutic potential offers a promising approach for the treatment of several human diseases that reached already for medical use [1–5]. Availability of efficient and safe delivery systems is of primary importance for wider spread of successful gene-based therapies. Due to large size, biodegradability and the negative charge of exogenous nucleic acids (NA) such as plasmid DNA (pDNA), mRNA, small interfering RNA (siRNA), microRNA (miRNA), or antisense oligonucleotides, transfer of therapeutic NAs to target cells requires help of viral and nonviral gene delivery systems. Although in current therapeutic clinical trials viral vectors dominate due to their higher efficiency, synthetic carriers show their advantages in the type of nucleic acid cargo (including also artificial chemically modified forms) [6,7], manner of production, formulation property, and storage [8–10]. Research on lipidic, peptide or polymer-based carriers that complex therapeutic nucleic acid by electrostatic interaction, is of particular interest for nonviral delivery. These vehicles should complex nucleic acids by formation of stabile polyplexes or lipoplexes [11] and protect against degradation in the bloodstream and reach target cells. The next requirement is an efficient intracellular delivery by entering via endocytosis into the intracellular space [9,12,13]. Endocytosis via invagination of nanoparticles by the lipid cell membrane into endosomal vesicles requests later escape from endosome instead of endolysosomal degradation [14–16]. In case of pDNA, either the whole polyplexes or the released nucleic acid must subsequently enter the nucleus via passive, active, or cell-cycle dependent mechanisms [17–21] and be transcribed [22]. Nucleic acids such as siRNA, miRNA, or mRNA need to reach the cytoplasm for bioactivity. Although on the one side, stability of complexes is important in the time of extracellular delivery steps, on the other side, the carrier should release the NA in the intracellular space and should not influence its functionality. Thus, for a successful nucleic acid delivery, synthetic nucleic acid shuttles have to be responsive to a changing bioenvironment just like natural viruses. Chemistry, size, and topology (linear, branched, comb, hyperbranched, and dendritic) of the shuttle, as well as size and physicochemical characteristics of formed nanostructures can play a decisive role for the biological activity [23–33]. For carrier optimization under such complex situations, a careful structure–activity relationship of carriers and their nucleic acid delivery characteristics is mandatory. This also requests synthetic methods to produce carriers in chemical precise form. One option outlined in this review presents the application of solid-phase assisted synthesis (SPS). Synthesis of peptides by SPS was introduced by Merrifield in 1963 [34] and has been refined to a very potent technology, which has been even applicable for the assembly of whole proteins such erythropoietin [35]. Analogous progress has been made in the area of SPS of oligonucleotides, applying phosphoramidite chemistry as initially developed by Caruthers [36]. Synthesis of oligonucleotides nowadays is routine; even the synthesis and subsequent recombinant assembly of oligonucleotides into a whole bacterial DNA genome was possible [37]. By nature of chemistry, nucleic acid analogs with favorable characteristics over their natural counterparts were generated [6,7,38]. In the current review, we focus on the design of nucleic acid delivery carriers that were synthesized by SPS. We summarize recent progress in the optimization of carrier design, differences between the most common groups of sequence-defined carriers, and their application for nucleic acid delivery and therapy.
Solid-phase synthesis (SPS) of nucleic acid carriers
As outlined above, potent nucleic acid vehicles need to be bioresponsive and multifunctional. To enable clear-cut structure–activity relationships to be drawn, synthesis of precise carriers is needed. With improved chemistries, such as controlled radical polymer syntheses or specific ligation strategies, products with decreased polydispersity, and more controlled architectures of carriers can be achieved than before [39–44]. Nevertheless, the exact numbers of monomers and locations of attached subunits still are not under perfect control. As alternative, a series of researchers have applied the well-established method of SPS (see Figure 1) for developing linear [45–55] and branched [56–62] peptide-based, lipid-based [63–66], or artificial oligomer-based [66–78] sequence-defined nucleic acid carriers.
Figure 1. Precedure of SPS and exemplarily protected amino acids.
(A) Standard procedure of a solid-phase peptide synthesis cycle. tBoc as well as Fmoc strategy follow the same procedure of a repetitive coupling cycle. Resins are commonly swollen in DCM. Coupling requires activation of the carboxylic function of the amino acid either by carbodiimides or by formation of activated esters with PyBOP, HBTU, or HOBt and addition of DIPEA or TEA. Washing steps are performed with nonaqueous, peptide grade DMF, and DCM. A Kaiser test for detection of unprotected amines via ninhydrine reaction is done to verify successful coupling and deprotection. Nevertheless, tBoc and Fmoc strategies differ significantly regarding protecting groups, their removal (deprotection) as well as the final cleavage from the solid support. In tBoc strategy, α-amines of amino acids are tBoc protected, removal after coupling is performed with TFA and the final peptide cleavage is conducted with HF. In Fmoc strategy, α-amines of amino acids are Fmoc protected, removal after coupling is performed with a mixture of piperidine/DMF and the final peptide cleavage is conducted with a cleavage cocktail mainly consisting of TFA. (B) Protected lysine for tBoc (tBoc-L-Lys(Cbz)-OH) or (C) Fmoc (Fmoc-L-Lys(tBoc)-OH) strategy. (D) Fmoc, tBoc protected artificial oligoamino acids derived from polyethylenimine (PEI) repeating units; Cbz, benzyloxycarbonyl; Fmoc, 9-fluorenylmethoxycarbonyl; Gtp, glutaroyl-tetraethylene pentamine; Gtt, glutaroyl-triethylene tetramine; Sph, succinoyl pentaethylene hexamine; Stp, succinoyl tetraethylene pentamine; tBoc, tert-butyloxycarbonyl.
Merrifield introduced SPS as an alternative to liquid-phase peptide synthesis by selecting preactivated polystyrene as solid support for synthesis [34]. The first protected amino acid (AA) was loaded onto the resin. Orthogonally protected amino acids are coupled sequentially, with easy washing steps between coupling and removal of the protection group of the primary amine to constantly grow the macromolecule on the solid support, until the peptide and the remaining protecting groups (see Figure 1). In comparison with solution-phase synthesis, solid-phase synthesis offers three important advantages. First, easier purification of intermediates is possible, by simple removal of unreacted reagents by washing during synthesis. Second, reduction of side products (such as by repeated couplings or capping) leads to increased product yields. And third, due to the repetitive nature of the process, the whole assembly can be performed in automated form by peptide synthesizers.
The general procedure of a SPS cycle can be found in Figure 1A. Initially, tBoc chemistry was applied to the amino acid to protect the α-amino group. A solid support resin was introduced to sequentially assemble peptides [34]. The first amino acid with a tBoc α-amine (see Figure 1B) is linked to the solid support via the free, C-terminal carboxy group. The resin-bound amino acid is then treated with trifluoro acetic acid (TFA) to remove the tBoc protecting group and to free the α-amine. Now, the next tBoc protected α-amino acid can be coupled. For sequential amino acid coupling, the carboxylic acid group of the AA needs to be activated, most commonly by addition of dicyclohexylcarbodiimide (DCC). Dichloromethane (DCM) and dimethylformamide (DMF) are used as organic solvents, to create the required, nonaqueous environment for successful coupling, while facilitating swelling of the solid support during the reaction. At the end of the synthesis, the resin is treated with hydrofluoric acid (HF) to cleave the peptide from the resin and to remove all side chain protecting groups.
Replacement of classical tBoc chemistry by introducing the base-labile protecting group Fmoc (N-α-9-fluorenylmethyloxycarbonyl) [79–82] opened peptide manufacture to a wide range of operators as it no longer requires the application of the hazardous HF deprotection chemistry. The use of resins with novel acid labile linkers, like the hydroxymethyl-based Wang resin, the Rink amide resin, or the trityl chloride (especially 2-chlorotrityl chloride) [82] facilitated cleavage from the resin with TFA instead of HF. Instead of tBoc, Fmoc is now used to protect the α-amine of the amino acids (see an example in Figure 1C), which can easily be removed by non-nucleophilic bases like piperidine or diazabicycloundecene (DBU), while side chains of the amino acids are most commonly protected by acid labile protecting groups such as tBu, Trt, tBoc, or Pbf [83]. Also, sophisticated strategies for orthogonal synthesis of peptides are available [84]. The loading of the first Fmoc-protected AA onto the resin remains the initial step in Fmoc-peptide synthesis. After AA loading, free linkers on the resin need to be capped (e.g. with methanol) and the amount of AA loading needs to be determined by correlation of Fmoc absorbance [82]. The synthesis can then be continued in a defined matter by Fmoc deprotection of the first AA with a mixture of piperidine/DMF. The solvent, containing the cleaved Fmoc-group, is removed and the resin is washed with DMF and DCM. Then, the next Fmoc protected amino acid can be coupled. For this purpose, an excess of amino acid, and a weak, non-nucleophilic base like DIPEA (N,N-diisopropylethylamine) or TEA (triethylamine) are required. On the one hand, the base ensures a deprotonation of the amine. On the other hand, it deprotonates the carboxylic function to facilitate an easier active ester formation with an activation reagent. An activation reagent like PyBOP, HBTU, or HOBt, also preventing racemization, is usually dissolved in DMF to maintain nonaqueous environment. After coupling, the solvents are discarded, and the resin is washed with DMF and DCM. A ninhydrin reaction, detecting unreacted amines, can be now applied to validate successful amino acid coupling. This so-called Kaiser test [85] is based on the formation of a purple amino–ninhydrin complex (Ruhmanns purple), if free amines are present. After successful coupling, five cycles of Fmoc deprotection with piperidine/DMF are commonly applied. This cycle, from coupling to Fmoc removal, is conducted until the desired peptide is assembled. The resin is then dried and, dependent of the side chain protecting groups, a TFA-based cleavage cocktail, supplemented with the required scavengers like TIS (triisopropylsilane), water, or EDT (ethanedithiol), and the maintained products are purified via common purification methods such as SEC (size-exclusion chromatography).
Recently, the Fmoc peptide SPS strategy has been adopted for the synthesis of sequence-defined oligo(ethylenamino)amides (Figure 1D). Instead of natural amino acids, artificial oligoamino acids such as Stp (succinoyl tetraethylene pentamine), Gtp (glutaroyl-tetraethylene pentamine), or Sph (succinoyl pentaethylene hexamine) in Fmoc, tBoc-protected forms [75,86,87] can be used for manual or automated SPS, the latter requires a peptide synthesizer [88].
Carrier requirements: nucleic acid binding
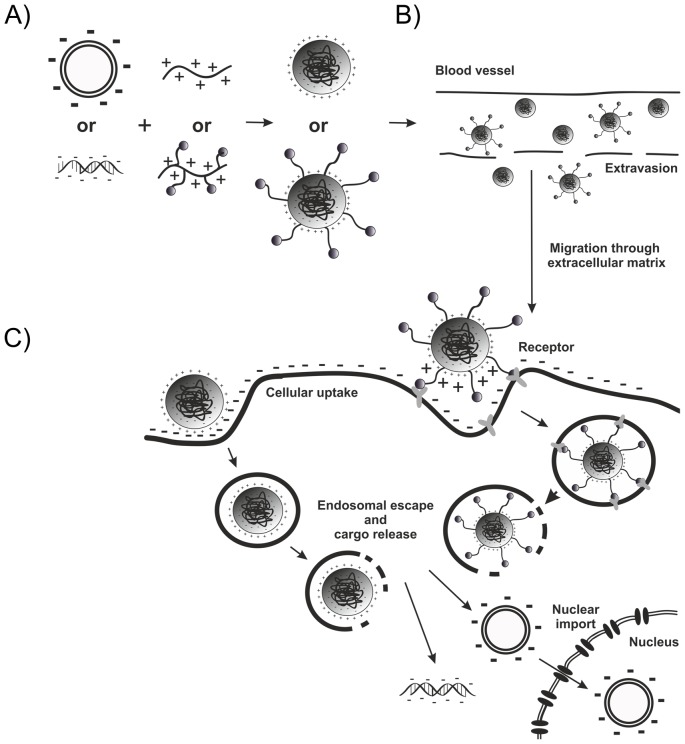
The multiple requirements for carriers to successfully deliver nucleic acids are described in Figure 2 in schematic form. Nonviral carriers tailored by solid-phase synthesis can be composed either solely of natural amino acids, solely of artificial building blocks, or of a combination of both (Figure 3). Especially homopolymers of the basic amino acid residues lysine (Figure 3A), ornithine, and arginine had shown ability to bind and condensate nucleic acid [89–92]. Later on, instead of polymerized amino acids, defined oligopeptides were developed via SPS [45–47,49,56]. Linear [45] as well as branched [56] oligolysine peptides were evaluated regarding nucleic acid binding and compaction as well as gene transfer. A minimum of six to eight cationic amino acids are required to compact pDNA into polyplexes active in gene delivery. The DNA binding and compaction ranked from arginine > lysine ∼ ornithine residues. Nucleic acid binding represents only one crucial step for successful gene delivery; not surprisingly, despite good nucleic acid binding oligolysine peptides could mediate gene transfer only to a limited extent, because of insufficient endosomal escape (see subsequent section). In several cases, combination with lysosomatropic chloroquine or lipidic helper molecules was necessary to mediate successful nucleic acid delivery [55,93–95].
Figure 2. Barriers for the nucleic acid delivery via polyplexes.
(A) Formation of stable polyplexes. (B) Protection against rapid clearance and unspecific interactions with blood components, and (C) overcoming cellular barriers
Figure 3. Oligopeptides and oligomers with nucleic acid binding motifs generated by SPS.
Nucleic acid binding motifs of (A) oligolysine and (B) acridine-modified oligolysine. (C) EHCO, a lipopeptide containing oleic acid, histidine, cysteine, and artificial aminoethyl blocks for nucleic acid binding. (D) Artificial amino acids derived from PEI repeat unit (see Figure 1) that are assembled by SPS to retrieve a nucleic acid binding domain within sequence-defined oligomers. (E) Example of HGFR/cMet targeted PEG-2-arm oligomer.
Branched peptides containing α,ε-modified lysines as branching points, and lysines and protonatable histidine as nucleic acid binding arms were found as very effective in either pDNA or siRNA transfer [57–61]. It had been observed that the type of nucleic acid cargo strongly influences the carrier performance [55,96,97]. Interestingly, combinatorial work pointed out that little changes in topology can decide on whether the carriers is effective for pDNA or siRNA delivery [60,61]. These peptides with incorporated histidines had significantly decreased cytotoxicity as compared with classical transfection polymers [98].
Introduction of cysteines into oligolysine peptides offered a biodegradable and cross-linking motif that allowed polymerization of Cys-Lys10-Cys corresponding to polylysine Lys205 [50,51]. Analogously, increased pDNA binding was obtained by introduction of cysteines via SPS into Trp-Lys18 peptides, which led to enhanced polyplex stability against salt induced stress [99]. Shorter peptides consisting of only six lysines mediated sufficient stability and notable gene transfer after cysteine dependent cross-linking [48]. With the help of convergent solid-phase synthesis, defined bioreducible polylysine derivatives comprising up to 74 lysines could be synthesized [100], revealing the possibilities of solid-phase synthesis.
In another approach, Rice and colleagues introduced acridine onto the ε-amine of a lysine suitable for SPS [101]. These acridinylated oligolysines complexed pDNA by charge dependent ionic interaction and also by polyintercalation (Figure 3B) [101–104].
Analogous to classical peptide synthesis, artificial building blocks such as triethylene tetramine or fatty acids were incorporated together with natural amino acids [53,66,67]. Wang et al. [66] designed a novel lipopeptide system (EHCO) based on (1-aminoethyl)iminobis [N-(oleoylcysteinylhistinyl-1-aminoethyl) propionamide] (Figure 3C) containing cysteines and oleic acids for siRNA nanoparticle stabilization, histidines for endosomal protonation, and (promoted by the fatty acids) endosomal membrane destabilization. The use of completely unnatural building blocks in SPS nucleic acid carriers was first introduced by Hartmann, Börner, and colleagues [68–74]. By alternating coupling of diamines (3,3′-diamino-N-methyl-dipropylamine or a bis-tBoc-protected spermine) and a diacid (succinic acid anhydride), the first sequence-defined oligo(amidoamines) were yielded. Optionally, disulfide linkage or a terminal PEG chain was introduced, and the sequence-defined oligomers were used for pDNA polyplex formation. Schaffert et al. [86] optimized the use of artificial amino acids for sequence-defined oligomer synthesis (Figure 3D). The design of the building blocks was based on the proton sponge diaminoethane motif of PEI. Triethylentetramine, tetraethylenpentamine, or pentaethylenhexamine were used with tBoc protection groups at the secondary amines and converted into artificial amino acids by introducing succinic acid onto one of the terminal primary amines, and Fmoc on the other primary amine [86,87]. With these novel artificial amino acids, oligomers were generated benefiting from the nucleic acid binding abilities as well as exhibiting a proton sponge effect, well known from PEI [23,105]. In combination with commercially available Fmoc α-amino acids, fatty acids, and also other artificial blocks introducing bioreducible breaking points [64], more than 1000 oligomers with different topologies for pDNA as well as siRNA delivery were synthesized. These topologies include linear [28,75], two-arm [75], three-arm [75,77,78,88], four-arm [75,87,106], comb architectures [29] as well as compounds with two cationic arms attached to a third arm of polyethylene glycol (PEG) of defined length and a targeting ligand (Figure 3E) [78,88,106–110].
With the precision of chemical design, in contrast with classical polymers like PEI or polylysine, oligomers could be generated to address simple questions on structure–activity relationships. For example, linear sequences of the building block Stp (succinyl tetraethylene pentamine, exhibiting three protonatable nitrogens per repetition) were prepared and the effect of increasing molecular weight of PEI-like oligomers on formed pDNA polyplexes could be investigated [28]. Very clearly, oligomers containing 20 Stp units (i.e. 100 nitrogen backbone) demonstrated good pDNA compaction, high marker gene transfer (6-fold higher than with gold standard LPEI 22kDa) in cell culture transfections, and an oligomer length-dependent 10-fold lower cytotoxicity than LPEI (containing in average an approximately 500 nitrogen backbone).
For further polyplex stabilization, terminal cysteines [75,77] or twin cysteines [111,112] served the formation of bioreducible disulfides. Optionally, further nanoparticle stabilization by incorporation of hydrophobic domains consisting of saturated as well as unsaturated fatty acids [64,75,77,113,114], or tyrosine trimers [64,113] at peripheral or central positions lead to T-shaped, i-shaped, or U-shaped oligomers with favorable properties for siRNA delivery in vitro as well as in vivo. Also the influence of different lengths of shielding agents in PEGylated two-arm structures on pDNA compaction and polyplex stability was examined [88]. An increased length of PEG (from 12 to 24 ethylene oxide units), resulting in a decreased polycation to PEG ratio, led to less compacted pDNA polyplexes as compared with unshielded polyplexes.
Remy and colleagues [115–117] took a completely different approach, designing a covalent incorporation of cationic carrier elements into nucleic acids. They adapted oligonucleotide SPS for synthesizing oligospermine–siRNA conjugates, which mediated efficient gene silencing in the absence of any other carrier. In course of their work, also lipidic elements were incorporated for improved efficacy [118].
Polyplex shielding
Nucleic acid complexation usually requires an excess of cationic charged carrier and thereby usually results in formation of nanoparticles with positive surface potential. This positive charge often displays an advantage for gene transfer efficacy in vitro due to unspecific binding to negatively charged cell surfaces [119,120] or by facilitating endosomal escape [121,122]. In the extracellular space, however, positively charged polyplexes depending on the applied cationic carrier may mediate undesired interactions with the complement system, blood cells, or other blood components [123–126]. Introduction of a hydrophilic surface shielding domain into artificial carriers has shown to reduce these interactions. PEG represents the most prominent and well-established shielding agent and has been successfully used for shielding of polyplexes in numerous instances, including SPS-designed nucleic acid carriers [12,103,123,127–131]. But also poly(N-(2-hydroxypropyl)methacrylamide) (pHPMA) [132,133], hydroxyethyl starch (HES) [134], polysarcosine [135], or repeats of Pro-Ala-Ser (PAS) [88] have been investigated as alternative hydrophilic shielding agents (Figure 4).
Figure 4. Chemical structures of the most prominent agents used for shielding.

Left: polyethylene glycol (PEG), right: poly(N-(2-hydroxypropyl)methacrylamide) (HPMA).
For example, Fmoc-PEGx-COOH was directly integrated into sequence-defined carriers during SPS (compare Figure 3D) [76,78,108,109,136]. Using folate or methotrexate (MTX) as folate receptor (FR) targeting ligands, small unimolecular siRNA nanoplexes were generated, which demonstrated FR-dependent in vivo gene silencing, and in case of MTX also therapeutic antitumor activity [76,136].
Although PEGylation may greatly improve pharmacokinetics and biodistribution to tumor target tissue, it may also negatively affect nucleic acid compaction (previous section) and intracellular performance [88,137,138]. The length of the PEG chain, and consequently the ratio of hydrophilic to cationic polymer within the polyplex, controls characteristics like nucleic acid compaction, polyplex size, and stability [88]. In a recent report by Kos et al. [78], systemic c-Met targeted gene transfer of pDNA polyplexes was successful, but only if combination polyplexes of a ligand-PEG carrier with a non-PEGylated compaction carrier were applied. Alternatively, to avoid difficulties with nucleic acid compaction, PEG was also introduced after pDNA [133,139] or siRNA [140–142] polyplex formation (“post-PEGylation”). For siRNA delivery, this approach led to increased tumor-specificity of RNA delivery in vivo, but only if tumor-specific ligands (EGFR binding peptide [140], transferrin protein [141], or folate [142], see next section) were applied.
Reduced intracellular efficacy is the second problem of the so-called “PEG-Dilemma”. As previously shown for other carriers, this problem can be overcome by introducing a pH-labile shield [130,143–145]. Removal of the shield at endosomal pH in the endolysosomal compartment was found to recover transfection activity in vitro and in vivo, also for pDNA polyplexes of sequence-defined oligomers [133].
Ligands for cellular targeting
After formulation, carriers loaded with nucleic acid have to be able to reach target cells. Physical concentration via adsorption, electrostatic interactions, and ligand–receptor interaction are possibilities for successful intracellular entry of vehicles. Nanoparticles, comprise nucleic acid and cationic core exhibiting target specific ligands, may facilitate specific binding to receptors expressed on the surface of target cells. Afterward, carriers can be taken up by the cell via receptor-mediated endocytosis [14]. When polyplexes are positively charged, unspecific ionic interactions can still reduce the value of targeting ligands. Hence, targeting ligands are introduced in combination with shielding agents described above. As mentioned, targeting ligands plus shielding agents can be included directly during the SPS, conjugated after the synthesis, or introduced after polyplex formation. Many different targeting ligands such as antibodies and their fragments, glycoproteins, peptides, and small molecules that can bind to receptors overexpressed in cancer or other target cells, have been investigated [13,146–149]. Up to now, several different receptor-targeted carriers based on SPS already showed favorable characteristics for enhanced nucleic acid delivery.
The group of Rice [150] designed an asialoglycoprotein receptor (ASGP-R) targeted carrier with triantennary galactose-terminated oligosaccharide as a ligand, which combined with the endosomalytical reagent chloroquine, enhanced DNA delivery on the HepG2 cell line. The same group showed receptor specific uptake of pDNA/polyacridine glycopeptides (Figure 3B). They introduced high-mannose N-glycane as a targeting ligand attached to modified forms of polyacridine peptides [151,152].
The ligand RGD (arginine–glycine–aspartic acid) is one of the most commonly used peptides for nucleic acid nanoparticle targeting cell–surface integrins [78,93,94,108,153,154]. RGD–oligolysine peptide in combination with lysosomatropic chloroquine or lipidic helper molecules mediated targeted nucleic acid delivery [55,59,93–95]. Leng et al. [59] developed a library of effective vehicles for siRNA delivery, branched peptides composed of histidines, and lysines (HK) with optionally attached RGD ligand. A promising integrin targeted siRNA delivery system, which showed efficient gene silencing in U87 glioma cells, was introduced by Wang et al. [154]. This system was based on (1-aminoethyl)iminobis [N-(oleoylcysteinylhistinyl-1-aminoethyl) propionamide] (EHCO), see Figure 3C. RGD was attached to siRNA nanoparticles via a PEG spacer. Analogously, bombesin was applied as another receptor ligand, which binds specifically to the gastrin-releasing peptide receptor, neuromedin B receptor, and the orphan receptor bombesin receptor subtype 3 that are overexpressed in various cancers. Systemic administration of the targeted nanoparticles loaded with anti-HIF-1α siRNA showed significant tumor growth inhibition in vivo [154].
Martin et al. [108] demonstrated ligand-dependent pDNA delivery by designing cyclic RGD-PEG-Stp 2-arm oligoaminoamides (Figure 3E); the same strategy was successfully developed for the targeting peptide B6, which was initially assumed to enhance uptake via the transferrin receptor (TfR) but later on was discovered as an TfR independent tumor cell uptake facilitator [155,156]. These initial conjugates were devoid of endosomal buffering histidines, therefore the presence of the endosomolytic reagent chloroquine was necessary for high level transfection. Subsequent work demonstrated a greatly improved transfection activity of PEGylated 2-arm structures upon incorporation of alternating histidines into the Stp carrier backbone [106]. This kind of oligomer, containing the peptide ligand cMBP2 binding to hepatocyte growth factor receptor/c-Met, showed enhanced gene delivery efficacy and target-specificity in vitro in HUH7 hepatoma and DU145 prostate carcinoma. Upon intravenous application in vivo in a hepatocellular carcinoma xenograft mouse model, specific and ligand-dependent gene transfer was detected, but only if combination polyplexes of a ligand-PEG carrier with a non-PEGylated compaction carrier were applied. Using a plasmid encoding the theranostic gene sodium iodide symporter (NIS), radioiodide-mediated tumor detection, and antitumoral activity were demonstrated [78,157].
In order to achieve improved selectivity and transfection activity, a dual-targeting concept, which simultaneously targets two different overexpressed receptors in tumors, was also investigated. Cyclic RGD peptide, B6 peptide, and the epidermal growth factor receptor targeting peptide GE11 were evaluated. In the investigated DU145 prostate cancer cell culture, which expresses all involved receptors, the most successful pDNA delivery was obtained by the combination of GE11 and B6 ligands [158]. EGFR targeting via peptide GE11 was also used for siRNA lipopolyplexes, which were surface-PEGylated with maleimide–PEG–GE11. These formulations showed potential for EGFR-specific siRNA and miRNA-200c delivery [140].
Transferrin (Tf) as an iron transport protein is targeting the transferrin receptor (TfR) overexpressed in many different malignant cells. Therefore, it was applied as ligand in pLys/pDNA polyplexes [159,160]. Previously, a Tf–pLys system was used for the preparation of IL-2 gene modified cancer vaccines in the first polyplex ex vivo human clinical gene therapy trial [161]. Tf–PEI conjugates were also shown to enhance gene transfection efficiency up to 1000-fold in TfR overexpressing cell lines [128,162–164]. A Tf–PEG-coated cationic cyclodextrin carrier was very effective in siRNA delivery, which was the basis for the first TfR-targeted in vivo siRNA human clinical trial [165]. Zhang et al. [166] combined sequence-defined, histidinylated 4-arm oligomers with Tf–PEI conjugates for efficient TfR-targeted pDNA delivery. An alternative TfR-targeted system was introduced by Prades et al. [167] with applying the retroenantio approach to a peptide that targets TfR; this was found capable to overcome the blood–brain barrier. Based on T-shaped lipo-oligomers, TfR-targeted siRNA polyplexes were generated by post-introduction of INF7 and PEG–Tf or PEG–TfR antibody (TfRab) onto the polyplex surface. These carriers mediated effective target-dependent gene silencing and potent tumor cell killing in vitro, as well as a tumor-target specific biodistribution in vivo, but limited in vivo stability [141].
Folic acid (FA), the vitamin with high-binding affinity to the FA receptor in many tumor types [168], was also effectively incorporated into 2-arm and 4-arm oligomers [106,107,109,169,170] or lipo-oligomers [142] for pDNA or siRNA delivery. FA–PEG–Stp 2-arms can formulate single influenza peptide INF7 conjugated-siRNA into very small nanoplexes [107]. The INF7 peptide was strictly required for endosomal escape. The analogous siRNA nanoplexes using MTX as targeting and cytotoxic ligand were able to cure mice from KB tumors after intratumoral application [76]. Combination of FA targeted PEGylated 2-arm oligomer with untargeted, 3-arm oligomer by directed disulfide exchange reaction resulted in generation of larger ∼100 nm TCP polyplexes, which enabled FA specific gene silencing in vivo also upon intravenous administration [170]. Optimization of FA–PEG containing carriers was extended in a library approach, evaluating 2-arms versus 4-arms, different building blocks, presence/absence of buffering histidines or polyplex-stabilizing tyrosine trimers. A two-arm folate-targeted oligomer containing histidines and tyrosine trimers was recognized as the most promising FA-containing carrier for the delivery of both pDNA and siRNA [109]. Folate receptor targeting by PEGylating siRNA lipopolyplexes was developed by Müller et al. [142] Tetra-γ-glutamyl FA had to be used as targeting ligand; PEGylation with standard FA–PEG (but not FA-free PEG) resulted in nanoparticle aggregation.
For targeting brain tumors, the blood–brain barrier (BBB) or at least the blood–tumor barrier presents a significant bottleneck. A combinatorial approach for effective glioma-targeted siRNA delivery was introduced by An and colleagues [171]. For siRNA lipopolyplex formation, a T-shaped oligoaminoamide was combined with an angiopep 2 (LRP-targeting peptide) attached via PEG to a sequence-defined 2-arm oligomer (compare Figure 3E). After intravenous delivery, receptor-enhanced accumulation in a brain tumor and enhanced gene silencing of a target gene were observed. Similarly, another glioma targeting ligand, I6P7, an interleukin-6 receptor binding peptide derived from IL-6, was included into a similar sequence defined carrier construct for glioma-targeted delivery of pDNA [110]. In this case, a histidinylated carrier version was applied and combined with a histidinylated compaction carrier analogously as described above for c-Met targeting [78]. In vitro and in vivo results demonstrated transfer across BBB as well as therapeutic antitumoral effects against the brain tumor when pING4 gene transfer was performed [110].
Endosomal escape
Effective endosomal escape to release the entrapped polyplexes into the cytosol is an important event for successful nucleic acid delivery. Otherwise, nucleic acid will be digested during the conversion of endosomes toward lysosomes or recycled to the cell surface and removed out of the cell. Endosomes are intracellular vesicles and mostly serve for sorting, trafficking, and recycling of endocytosed material. Active transport of protons from the cytosol into the vesicle generated by the action of the proton pump ATPase is a reason for acidification of a series of vesicles. Based on the proton sponge hypothesis (Figure 5A), Jean-Paul Behr and colleagues [105] screened a series of “proton-sponge” polymers which exhibit weakly basic functionalities with pKa values between physiological and endosomal pH. Thus during endocytic trafficking, such polymers would experience increase in protonation. Increased cationization and counterion concentration might be a reason for osmotic swelling and rupture of the endosomes membrane, causing the escape of polyplexes into the cytosol. Such considerations were the basis for the development of polyethylenimine (PEI) as transfection agent [23], or subsequent SPS-based oligoaminoamides [75,87] utilizing the aminoethylene motif of PEI. Uchida et al. [172] and later on Lächelt et al. [106] showed that oligoaminoethylene building blocks with even numbered amine groups (two or four protonatable nitrogens) have the highest buffer capacity around pH 5–6. Data accumulating during the last two decades rule out a purely osmotic effect for endosomal escape [121,126,143,173–176]. Direct interaction of protonated, cationized polymer domains with the endosomal phospholipid domain appear as essential for vesicle destabilization. In addition, free polycations (not bound to polyplexes) were found to critically contribute to gene delivery [177–180], and instead of complete lysis, only partial vesicle disruption was observed [176].
Figure 5. Strategies for endosomal escape.

(A) Schematic presentation of endosomal release by the proton sponge effect. Note that beyond osmotic swelling, direct destabilization of the phospholipid domain by the cationized polymer domains contributes to endosomal escape. (B) Membrane destabilization by amphiphilic lytic peptides.
Nonprotonatable polymers such as polylysine can be converted into proton sponges. It is known that histidinylation of polylysine or PEI offers higher endosomal buffer capacity based on a pKa around 6 of the imidazole groups; therefore, protonatable histidines were introduced into sequence-defined oligolysine-based carriers [48,57–61,78,106,109,181–185]. Consequently, total buffer capacities as well as nucleic acid transfer increased both in vitro and in vivo. Several groups reported about positive effect of histidines in the structures. Incorporation of histidines into a peptide of Cys-His-(Lys)6-His-Cys improved in vitro gene expression also in the absence of chloroquine as described by McKenzie et al. [48]. Read and colleagues reported efficient intracellular delivery of siRNA with histidine-rich reducible polycations [51]. The lab of Mixson developed a series of branched (HK) peptides containing lysines for nucleic acid binding and histidines for endosomal-buffering [57]. They further modified HK peptides of different length by adding histidine-rich tails. Thus, increased buffer capacity further improved transfection efficiency [60].
The proton sponge effect is not the only solution to overcome the endolysosomal entrapment. In fact, previous studies with (nonproton sponge) polylysine carriers already had shown that integration of fusogenic peptides (Figure 5B) such as influenza-derived INF1-7, JTS-1, or H5WYG into polylysine/pDNA polyplexes improved gene transfer significantly. The latter mentioned peptides mimic the functions of viral proteins and enable permeabilization of the endosomal membrane triggered by acidification of endosomes [186–188]. As reported by Dohmen et al. [107], the endosomolytic peptide INF7, originally designed as the glutamic acid-enriched analog of the influenza hemagglutinin membrane protein HA2 N-terminus, was coupled to the 5′-end of the siRNA sense strand, which maintains its silencing efficiency with increased endosomal escape when formulated into nanoplexes INF7 also greatly improved TfR-targeted siRNA lipoplexes when incorporated by post-modification of lipoplex surface [141] Artificial amphipathic cationic peptides such as KALA and LAH4, or derivatives of the bee venom melittin facilitated significantly improved gene transfer [189–191]. The latter peptides own two important properties for efficient gene transfer—possibility of DNA binding and destabilization of membranes. The positive charge of KALA allows electrostatic interactions with the negatively charged pDNA. However, the positive charged amphiphile KALA can also interact with the endosomal membrane and consequently can cause membrane leakage [189 ]. Next, partially mimicking the proton sponge activity of PEI and presence of histidine residues are responsible for improved endosomal escape in the case of LAH4 [190]. Boeckle et al. [191] showed that melittin–PEI conjugates can enhance gene transfer, but also cause high toxicity due to lysis of the plasma membrane. Therefore, modifications with acidic residues (glutamic acid or histidine) should allow high lytic activity at acidic pH to induce membrane destabilization in endosomes. Polyacridine peptides modified with melittin (by either a maleimide-Cys or a thiopyridine-Cys linkage) were used in pDNA transfection with efficacies as high as for PEI [101]. Also others peptides called cell-penetrating peptides (CPPs) promote endosomal escape, for example, PepFect6 [53] and PepFect14 [54].
In case of cationic lipoplexes, endosomal escape may occur through local, transient perturbations of the endosomal membrane by lipid mixing; cationic lipids possess the ability to form nonbilayer structures and charge neutral ion pairs with the negatively charged phospholipids (shift to the inner part of endosome caused by lipoplexes) [192]. Analogously, incorporation of fatty acids into polycation structures presents another option for generating amphiphilic characteristics that facilitate endosomal escape. The group of Lu generated lipo-oligomer carriers for pDNA and siRNA delivery, with two oleic acid residues triggering a pH-dependent disruption of lipid membranes [66]. Also Schaffert, Fröhlich, and colleagues generated lipo-oligomer carriers based on oligoaminoamides, which were modified with pairs of fatty acids incorporated at terminal lysine amines in i-, T-, or U-shaped topologies [75,77,114]. The type of incorporated fatty acid had more influence on the performance than the topology. Oligomers modified with the unsaturated (C18) fatty acids oleic acid and linoleic acids demonstrated best transfection efficiency due to endosomal pH-specific lytic activity. Furthermore, myristic acids (C14) caused high, but pH-independent lytic activity but also cytotoxic effects. Recently, Klein et al. [64] designed T-shaped oligomers containing a bioreducible disulfide bond between the cationic and lipid building block. Thus, the carriers would dissociate via GSH-mediated cleavage in the cytosol into nontoxic fragments leading to enhanced intracellular nucleic acid release while improving polyplex stability in the extracellular space. Using this strategy, bis-myristyl and bis-cholanic acid based lipo-oligomers should enable high lytic activity, high siRNA, delivery and silencing activity in the absence of cytotoxicity.
Cargo release and nuclear delivery
Polyplex stability is a critical issue for extracellular delivery, where high stability is of highest importance; it also is a critical parameter in intracellular delivery and subsequent cargo release at the target site, where nucleic acid release or at least exposure in bioactive form is important. To mediate gene silencing, siRNA and miRNA need only to reach the cytoplasm for incorporation into the RISC complex. For pDNA, further transport through the cytoplasm toward the nucleus (before or after endosomal escape, with or without complexation with cationic carrier), entry across the nuclear envelope, and accessibility for transcription are required.
Events following endosomal escape (fate of the polymer, nucleic acid, and different sortings of endosome) are still poorly understood. In fact, cargo release and productive delivery very much depend on the specific cargo size, the carrier, cell type, and different intracellular routes [193,194]; it is impossible to provide a general statement on the fates. First of all, even with effective nanoparticle systems, endosomal release is a rare event and bottleneck in the delivery process, therefore subsequent steps are difficult to track [176,195,196]. Even with potent siRNA LNPs, only 1–3% of internalized siRNA molecules were delivered into the cytosol [197,198]. For these LNPs, a narrow window of siRNA release from maturating endosomes approximately 5–15 min after internalization was observed. Releasing endosomes were recognized by cytosolic galectin-8/-9, which target them for autophagy [198]. Moreover, exocytosis of recycling siRNA nanoparticle-loaded vesicles was identified as a limitation [199]. In a different study, gene silencing potency correlated with intracellular siRNA lipopolyplex stabilization instead of early endosomal exit [200].
Only few studies have been performed comparing lipoplexes (e.g. lipofectamine) and polyplexes (with PEI), but significant differences were observed in the intracellular delivery steps [176,192,201,202]. Endosomal escape of lipoplexes by mixing of cationic lipids with the negatively charged phospholipids of endosomal membranes should release nucleic acids in lipid-free form [192,203]. For some lipoplex-mediated transfection using oligocationic lipids, however, despite effective nuclear delivery of pDNA, an insufficient release and availability for transcription were reported as possible limitation for gene transfer [204]. For polyplexes, the site of release from polycations such as PEI is even less clear, although delivery of small polyplexes was been reported. Interestingly, free PEI was found to not only enhance endosomal escape, but also assist in transfer of pDNA into the nucleus (by ∼5-fold), enhance the pDNA-to-mRNA transcription efficiency (by ∼4-fold), and facilitate the nucleus-to-cytosol translocation of mRNA (by 7–8-fold) [180].
Nuclear import is a crucial size-dependent process, and presents the next important barrier for delivery of larger nucleic acids such as pDNA [12]. The nuclear pore complex (NPC) only allows the passage of small molecules such as oligonucleotides [176,205,206]. whereas polyplexes greater than ∼50 nm do not have this capacity. In that case, nuclear entry relies on nuclear membrane breakdown during cell division process [207]. The importance of the nuclear import step has been demonstrated in cell cycle studies. Transfection efficiency of branched PEI polyplexes was strongly enhanced in the G2/M phase, when the nuclear envelope breaks down. In contrast, linear PEI polyplexes showed lower cell cycle dependence. The same was observed for c-Met-targeted, PEG shielded minicircle (MC) DNA polyplexes, which were well compacted into 35–40 nm rod structures by tyrosine trimer-containing Stp oligoaminoamides [208]. Conjugation of short cationic nuclear localization signals (NLS) peptide for an active, targeted transport through the NPC has been evaluated as a possible solution for cell-cycle independent gene transfer [12,18–20,209]. The exact conditions to successfully utilize the properties of NLS peptides are still unclear and therefore only a small number of carriers which could reach the nucleus have been described [153,210–215]. Further optimizations of nuclear import are required for improved pDNA delivery into nondividing cells.
Challenges of in vivo delivery
In vivo delivery faces several additional hurdles. As mentioned in previous sections, polyplex shielding and receptor-targeting are possible measures to avoid undesired reactions such as innate immune responses and to provide some specificity upon systemic administration, for example, in passive or active tumor targeting [123,124]. For this purpose, numerous targeting ligands for various cell surface receptors have been evaluated in vivo [9,55,62,65,78,110,128,156,157,165,171,200,216–221]. The polyplex size may be at least as crucial for in vivo performance as the ligand selection; for example, free siRNA or nanoparticles exhibiting a size of approximately 6 nm are quickly cleared by the kidney [107,222]. Passive targeting of blood-circulating nanoparticles by the EPR effect (enhanced permeability and retention of tumor tissue) offers polyplexes of a size of 20 nm up to 400 nm distribution into solid tumors via leaky vasculature [223,224]; the EPR effect, however, can be tumor type- and patient-specific and also heterogeneous within tumors. Polyplex delivery may be ineffective in less vascularized tumors [225]. For tumors such as stroma-rich pancreatic cancer, only smaller nanoparticles were effective [226]. Despite the many efforts, the efficiency of tumor targeting is still low; Chan and colleagues reviewed published work and concluded that on average only 0.7% of the dose is accumulating at the target tumor site [227].
Apart from targeting, shielding, and nanoparticle size, the stability of polyplexes is an additional challenge for in vivo performance; thus, additional measures such as bioreversible internal covalent cross-linkage of polyplexes or incorporation of bioresponsive domains into carriers for noncovalent stabilization have to be investigated [125,139,228–231]. Another critical aspect for in vivo gene delivery is the reduction of polyplex- and carrier-triggered toxicity. The transfection efficiency of frequently used high molecular weight PEI goes hand in hand with an N/P dependent cytotoxicity; mechanistic details are reviewed in Hall et al. [126]. Nevertheless, linear PEI has already been developed for clinical application with encouraging results [232]. The therapeutic window in systemic administration and wider therapeutic use still would strongly benefit from a reduced carrier cytotoxicity. In that view, degradable PEI analogs are highly desirable [233]. In this regard, SPS offers excellent opportunities to design structurally precise carriers with cysteine residues for cleavable linkages. During polyplex formation, the cysteines form bioreducible disulfides and thus enhance stability in the extracellular part of the gene delivery process. When having reached the bioreductive environment of the cytosol, bioreducibility of the polyplexes enhances cargo release and also cause fragmentation of the carriers into smaller less toxic pieces [46,48,50,51,75,100,111].
Conclusion and prospects
Over the past decades, significant progress has been made in the field of nucleic acid delivery vehicles. Sequence-defined macromolecular carriers synthesized by SPS play a significant role in this development. As several different extracellular and intracellular barriers must be overcome for successful transfer, the multifunctional nature of such carriers is of greatest importance. Carriers need to be dynamic [234,235]. On the one hand, stability of complexes is important at the time of extracellular delivery steps, while on the other hand, the carrier must release therapeutic nucleic acid after delivery inside the cell. SPS offers excellent opportunities to develop structural precise carriers, which is crucial for establishing appropriate structure–activity relationships. Still further optimization of delivery carriers is required. A better understanding of structures characteristics in nucleic acid complexation, target cell recognition, endosomal escape, nuclear delivery, and transgene expression or toxicity is necessary. In sum, sequence-defined delivery carriers containing natural and/or artificial building blocks represent a valuable part in the development of “smart” delivery systems, which will have great impact in the medicine of tomorrow.
Abbreviations
- DCC
dicyclohexylcarbodiimide
- DCM
dichloromethane
- GSH
Glutathione
- HBTU
2-(1H-Benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- HOBt
Hydroxybenzotriazole
- HF
hydrofluoric acid
- INF7
glutamic acid-enriched analogue of the influenza hemagglutinin membrane protein HA2
- LPEI
linear polyethylenimine
- PEG
polyethylene glycol
- PEI
polyethylenimine
- PyBOP
Benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate
- SPS
solid-phase assisted synthesis
- TFA
trifluoro acetic acid
- RISC
RNA-induced silencing complex
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) [Cluster of Excellence Nanosystems Initiative Munich (NIM), SFB1032 (project B 4), SFB1066 (project B 5), SFB824 (project C 8)] and Sino-German Center research grant GZ995.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Friedmann T. and Roblin R. (1972) Gene therapy for human genetic disease? Science 175, 949. [DOI] [PubMed] [Google Scholar]
- 2.Wirth T., Parker N. and Yla-Herttuala S. (2013) History of gene therapy. Gene 525, 162. [DOI] [PubMed] [Google Scholar]
- 3.Touchot N. and Flume M. (2017) Early insights from commercialization of gene therapies in Europe. Genes 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Titze-de-Almeida R., David C. and Titze-de-Almeida S.S. (2017) The race of 10 synthetic RNAi-based drugs to the pharmaceutical market. Pharm. Res. 34, 1339. [DOI] [PubMed] [Google Scholar]
- 5.Stein C.A. and Castanotto D. (2017) FDA-approved oligonucleotide therapies in 2017. Mol. Ther. 25, 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parmar R., Willoughby J.L., Liu J., Foster D.J., Brigham B., Theile C.S. et al. (2016) 5′-(E)-Vinylphosphonate: a stable phosphate mimic can improve the RNAi activity of siRNA-GalNAc conjugates. ChemBioChem 17, 985. [DOI] [PubMed] [Google Scholar]
- 7.Behlke M.A. (2008) Chemical modification of siRNAs for in vivo use. Oligonucleotides 18, 305. [DOI] [PubMed] [Google Scholar]
- 8.Hardee C.L., Arevalo-Soliz L.M., Hornstein B.D. and Zechiedrich L. (2017) Advances in non-viral DNA vectors for gene therapy. Genes 8, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lächelt U. and Wagner E. (2015) Nucleic acid therapeutics using polyplexes: a journey of 50 years (and beyond). Chem. Rev. 115, 11043. [DOI] [PubMed] [Google Scholar]
- 10.Walther W., Schmeer M., Kobelt D., Baier R., Harder A., Walhorn V. et al. (2013) A seven-year storage report of good manufacturing practice-grade naked plasmid DNA: stability, topology, and in vitro/in vivo functional analysis. Hum. Gene Ther.: Clin. Dev. 24, 147. [DOI] [PubMed] [Google Scholar]
- 11.Felgner P.L., Barenholz Y., Behr J.P., Cheng S.H., Cullis P., Huang L. et al. (1997) Nomenclature for synthetic gene delivery systems. Hum. Gene Ther. 8, 511. [DOI] [PubMed] [Google Scholar]
- 12.Pack D.W., Hoffman A.S., Pun S. and Stayton P.S. (2005) Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 4, 581. [DOI] [PubMed] [Google Scholar]
- 13.Zhang P. and Wagner E. (2017) History of polymeric gene delivery systems. Top. Curr. Chem. 375, 26. [DOI] [PubMed] [Google Scholar]
- 14.Rejman J., Oberle V., Zuhorn I.S. and Hoekstra D. (2004) Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 377, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoekstra D., Rejman J., Wasungu L., Shi F. and Zuhorn I. (2007) Gene delivery by cationic lipids: in and out of an endosome. Biochem. Soc. Trans. 35, 68. [DOI] [PubMed] [Google Scholar]
- 16.Lechardeur D., Verkman A.S. and Lukacs G.L. (2005) Intracellular routing of plasmid DNA during non-viral gene transfer. Adv. Drug Delivery Rev. 57, 755. [DOI] [PubMed] [Google Scholar]
- 17.Vacik J., Dean B.S., Zimmer W.E. and Dean D.A. (1999) Cell-specific nuclear import of plasmid DNA 927. Gene Ther. 6, 1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunner S., Furtbauer E., Sauer T., Kursa M. and Wagner E. (2002) Overcoming the nuclear barrier: cell cycle independent nonviral gene transfer with linear polyethylenimine or electroporation. Mol. Ther. 5, 80. [DOI] [PubMed] [Google Scholar]
- 19.Brunner S., Sauer T., Carotta S., Cotten M., Saltik M. and Wagner E. (2000) Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther. 7, 401. [DOI] [PubMed] [Google Scholar]
- 20.Grandinetti G., Smith A.E. and Reineke T.M. (2012) Membrane and nuclear permeabilization by polymeric pDNA vehicles: efficient method for gene delivery or mechanism of cytotoxicity? Mol. Pharmaceutics 9, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen H., Parhamifar L., Hunter A.C., Shahin V. and Moghimi S.M. (2016) AFM visualization of sub-50nm polyplex disposition to the nuclear pore complex without compromising the integrity of the nuclear envelope. J. Controlled Release 244, 24. [DOI] [PubMed] [Google Scholar]
- 22.Akita H., Kurihara D., Schmeer M., Schleef M. and Harashima H. (2015) Effect of the compaction and the size of DNA on the nuclear transfer efficiency after microinjection in synchronized cells. Pharmaceutics 7, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boussif O., Lezoualc’h F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B. et al. (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U.S.A. 92, 7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coll J.L., Chollet P., Brambilla E., Desplanques D., Behr J.P. and Favrot M. (1999) In vivo delivery to tumors of DNA complexed with linear polyethylenimine. Hum. Gene Ther. 10, 1659. [DOI] [PubMed] [Google Scholar]
- 25.Kadlecova Z., Rajendra Y., Matasci M., Baldi L., Hacker D.L., Wurm F.M. et al. (2013) DNA delivery with hyperbranched polylysine: a comparative study with linear and dendritic polylysine. J. Controlled Release 169, 276. [DOI] [PubMed] [Google Scholar]
- 26.Werth S., Urban-Klein B., Dai L., Hobel S., Grzelinski M., Bakowsky U. et al. (2006) A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J. Controlled Release 112, 257. [DOI] [PubMed] [Google Scholar]
- 27.Jeong G.J., Byun H.M., Kim J.M., Yoon H., Choi H.G., Kim W.K. et al. (2007) Biodistribution and tissue expression kinetics of plasmid DNA complexed with polyethylenimines of different molecular weight and structure. J. Controlled Release 118, 118–125 [DOI] [PubMed] [Google Scholar]
- 28.Scholz C., Kos P., Leclercq L., Jin X., Cottet H. and Wagner E. (2014) Correlation of length of linear oligo(ethanamino) amides with gene transfer and cytotoxicity. ChemMedChem 9, 2104. [DOI] [PubMed] [Google Scholar]
- 29.Scholz C., Kos P. and Wagner E. (2014) Comb-like oligoaminoethane carriers: change in topology improves pDNA delivery. Bioconjugate Chem. 25, 251. [DOI] [PubMed] [Google Scholar]
- 30.Tang R., Palumbo R.N., Nagarajan L., Krogstad E. and Wang C. (2010) Well-defined block copolymers for gene delivery to dendritic cells: probing the effect of polycation chain-length. J. Controlled Release 142, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson R.N., Chu D.S., Shi J., Schellinger J.G., Carlson P.M. and Pun S.H. (2011) HPMA-oligolysine copolymers for gene delivery: optimization of peptide length and polymer molecular weight. J. Controlled Release 155, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei H., Pahang J.A. and Pun S.H. (2013) Optimization of brush-like cationic copolymers for nonviral gene delivery. Biomacromolecules 14, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprouse D. and Reineke T.M. (2014) Investigating the effects of block versus statistical glycopolycations containing primary and tertiary amines for plasmid DNA delivery. Biomacromolecules 15, 2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merrifield R.B. (1963) Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 85, 2149 [Google Scholar]
- 35.Wang P., Dong S., Shieh J.H., Peguero E., Hendrickson R., Moore M.A.S. et al. (2013) Erythropoietin derived by chemical synthesis. Science 342, 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caruthers M.H., Beaucage S.L., Becker C., Efcavitch J.W., Fisher E.F., Galluppi G. et al. (1983) Deoxyoligonucleotide synthesis via the phosphoramidite method. Gene Amplif. Anal. 3, 1. [PubMed] [Google Scholar]
- 37.Gibson D.G., Glass J.I., Lartigue C., Noskov V.N., Chuang R.Y., Algire M.A. et al. (2010) Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329, 52. [DOI] [PubMed] [Google Scholar]
- 38.Wagner E. (2013) Biomaterials in RNAi therapeutics: quo vadis? Biomater. Sci. 1, 804. [DOI] [PubMed] [Google Scholar]
- 39.Lutz J.-F. (2010) Sequence-controlled polymerizations: the next Holy Grail in polymer science? Polym. Chem. 1, 55 [Google Scholar]
- 40.Lutz J.F., Ouchi M., Liu D.R. and Sawamoto M. (2013) Sequence-controlled polymers. Science 341, 1238149. [DOI] [PubMed] [Google Scholar]
- 41.Stenzel M.H. (2008) RAFT polymerization: an avenue to functional polymeric micelles for drug delivery. Chem. Commun. 3486. [DOI] [PubMed] [Google Scholar]
- 42.Vazquez-Dorbatt V., Lee J., Lin E.W. and Maynard H.D. (2012) Synthesis of glycopolymers by controlled radical polymerization techniques and their applications. ChemBioChem 13, 2478. [DOI] [PubMed] [Google Scholar]
- 43.Mori H. and Endo T. (2012) Amino-acid-based block copolymers by RAFT polymerization. Macromol. Rapid Commun. 33, 1090. [DOI] [PubMed] [Google Scholar]
- 44.Boyer C., Bulmus V., Davis T.P., Ladmiral V., Liu J. and Perrier S. (2009) Bioapplications of RAFT polymerization. Chem. Rev. 109, 5402. [DOI] [PubMed] [Google Scholar]
- 45.Wadhwa M.S., Collard W.T., Adami R.C., McKenzie D.L. and Rice K.G. (1997) Peptide-mediated gene delivery: influence of peptide structure on gene expression. Bioconjugate Chem. 8, 81. [DOI] [PubMed] [Google Scholar]
- 46.Adami R.C., Collard W.T., Gupta S.A., Kwok K.Y., Bonadio J. and Rice K.G. (1998) Stability of peptide-condensed plasmid DNA formulations. J. Pharm. Sci. 87, 678. [DOI] [PubMed] [Google Scholar]
- 47.van Rossenberg S.M., van Keulen A.C., Drijfhout J.W., Vasto S., Koerten H.K., Spies F. et al. (2004) Stable polyplexes based on arginine-containing oligopeptides for in vivo gene delivery. Gene Ther. 11, 457. [DOI] [PubMed] [Google Scholar]
- 48.McKenzie D.L., Smiley E., Kwok K.Y. and Rice K.G. (2000) Low molecular weight disulfide cross-linking peptides as nonviral gene delivery carriers. Bioconjugate Chem. 11, 901. [DOI] [PubMed] [Google Scholar]
- 49.Parker A.L., Fisher K.D., Oupicky D., Read M.L., Nicklin S.A., Baker A.H. et al. (2005) Enhanced gene transfer activity of peptide-targeted gene-delivery vectors. J. Drug Targeting 13, 39. [DOI] [PubMed] [Google Scholar]
- 50.Read M.L., Bremner K.H., Oupicky D., Green N.K., Searle P.F. and Seymour L.W. (2003) Vectors based on reducible polycations facilitate intracellular release of nucleic acids. J. Gene Med. 5, 232. [DOI] [PubMed] [Google Scholar]
- 51.Read M.L., Singh S., Ahmed Z., Stevenson M., Briggs S.S., Oupicky D. et al. (2005) A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Res. 33, e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehto T., Abes R., Oskolkov N., Suhorutsenko J., Copolovici D.M., Mager I. et al. (2010) Delivery of nucleic acids with a stearylated (RxR)4 peptide using a non-covalent co-incubation strategy. J. Controlled Release 141, 42. [DOI] [PubMed] [Google Scholar]
- 53.Andaloussi S.E., Lehto T., Mager I., Rosenthal-Aizman K., Oprea II, Simonson O.E. et al. (2011) Design of a peptide-based vector, PepFect6, for efficient delivery of siRNA in cell culture and systemically in vivo. Nucleic Acids Res. 39, 3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ezzat K., Andaloussi S.E., Zaghloul E.M., Lehto T., Lindberg S., Moreno P.M. et al. (2011) PepFect 14, a novel cell-penetrating peptide for oligonucleotide delivery in solution and as solid formulation. Nucleic Acids Res. 39, 5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwok A., McCarthy D., Hart S.L. and Tagalakis A.D. (2016) Systematic comparisons of formulations of linear oligolysine peptides with siRNA and plasmid DNA. Chem. Biol. Drug Des. 87, 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Plank C., Tang M.X., Wolfe A.R. and Szoka F.C. Jr (1999) Branched cationic peptides for gene delivery: role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum. Gene Ther. 10, 319. [DOI] [PubMed] [Google Scholar]
- 57.Chen Q.R., Zhang L., Stass S.A. and Mixson A.J. (2001) Branched co-polymers of histidine and lysine are efficient carriers of plasmids. Nucleic Acids Res. 29, 1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leng Q. and Mixson A.J. (2005) Small interfering RNA targeting Raf-1 inhibits tumor growth in vitro and in vivo. Cancer Gene Ther. 12, 682. [DOI] [PubMed] [Google Scholar]
- 59.Leng Q., Scaria P., Zhu J., Ambulos N., Campbell P. and Mixson A.J. (2005) Highly branched HK peptides are effective carriers of siRNA. J. Gene Med. 7, 977. [DOI] [PubMed] [Google Scholar]
- 60.Leng Q. and Mixson A.J. (2005) Modified branched peptides with a histidine-rich tail enhance in vitro gene transfection. Nucleic Acids Res. 33, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chou S.T., Hom K., Zhang D., Leng Q., Tricoli L.J., Hustedt J.M. et al. (2014) Enhanced silencing and stabilization of siRNA polyplexes by histidine-mediated hydrogen bonds. Biomaterials 35, 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leng Q. and Mixson A.J. (2016) The neuropilin-1 receptor mediates enhanced tumor delivery of H2K polyplexes. J. Gene Med. 18, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dauty E., Remy J.-S., Blessing T. and Behr J.-P. (2001) Dimerizable cationic detergents with a low cmc condense plasmid DNA into nanometric particles and transfect cells in culture. J. Am. Chem. Soc. 123, 9227. [DOI] [PubMed] [Google Scholar]
- 64.Klein P.M., Reinhard S., Lee D.J., Müller K., Ponader D., Hartmann L. et al. (2016) Precise redox-sensitive cleavage sites for improved bioactivity of siRNA lipopolyplexes. Nanoscale 8, 18098. [DOI] [PubMed] [Google Scholar]
- 65.Wang X.L., Xu R. and Lu Z.R. (2009) A peptide-targeted delivery system with pH-sensitive amphiphilic cell membrane disruption for efficient receptor-mediated siRNA delivery. J. Controlled Release 134, 207. [DOI] [PubMed] [Google Scholar]
- 66.Wang X.L., Ramusovic S., Nguyen T. and Lu Z.R. (2007) Novel polymerizable surfactants with pH-sensitive amphiphilicity and cell membrane disruption for efficient siRNA delivery. Bioconjugate Chem. 18, 2169. [DOI] [PubMed] [Google Scholar]
- 67.Wang X.L., Jensen R. and Lu Z.R. (2007) A novel environment-sensitive biodegradable polydisulfide with protonatable pendants for nucleic acid delivery. J. Controlled Release 120, 250. [DOI] [PubMed] [Google Scholar]
- 68.Hartmann L., Krause E., Antonietti M. and Borner H.G. (2006) Solid-phase supported polymer synthesis of sequence-defined, multifunctional poly(amidoamines). Biomacromolecules 7, 1239. [DOI] [PubMed] [Google Scholar]
- 69.Hartmann L., Häfele S., Peschka-Süss R., Antonietti M. and Börner H.G. (2007) Sequence positioning of disulfide linkages to program the degradation of monodisperse poly(amidoamines). Macromolecules 40, 7771 [Google Scholar]
- 70.Hartmann L., Hafele S., Peschka-Suss R., Antonietti M. and Borner H.G. (2008) Tailor-made poly(amidoamine)s for controlled complexation and condensation of DNA. Chemistry 14, 2025. [DOI] [PubMed] [Google Scholar]
- 71.Hartmann L. and Borner H.G. (2009) Precision polymers: monodisperse, monomer-sequence-defined segments to target future demands of polymers in medicine. Adv. Mater. 21, 3425. [DOI] [PubMed] [Google Scholar]
- 72.Mosca S., Wojcik F. and Hartmann L. (2011) Precise positioning of chiral building blocks in monodisperse, sequence-defined polyamides. Macromol. Rapid Commun. 32, 197. [DOI] [PubMed] [Google Scholar]
- 73.Wojcik F., Mosca S. and Hartmann L. (2012) Solid-phase synthesis of asymmetrically branched sequence-defined poly/oligo(amidoamines). J. Org. Chem. 77, 4226. [DOI] [PubMed] [Google Scholar]
- 74.Ponader D., Wojcik F., Beceren-Braun F., Dernedde J. and Hartmann L. (2012) Sequence-defined glycopolymer segments presenting mannose: synthesis and lectin binding affinity. Biomacromolecules 13, 1845. [DOI] [PubMed] [Google Scholar]
- 75.Schaffert D., Troiber C., Salcher E.E., Fröhlich T., Martin I., Badgujar N. et al. (2011) Solid-phase synthesis of sequence-defined T-, i-, and U-shape polymers for pDNA and siRNA delivery. Angew. Chem., Int. Ed. 50, 8986. [DOI] [PubMed] [Google Scholar]
- 76.Lee D.J., Kessel E., Edinger D., He D., Klein P.M., Voith von Voithenberg L. et al. (2016) Dual antitumoral potency of EG5 siRNA nanoplexes armed with cytotoxic bifunctional glutamyl-methotrexate targeting ligand. Biomaterials 77, 98. [DOI] [PubMed] [Google Scholar]
- 77.Fröhlich T., Edinger D., Kläger R., Troiber C., Salcher E., Badgujar N. et al. (2012) Structure-activity relationships of siRNA carriers based on sequence-defined oligo (ethane amino) amides. J. Controlled Release 160, 532. [DOI] [PubMed] [Google Scholar]
- 78.Kos P., Lächelt U., Herrmann A., Mickler F.M., Döblinger M., He D. et al. (2015) Histidine-rich stabilized polyplexes for cMet-directed tumor-targeted gene transfer. Nanoscale 7, 5350. [DOI] [PubMed] [Google Scholar]
- 79.Carpino L.A. and Han G.Y. (1970) 9-Fluorenylmethoxycarbonyl function, a new base-sensitive amino-protecting group. J. Am. Chem. Soc. 92, 5748 [Google Scholar]
- 80.Atherton E., Fox H., Harkiss D., Logan C.J., Sheppard R.C. and Williams B.J. (1978) A mild procedure for solid phase peptide synthesis: use of fluorenylmethoxycarbonylamino-acids. Chem. Commun., 0, 537 [Google Scholar]
- 81.Chang C.D. and Meienhofer J. (1978) Solid-phase peptide synthesis using mild base cleavage of N alpha-fluorenylmethyloxycarbonylamino acids, exemplified by a synthesis of dihydrosomatostatin. Int. J. Pept. Protein Res. 11, 246. [DOI] [PubMed] [Google Scholar]
- 82.Chan W.C. and White P.D. (2000) Fmoc solid phase peptide synthesis: a practical approach, Oxford University Press, 1 [Google Scholar]
- 83.Orain D., Ellard J. and Bradley M. (2002) Protecting groups in solid-phase organic synthesis. J. Comb. Chem. 4, 1. [DOI] [PubMed] [Google Scholar]
- 84.Nash A., Bycroft B.W. and Chan W.C. (1996) Dde – a selective primary amine protecting group: a facile solid phase synthetic approach to polyamine conjugates. Tetrahedron Lett. 37, 2625 [Google Scholar]
- 85.Kaiser E., Colescott R.L., Bossinger C.D. and Cook P.I. (1970) Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 34, 595. [DOI] [PubMed] [Google Scholar]
- 86.Schaffert D., Badgujar N. and Wagner E. (2011) Novel Fmoc-polyamino acids for solid-phase synthesis of defined polyamidoamines. Org. Lett. 13, 1586. [DOI] [PubMed] [Google Scholar]
- 87.Salcher E.E., Kos P., Fröhlich T., Badgujar N., Scheible M. and Wagner E. (2012) Sequence-defined four-arm oligo(ethanamino)amides for pDNA and siRNA delivery: Impact of building blocks on efficacy. J. Controlled Release 164, 380. [DOI] [PubMed] [Google Scholar]
- 88.Morys S., Krhac Levacic A., Urnauer S., Kempter S., Kern S., Rädler J.O. et al. (2017) Influence of defined hydrophilic blocks within oligoaminoamide copolymers: Compaction versus shielding of pDNA nanoparticles. Polymers 9, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wagner K.G. and Arav R. (1968) On the interaction of nucleotides with poly-L-lysine and poly-L-arginine. I. The influence of the nucleotide base on the binding behavior. Biochemistry 7, 1771. [DOI] [PubMed] [Google Scholar]
- 90.Farber F.E., Melnick J.L. and Butel J.S. (1975) Optimal conditions for uptake of exogenous DNA by Chinese hamster lung cells deficient in hypoxanthine-guanine phosphoribosyltransferase. Biochim. Biophys. Acta 390, 298. [DOI] [PubMed] [Google Scholar]
- 91.Wu G.Y. and Wu C.H. (1988) Receptor-mediated gene delivery and expression in vivo. J. Biol. Chem. 262, 14621. [PubMed] [Google Scholar]
- 92.Wagner E., Cotten M., Foisner R. and Birnstiel M.L. (1991) Transferrin-polycation-DNA complexes: the effect of polycations on the structure of the complex and DNA delivery to cells. Proc. Natl. Acad. Sci. U.S.A. 88, 4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hart S.L., Harbottle R.P., Cooper R., Miller A., Williamson R. and Coutelle C. (1995) Gene delivery and expression mediated by an integrin-binding peptide 274. Gene Ther. 2, 552. [PubMed] [Google Scholar]
- 94.Harbottle R.P., Cooper R.G., Hart S.L., Ladhoff A., McKay T., Knight A.M. et al. (1998) An RGD-oligolysine peptide: a prototype construct for integrin-mediated gene delivery. Hum. Gene Ther. 9, 1037. [DOI] [PubMed] [Google Scholar]
- 95.Tagalakis A.D., Lee D.H., Bienemann A.S., Zhou H., Munye M.M., Saraiva L. et al. (2014) Multifunctional, self-assembling anionic peptide-lipid nanocomplexes for targeted siRNA delivery. Biomaterials 35, 8406. [DOI] [PubMed] [Google Scholar]
- 96.Scholz C. and Wagner E. (2012) Therapeutic plasmid DNA versus siRNA delivery: common and different tasks for synthetic carriers. J. Controlled Release 161, 554. [DOI] [PubMed] [Google Scholar]
- 97.Kwok A. and Hart S.L. (2011) Comparative structural and functional studies of nanoparticle formulations for DNA and siRNA delivery. Nanomedicine 7, 210. [DOI] [PubMed] [Google Scholar]
- 98.Leng Q., Goldgeier L., Zhu J., Cambell P., Ambulos N. and Mixson A.J. (2007) Histidine-lysine peptides as carriers of nucleic acids. Drug News Perspect. 20, 77. [DOI] [PubMed] [Google Scholar]
- 99.McKenzie D.L., Kwok K.Y. and Rice K.G. (2000) A potent new class of reductively activated peptide gene delivery agents. J. Biol. Chem. 275, 9970. [DOI] [PubMed] [Google Scholar]
- 100.Ericson M.D. and Rice K.G. (2013) A convergent synthesis of homogeneous reducible polypeptides. Tetrahedron Lett. 54, 4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baumhover N.J., Anderson K., Fernandez C.A. and Rice K.G. (2010) Synthesis and in vitro testing of new potent polyacridine-melittin gene delivery peptides. Bioconjugate Chem. 21, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kizzire K., Khargharia S. and Rice K.G. (2013) High-affinity PEGylated polyacridine peptide polyplexes mediate potent in vivo gene expression. Gene Ther. 20, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khargharia S., Kizzire K., Ericson M.D., Baumhover N.J. and Rice K.G. (2013) PEG length and chemical linkage controls polyacridine peptide DNA polyplex pharmacokinetics, biodistribution, metabolic stability and in vivo gene expression. J. Controlled Release 170, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fernandez C.A., Baumhover N.J., Duskey J.T., Khargharia S., Kizzire K., Ericson M.D. et al. (2011) Metabolically stabilized long-circulating PEGylated polyacridine peptide polyplexes mediate hydrodynamically stimulated gene expression in liver. Gene Ther. 18, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Behr J.P. (1997) The proton sponge: a trick to enter cells the viruses did not exploit. Chimia 51, 34 [Google Scholar]
- 106.Lächelt U., Kos P., Mickler F.M., Herrmann A., Salcher E.E., Rödl W. et al. (2014) Fine-tuning of proton sponges by precise diaminoethanes and histidines in pDNA polyplexes. Nanomedicine 10, 35. [DOI] [PubMed] [Google Scholar]
- 107.Dohmen C., Edinger D., Fröhlich T., Schreiner L., Lächelt U., Troiber C. et al. (2012) Nanosized multifunctional polyplexes for receptor-mediated siRNA delivery. ACS Nano 6, 5198. [DOI] [PubMed] [Google Scholar]
- 108.Martin I., Dohmen C., Mas-Moruno C., Troiber C., Kos P., Schaffert D. et al. (2012) Solid-phase-assisted synthesis of targeting peptide-PEG-oligo(ethane amino)amides for receptor-mediated gene delivery. Org. Biomol. Chem. 10, 3258. [DOI] [PubMed] [Google Scholar]
- 109.He D., Müller K., Krhac Levacic A., Kos P., Lächelt U. and Wagner E. (2016) Combinatorial optimization of sequence-defined oligo(ethanamino)amides for folate receptor-targeted pDNA and siRNA delivery. Bioconjugate Chem. 27, 647. [DOI] [PubMed] [Google Scholar]
- 110.Wang S., Reinhard S., Li C., Qian M., Jiang H., Du Y. et al. (2017) Antitumoral cascade-targeting ligand for IL-6 receptor-mediated gene delivery to glioma. Mol. Ther. 25, 1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Klein P.M. and Wagner E. (2014) Bioreducible polycations as shuttles for therapeutic nucleic acid and protein transfection. Antioxid. Redox Signaling 21, 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klein P.M., Müller K., Gutmann C., Kos P., Krhac Levacic A., Edinger D. et al. (2015) Twin disulfides as opportunity for improving stability and transfection efficiency of oligoaminoethane polyplexes. J. Controlled Release 205, 109. [DOI] [PubMed] [Google Scholar]
- 113.Troiber C., Edinger D., Kos P., Schreiner L., Kläger R., Herrmann A. et al. (2013) Stabilizing effect of tyrosine trimers on pDNA and siRNA polyplexes. Biomaterials 34, 1624. [DOI] [PubMed] [Google Scholar]
- 114.Schaffert D., Troiber C. and Wagner E. (2012) New sequence-defined polyaminoamides with tailored endosomolytic properties for plasmid DNA delivery. Bioconjugate Chem. 23, 1157. [DOI] [PubMed] [Google Scholar]
- 115.Nothisen M., Kotera M., Voirin E., Remy J.S. and Behr J.P. (2009) Cationic siRNAs provide carrier-free gene silencing in animal cells. J. Am. Chem. Soc. 131, 17730. [DOI] [PubMed] [Google Scholar]
- 116.Perche P., Kotera M. and Remy J.S. (2011) MMT, Npeoc-protected spermine, a valuable synthon for the solid phase synthesis of oligonucleotide oligospermine conjugates via guanidine linkers. Bioorg. Med. Chem. 19, 1972. [DOI] [PubMed] [Google Scholar]
- 117.Nothisen M., Bagilet J., Behr J.P., Remy J.S. and Kotera M. (2016) Structure tuning of cationic oligospermine-siRNA conjugates for carrier-free gene silencing. Mol. Pharmaceutics 13, 2718. [DOI] [PubMed] [Google Scholar]
- 118.Perche P., Nothisen M., Bagilet J., Behr J.P., Kotera M. and Remy J.S. (2013) Cell-penetrating cationic siRNA and lipophilic derivatives efficient at nanomolar concentrations in the presence of serum and albumin. J. Controlled Release 170, 92. [DOI] [PubMed] [Google Scholar]
- 119.Mislick K.A. and Baldeschwieler J.D. (1996) Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc. Natl. Acad. Sci. U.S.A. 93, 12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kopatz I., Remy J.S. and Behr J.P. (2004) A model for non-viral gene delivery: through syndecan adhesion molecules and powered by actin. J. Gene Med. 6, 769. [DOI] [PubMed] [Google Scholar]
- 121.Wagner E. (2012) Polymers for siRNA delivery: inspired by viruses to be targeted, dynamic, and precise. Acc. Chem. Res. 45, 1005. [DOI] [PubMed] [Google Scholar]
- 122.Yousefi A., Storm G., Schiffelers R. and Mastrobattista E. (2013) Trends in polymeric delivery of nucleic acids to tumors. J. Controlled Release 170, 209. [DOI] [PubMed] [Google Scholar]
- 123.Plank C., Mechtler K., Szoka F.C. Jr and Wagner E. (1996) Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum. Gene Ther. 7, 1437. [DOI] [PubMed] [Google Scholar]
- 124.Merkel O.M., Urbanics R., Bedocs P., Rozsnyay Z., Rosivall L., Toth M. et al. (2011) In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine-graft-poly(ethylene glycol) block copolymers. Biomaterials 32, 4936. [DOI] [PubMed] [Google Scholar]
- 125.Burke R.S. and Pun S.H. (2008) Extracellular barriers to in vivo PEI and PEGylated PEI polyplex-mediated gene delivery to the liver. Bioconjugate Chem. 19, 693. [DOI] [PubMed] [Google Scholar]
- 126.Hall A., Lächelt U., Bartek J., Wagner E. and Moghimi S.M. (2017) Polyplex evolution: understanding biology, optimizing performance. Mol. Ther. 25, 1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Knop K., Hoogenboom R., Fischer D. and Schubert U.S. (2010) Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem., Int. Ed. 49, 6288. [DOI] [PubMed] [Google Scholar]
- 128.Kursa M., Walker G.F., Roessler V., Ogris M., Rödl W., Kircheis R. et al. (2003) Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. Bioconjugate Chem. 14, 222. [DOI] [PubMed] [Google Scholar]
- 129.DeRouchey J., Walker G.F., Wagner E. and Rädler J.O. (2006) Decorated rods: a “bottom-up” self-assembly of monomolecular DNA complexes. J. Phys. Chem. B 110, 4548. [DOI] [PubMed] [Google Scholar]
- 130.Fella C., Walker G.F., Ogris M. and Wagner E. (2008) Amine-reactive pyridylhydrazone-based PEG reagents for pH-reversible PEI polyplex shielding. Eur. J. Pharm. Sci. 34, 309. [DOI] [PubMed] [Google Scholar]
- 131.Merkel O.M., Librizzi D., Pfestroff A., Schurrat T., Buyens K., Sanders N.N. et al. (2009) Stability of siRNA polyplexes from poly(ethylenimine) and poly(ethylenimine)-g-poly(ethylene glycol) under in vivo conditions: effects on pharmacokinetics and biodistribution measured by Fluorescence Fluctuation Spectroscopy and Single Photon Emission Computed Tomography (SPECT) imaging. J. Controlled Release 138, 148. [DOI] [PubMed] [Google Scholar]
- 132.Burke R.S. and Pun S.H. (2010) Synthesis and characterization of biodegradable HPMA-oligolysine copolymers for improved gene delivery. Bioconjugate Chem. 21, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Beckert L., Kostka L., Kessel E., Krhac Levacic A., Kostkova H., Etrych T. et al. (2016) Acid-labile pHPMA modification of four-arm oligoaminoamide pDNA polyplexes balances shielding and gene transfer activity in vitro and in vivo. Eur. J. Pharm. Biopharm. 105, 85. [DOI] [PubMed] [Google Scholar]
- 134.Noga M., Edinger D., Kläger R., Wegner S.V., Spatz J.P., Wagner E. et al. (2013) The effect of molar mass and degree of hydroxyethylation on the controlled shielding and deshielding of hydroxyethyl starch-coated polyplexes. Biomaterials 34, 2530. [DOI] [PubMed] [Google Scholar]
- 135.Heller P., Birke A., Huesmann D., Weber B., Fischer K., Reske-Kunz A. et al. (2014) Introducing PeptoPlexes: polylysine-block-polysarcosine based polyplexes for transfection of HEK 293T cells. Macromol. Biosci. 14, 1380. [DOI] [PubMed] [Google Scholar]
- 136.Dohmen C., Fröhlich T., Lächelt U., Rohl I., Vornlocher H.P., Hadwiger P. et al. (2012) Defined folate-PEG-siRNA conjugates for receptor-specific gene silencing. Mol. Ther. Nucleic Acids 1, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hatakeyama H., Akita H. and Harashima H. (2011) A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv. Drug Deliv. Rev. 63, 152. [DOI] [PubMed] [Google Scholar]
- 138.Tockary T.A., Osada K., Motoda Y., Hiki S., Chen Q., Takeda K.M. et al. (2016) Rod-to-globule transition of pDNA/PEG-poly(l-lysine) polyplex micelles induced by a collapsed balance between DNA rigidity and PEG crowdedness. Small 12, 1193. [DOI] [PubMed] [Google Scholar]
- 139.Morys S., Urnauer S., Spitzweg C. and Wagner E. (2017) EGFR targeting and shielding of pDNA lipopolyplexes via bivalent attachment of a sequence-defined PEG agent. Macromol. Biosci., 10.1002/mabi.20170020 [DOI] [PubMed] [Google Scholar]
- 140.Müller K., Klein P.M., Heissig P., Roidl A. and Wagner E. (2016) EGF receptor targeted lipo-oligocation polyplexes for antitumoral siRNA and miRNA delivery. Nanotechnology 27, 464001. [DOI] [PubMed] [Google Scholar]
- 141.Zhang W., Müller K., Kessel E., Reinhard S., He D., Klein P.M. et al. (2016) Targeted siRNA delivery using a lipo-oligoaminoamide nanocore with an influenza peptide and transferrin shell. Adv. Healthcare Mater. 5, 1493. [DOI] [PubMed] [Google Scholar]
- 142.Müller K., Kessel E., Klein P.M., Höhn M. and Wagner E. (2016) Post-PEGylation of siRNA lipo-oligoamino amide polyplexes using tetra-glutamylated folic acid as ligand for receptor-targeted delivery. Mol. Pharmaceutics 13, 2332. [DOI] [PubMed] [Google Scholar]
- 143.Walker G.F., Fella C., Pelisek J., Fahrmeir J., Boeckle S., Ogris M. et al. (2005) Toward synthetic viruses: endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Mol. Ther. 11, 418. [DOI] [PubMed] [Google Scholar]
- 144.Knorr V., Allmendinger L., Walker G.F., Paintner F.F. and Wagner E. (2007) An acetal-based PEGylation reagent for pH-sensitive shielding of DNA polyplexes. Bioconjugate Chem. 18, 1218. [DOI] [PubMed] [Google Scholar]
- 145.Rozema D.B., Lewis D.L., Wakefield D.H., Wong S.C., Klein J.J., Roesch P.L. et al. (2007) Dynamic polyconjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 104, 12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Das M., Mohanty C. and Sahoo S.K. (2009) Ligand-based targeted therapy for cancer tissue. Expert Opin. Drug Deliv. 6, 285. [DOI] [PubMed] [Google Scholar]
- 147.Ogris M. and Wagner E. (2011) To be targeted: is the magic bullet concept a viable option for synthetic nucleic acid therapeutics? Hum. Gene Ther. 22, 799. [DOI] [PubMed] [Google Scholar]
- 148.Wagner E. (2011) Functional polymer conjugates for medicinal nucleic acid delivery.. In Polymers in Nanomedicine. Advances in Polymer Science. (Kunugi S., Yamaoka T., eds), Vol. 247, pp. 1 [Google Scholar]
- 149.Duskey J.T. and Rice K.G. (2014) Nanoparticle ligand presentation for targeting solid tumors. AAPS Pharm. Sci. Tech. 15, 1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wadhwa M.S., Knoell D.L., Young A.P. and Rice K.G. (1995) Targeted gene delivery with a low molecular weight glycopeptide carrier. Bioconjugate Chem. 6, 283. [DOI] [PubMed] [Google Scholar]
- 151.Ueyama H., Takagi M., Waki M. and Takenaka S. (2001) DNA binding behavior of peptides carrying acridinyl units: First example of effective poly-intercalation. Nucleic Acids Res. 163. [DOI] [PubMed] [Google Scholar]
- 152.Anderson K., Fernandez C. and Rice K.G. (2010) N-glycan targeted gene delivery to the dendritic cell SIGN receptor. Bioconjugate Chem. 21, 1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Colin M., Moritz S., Fontanges P., Kornprobst M., Delouis C., Keller M. et al. (2001) The nuclear pore complex is involved in nuclear transfer of plasmid DNA condensed with an oligolysine-RGD peptide containing nuclear localisation properties. Gene Ther. 8, 1643. [DOI] [PubMed] [Google Scholar]
- 154.Wang X.L., Xu R., Wu X., Gillespie D., Jensen R. and Lu Z.R. (2009) Targeted systemic delivery of a therapeutic siRNA with a multifunctional carrier controls tumor proliferation in mice. Mol. Pharmaceutics 6, 738. [DOI] [PubMed] [Google Scholar]
- 155.Broda E., Mickler F.M., Lächelt U., Morys S., Wagner E. and Bräuchle C. (2015) Assessing potential peptide targeting ligands by quantification of cellular adhesion of model nanoparticles under flow conditions. J. Controlled Release 213, 79. [DOI] [PubMed] [Google Scholar]
- 156.Urnauer S., Klutz K., Grünwald G.K., Morys S., Schwenk N., Zach C. et al. (2017) Systemic tumor-targeted sodium iodide symporter (NIS) gene therapy of hepatocellular carcinoma mediated by B6 peptide polyplexes. J. Gene Med. 19, 10.1002/jgm.2957 [DOI] [PubMed] [Google Scholar]
- 157.Urnauer S., Morys S., Krhac Levacic A., Müller A.M., Schug C., Schmohl K.A. et al. (2016) Sequence-defined cMET/HGFR-targeted polymers as gene delivery vehicles for the theranostic sodium iodide symporter (NIS) gene. Mol. Ther. 24, 1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kos P., Lächelt U., He D., Nie Y., Gu Z. and Wagner E. (2015) Dual-targeted polyplexes based on sequence-defined peptide-PEG-oligoamino amides. J. Pharm. Sci. 104, 464. [DOI] [PubMed] [Google Scholar]
- 159.Wagner E., Zenke M., Cotten M., Beug H. and Birnstiel M.L. (1990) Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc. Natl. Acad. Sci. U.S.A. 87, 3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wagner E., Cotten M., Mechtler K., Kirlappos H. and Birnstiel M.L. (1991) DNA-binding transferrin conjugates as functional gene-delivery agents: synthesis by linkage of polylysine or ethidium homodimer to the transferrin carbohydrate moiety. Bioconjugate Chem. 2, 226. [DOI] [PubMed] [Google Scholar]
- 161.Schreiber S., Kampgen E., Wagner E., Pirkhammer D., Trcka J., Korschan H. et al. (1999) Immunotherapy of metastatic malignant melanoma by a vaccine consisting of autologous interleukin 2-transfected cancer cells: outcome of a phase I study. Hum. Gene Ther. 10, 983. [DOI] [PubMed] [Google Scholar]
- 162.Kircheis R., Kichler A., Wallner G., Kursa M., Ogris M., Felzmann T. et al. (1997) Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Ther. 4, 409. [DOI] [PubMed] [Google Scholar]
- 163.Kircheis R., Schuller S., Brunner S., Ogris M., Heider K.H., Zauner W. et al. (1999) Polycation-based DNA complexes for tumor-targeted gene delivery in vivo. J. Gene Med. 1, 111. [DOI] [PubMed] [Google Scholar]
- 164.Kircheis R., Wightman L., Schreiber A., Robitza B., Rossler V., Kursa M. et al. (2001) Polyethylenimine/DNA complexes shielded by transferrin target gene expression to tumors after systemic application. Gene Ther. 8, 28. [DOI] [PubMed] [Google Scholar]
- 165.Davis M.E., Zuckerman J.E., Choi C.H., Seligson D., Tolcher A., Alabi C.A. et al. (2010) Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464, 1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang W., Rödl W., He D., Doblinger M., Lächelt U. and Wagner E. (2015) Combination of sequence-defined oligoaminoamides with transferrin-polycation conjugates for receptor-targeted gene delivery. J. Gene Med. 17, 161. [DOI] [PubMed] [Google Scholar]
- 167.Prades R., Oller-Salvia B., Schwarzmaier S.M., Selva J., Moros M., Balbi M. et al. (2015) Applying the retro-enantio approach to obtain a peptide capable of overcoming the blood-brain barrier. Angew. Chem., Int. Ed. 54, 3967. [DOI] [PubMed] [Google Scholar]
- 168.Hilgenbrink A.R. and Low P.S. (2005) Folate receptor-mediated drug targeting: from therapeutics to diagnostics. J. Pharm. Sci. 94, 2135. [DOI] [PubMed] [Google Scholar]
- 169.Zhang C.Y., Kos P., Müller K., Schrimpf W., Troiber C., Lächelt U. et al. (2014) Native chemical ligation for conversion of sequence-defined oligomers into targeted pDNA and siRNA carriers. J. Controlled Release 180, 42. [DOI] [PubMed] [Google Scholar]
- 170.Lee D.J., He D., Kessel E., Padari K., Kempter S., Lächelt U. et al. (2016) Tumoral gene silencing by receptor-targeted combinatorial siRNA polyplexes. J. Controlled Release 244, 280. [DOI] [PubMed] [Google Scholar]
- 171.An S., He D., Wagner E. and Jiang C. (2015) Peptide-like polymers exerting effective glioma-targeted siRNA delivery and release for therapeutic application. Small 11, 5142. [DOI] [PubMed] [Google Scholar]
- 172.Uchida H., Miyata K., Oba M., Ishii T., Suma T., Itaka K. et al. (2011) Odd-even effect of repeating aminoethylene units in the side chain of N-substituted polyaspartamides on gene transfection profiles. J. Am. Chem. Soc. 133, 15524. [DOI] [PubMed] [Google Scholar]
- 173.Miyata K., Nishiyama N. and Kataoka K. (2012) Rational design of smart supramolecular assemblies for gene delivery: chemical challenges in the creation of artificial viruses. Chem. Soc. Rev. 41, 2562. [DOI] [PubMed] [Google Scholar]
- 174.Benjaminsen R.V., Mattebjerg M.A., Henriksen J.R., Moghimi S.M. and Andresen T.L. (2013) The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 21, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Funhoff A.M., van Nostrum C.F., Koning G.A., Schuurmans-Nieuwenbroek N.M., Crommelin D.J. and Hennink W.E. (2004) Endosomal escape of polymeric gene delivery complexes is not always enhanced by polymers buffering at low pH. Biomacromolecules 5, 32. [DOI] [PubMed] [Google Scholar]
- 176.ur Rehman Z., Hoekstra D. and Zuhorn I.S. (2013) Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano 7, 3767. [DOI] [PubMed] [Google Scholar]
- 177.Boeckle S., von Gersdorff K., van der Piepen S., Culmsee C., Wagner E. and Ogris M. (2004) Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J. Gene Med. 6, 1102. [DOI] [PubMed] [Google Scholar]
- 178.Yue Y., Jin F., Deng R., Cai J., Chen Y., Lin M.C. et al. (2011) Revisit complexation between DNA and polyethylenimine – effect of uncomplexed chains free in the solution mixture on gene transfection. J. Controlled Release 155, 67. [DOI] [PubMed] [Google Scholar]
- 179.Yue Y., Jin F., Deng R., Cai J., Dai Z., Lin M.C. et al. (2011) Revisit complexation between DNA and polyethylenimine–effect of length of free polycationic chains on gene transfection. J. Controlled Release 152, 143. [DOI] [PubMed] [Google Scholar]
- 180.Cai J., Yue Y., Wang Y., Jin Z., Jin F. and Wu C. (2016) Quantitative study of effects of free cationic chains on gene transfection in different intracellular stages. J. Controlled Release 238, 71. [DOI] [PubMed] [Google Scholar]
- 181.Midoux P. and Monsigny M. (1999) Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjugate Chem. 10, 406. [DOI] [PubMed] [Google Scholar]
- 182.Pichon C., Roufai M.B., Monsigny M. and Midoux P. (2000) Histidylated oligolysines increase the transmembrane passage and the biological activity of antisense oligonucleotides. Nucleic Acids Res. 28, 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bertrand E., Goncalves C., Billiet L., Gomez J.P., Pichon C., Cheradame H. et al. (2011) Histidinylated linear PEI: a new efficient non-toxic polymer for gene transfer. Chem. Commun. 47, 12547. [DOI] [PubMed] [Google Scholar]
- 184.Gomez J.P., Pichon C. and Midoux P. (2013) Ability of plasmid DNA complexed with histidinylated lPEI and lPEI to cross in vitro lung and muscle vascular endothelial barriers. Gene 525, 182. [DOI] [PubMed] [Google Scholar]
- 185.Stevenson M., Ramos-Perez V., Singh S., Soliman M., Preece J.A., Briggs S.S. et al. (2008) Delivery of siRNA mediated by histidine-containing reducible polycations. J. Controlled Release 130, 46. [DOI] [PubMed] [Google Scholar]
- 186.Plank C., Oberhauser B., Mechtler K., Koch C. and Wagner E. (1994) The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J. Biol. Chem. 269, 12918. [PubMed] [Google Scholar]
- 187.Gottschalk S., Sparrow J.T., Hauer J., Mims M.P., Leland F.E., Woo S.L. et al. (1996) A novel DNA-peptide complex for efficient gene transfer and expression in mammalian cells 844. Gene Ther. 3, 48. [PubMed] [Google Scholar]
- 188.Midoux P., Kichler A., Boutin V., Maurizot J.C. and Monsigny M. (1998) Membrane permeabilization and efficient gene transfer by a peptide containing several histidines 878. Bioconjugate Chem. 9, 260. [DOI] [PubMed] [Google Scholar]
- 189.Wyman T.B., Nicol F., Zelphati O., Scaria P.V., Plank C. and Szoka F.C. Jr (1997) Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry 36, 3008. [DOI] [PubMed] [Google Scholar]
- 190.Kichler A., Leborgne C., Marz J., Danos O. and Bechinger B. (2003) Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 100, 1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Boeckle S., Fahrmeir J., Rödl W., Ogris M. and Wagner E. (2006) Melittin analogs with high lytic activity at endosomal pH enhance transfection with purified targeted PEI polyplexes. J. Controlled Release 112, 240. [DOI] [PubMed] [Google Scholar]
- 192.Xu Y. and Szoka F.C. Jr (1996) Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry 35, 5616. [DOI] [PubMed] [Google Scholar]
- 193.von Gersdorff K., Sanders N.N., Vandenbroucke R., De Smedt S.C., Wagner E. and Ogris M. (2006) The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Mol. Ther. 14, 745. [DOI] [PubMed] [Google Scholar]
- 194.Rejman J., Bragonzi A. and Conese M. (2005) Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol. Ther. 12, 468. [DOI] [PubMed] [Google Scholar]
- 195.Remaut K., Lucas B., Raemdonck K., Braeckmans K., Demeester J. and De Smedt S.C. (2007) Can we better understand the intracellular behavior of DNA nanoparticles by fluorescence correlation spectroscopy? J. Controlled Release 121, 49. [DOI] [PubMed] [Google Scholar]
- 196.Lucas B., Remaut K., Sanders N.N., Braeckmans K., De Smedt S.C. and Demeester J. (2005) Towards a better understanding of the dissociation behavior of liposome-oligonucleotide complexes in the cytosol of cells. J. Controlled Release 103, 435. [DOI] [PubMed] [Google Scholar]
- 197.Gilleron J., Querbes W., Zeigerer A., Borodovsky A., Marsico G., Schubert U. et al. (2013) Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 31, 638. [DOI] [PubMed] [Google Scholar]
- 198.Wittrup A., Ai A., Liu X., Hamar P., Trifonova R., Charisse K. et al. (2015) Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 33, 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sahay G., Querbes W., Alabi C., Eltoukhy A., Sarkar S., Zurenko C. et al. (2013) Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 31, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee D.J., Kessel E., Lehto T., Liu X., Yoshinaga N., Padari K. et al. (2017) Systemic delivery of folate-PEG siRNA lipopolyplexes with enhanced intracellular stability for in vivo gene silencing in leukemia. Bioconjugate Chem. 28, 2393. [DOI] [PubMed] [Google Scholar]
- 201.Wasungu L. and Hoekstra D. (2006) Cationic lipids, lipoplexes and intracellular delivery of genes. J. Controlled Release 116, 255. [DOI] [PubMed] [Google Scholar]
- 202.Conese M., Biffi A., Dina G., Marziliano N. and Villa A. (2009) Comparison between cationic polymer and lipid in plasmidic DNA delivery to the cell nucleus. Open Gene Ther. J. 2, 21 [Google Scholar]
- 203.Zelphati O. and Szoka F.C. Jr (1996) Mechanism of oligonucleotide release from cationic liposomes. Proc. Natl. Acad. Sci. U.S.A. 93, 11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hama S., Akita H., Ito R., Mizuguchi H., Hayakawa T. and Harashima H. (2006) Quantitative comparison of intracellular trafficking and nuclear transcription between adenoviral and lipoplex systems. Mol. Ther. 13, 786. [DOI] [PubMed] [Google Scholar]
- 205.Sixou S., Szoka F.C. Jr, Green G.A., Giusti B., Zon G. and Chin D.J. (1994) Intracellular oligonucleotide hybridization detected by fluorescence resonance energy transfer (FRET) 913. Nucleic Acids Res. 22, 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Heissig P., Schrimpf W., Hadwiger P., Wagner E. and Lamb D.C. (2017) Monitoring integrity and localization of modified single-stranded RNA oligonucleotides using ultrasensitive fluorescence methods. PLoS One 12, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Subramanian A., Ranganathan P. and Diamond S.L. (1999) Nuclear targeting peptide scaffolds for lipofection of nondividing mammalian cells. Nat. Biotechnol. 17, 873. [DOI] [PubMed] [Google Scholar]
- 208.Krhac Levacic A., Morys S., Kempter S., Lächelt U. and Wagner E. (2017) Minicircle versus plasmid DNA delivery by receptor-targeted polyplexes. Hum. Gene Ther. [DOI] [PubMed] [Google Scholar]
- 209.Zanta M.A., Belguise V.P. and Behr J.P. (1999) Gene delivery: A single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc. Natl. Acad. Sci. U.S.A. 96, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tachibana R., Harashima H., Shinohara Y. and Kiwada H. (2001) Quantitative studies on the nuclear transport of plasmid DNA and gene expression employing nonviral vectors. Adv. Drug Deliv. Rev. 52, 219. [DOI] [PubMed] [Google Scholar]
- 211.Cartier R. and Reszka R. (2002) Utilization of synthetic peptides containing nuclear localization signals for nonviral gene transfer systems. Gene Ther. 9, 157. [DOI] [PubMed] [Google Scholar]
- 212.Chan C.K., Senden T. and Jans D.A. (2000) Supramolecular structure and nuclear targeting efficiency determine the enhancement of transfection by modified polylysines. Gene Ther. 7, 1690. [DOI] [PubMed] [Google Scholar]
- 213.Branden L.J., Mohamed A.J. and Smith C.I. (1999) A peptide nucleic acid-nuclear localization signal fusion that mediates nuclear transport of DNA. Nat. Biotechnol. 17, 784. [DOI] [PubMed] [Google Scholar]
- 214.Ciolina C., Byk G., Blanche F., Thuillier V., Scherman D. and Wils P. (1999) Coupling of nuclear localization signals to plasmid DNA and specific interaction of the conjugates with importin alpha. Bioconjugate Chem. 10, 49. [DOI] [PubMed] [Google Scholar]
- 215.Bremner K.H., Seymour L.W., Logan A. and Read M.L. (2004) Factors influencing the ability of nuclear localization sequence peptides to enhance nonviral gene delivery. Bioconjugate Chem. 15, 152. [DOI] [PubMed] [Google Scholar]
- 216.Schiffelers R.M., Ansari A., Xu J., Zhou Q., Tang Q., Storm G. et al. (2004) Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res. 32, e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li S.D. and Huang L. (2006) Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol. Pharmaceutics 3, 579. [DOI] [PubMed] [Google Scholar]
- 218.Low P.S., Henne W.A. and Doorneweerd D.D. (2008) Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 41, 120. [DOI] [PubMed] [Google Scholar]
- 219.Schäfer A., Pahnke A., Schaffert D., van Weerden W.M., de Ridder C.M., Rödl W. et al. (2011) Disconnecting the yin and yang relation of epidermal growth factor receptor (EGFR)-mediated delivery: a fully synthetic, EGFR-targeted gene transfer system avoiding receptor activation. Hum. Gene Ther. 22, 1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Klutz K., Schaffert D., Willhauck M.J., Grunwald G.K., Haase R., Wunderlich N. et al. (2011) Epidermal growth factor receptor-targeted (131)I-therapy of liver cancer following systemic delivery of the sodium iodide symporter gene. Mol. Ther. 19, 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Abourbeh G., Shir A., Mishani E., Ogris M., Rödl W., Wagner E. et al. (2012) PolyIC GE11 polyplex inhibits EGFR-overexpressing tumors. IUBMB Life 64, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Choi H.S., Liu W., Liu F., Nasr K., Misra P., Bawendi M.G. et al. (2010) Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Maeda H. (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 41, 189. [DOI] [PubMed] [Google Scholar]
- 224.Maeda H., Bharate G.Y. and Daruwalla J. (2009) Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 71, 409. [DOI] [PubMed] [Google Scholar]
- 225.Smrekar B., Wightman L., Wolschek M.F., Lichtenberger C., Ruzicka R., Ogris M. et al. (2003) Tissue-dependent factors affect gene delivery to tumors in vivo. Gene Ther. 10, 1079. [DOI] [PubMed] [Google Scholar]
- 226.Cabral H., Matsumoto Y., Mizuno K., Chen Q., Murakami M., Kimura M. et al. (2011) Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 6, 815. [DOI] [PubMed] [Google Scholar]
- 227.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F. et al. (2016) Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 [Google Scholar]
- 228.Merdan T., Kunath K., Petersen H., Bakowsky U., Voigt K.H., Kopecek J. et al. (2005) PEGylation of poly(ethylene imine) affects stability of complexes with plasmid DNA under in vivo conditions in a dose-dependent manner after intravenous injection into mice. Bioconjugate Chem. 16, 785. [DOI] [PubMed] [Google Scholar]
- 229.Neu M., Germershaus O., Behe M. and Kissel T. (2007) Bioreversibly crosslinked polyplexes of PEI and high molecular weight PEG show extended circulation times in vivo. J. Controlled Release 124, 69. [DOI] [PubMed] [Google Scholar]
- 230.Wei H., Volpatti L.R., Sellers D.L., Maris D.O., Andrews I.W., Hemphill A.S. et al. (2013) Dual responsive, stabilized nanoparticles for efficient in vivo plasmid delivery. Angew. Chem., Int. Ed. 52, 5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Cheng Y., Yumul R.C. and Pun S.H. (2016) Virus-inspired polymer for efficient in vitro and in vivo gene delivery. Angew. Chem., Int. Ed. 55, 12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Neuberg P. and Kichler A. (2014) Recent developments in nucleic acid delivery with polyethylenimines. Adv. Genet. 88, 263. [DOI] [PubMed] [Google Scholar]
- 233.Breunig M., Lungwitz U., Liebl R. and Goepferich A. (2007) Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. Proc. Natl. Acad. Sci. U.S.A. 104, 14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wagner E. (2007) Programmed drug delivery: nanosystems for tumor targeting. Expert Opin. Biol. Ther. 7, 587. [DOI] [PubMed] [Google Scholar]
- 235.Wolff J.A. and Rozema D.B. (2008) Breaking the bonds: non-viral vectors become chemically dynamic. Mol. Ther. 16, 8. [DOI] [PubMed] [Google Scholar]